Assessment of Biological Toxicity and Ecological Safety for Urban Black-Odor River Remediation
Abstract
:1. Introduction
2. Materials and Methods
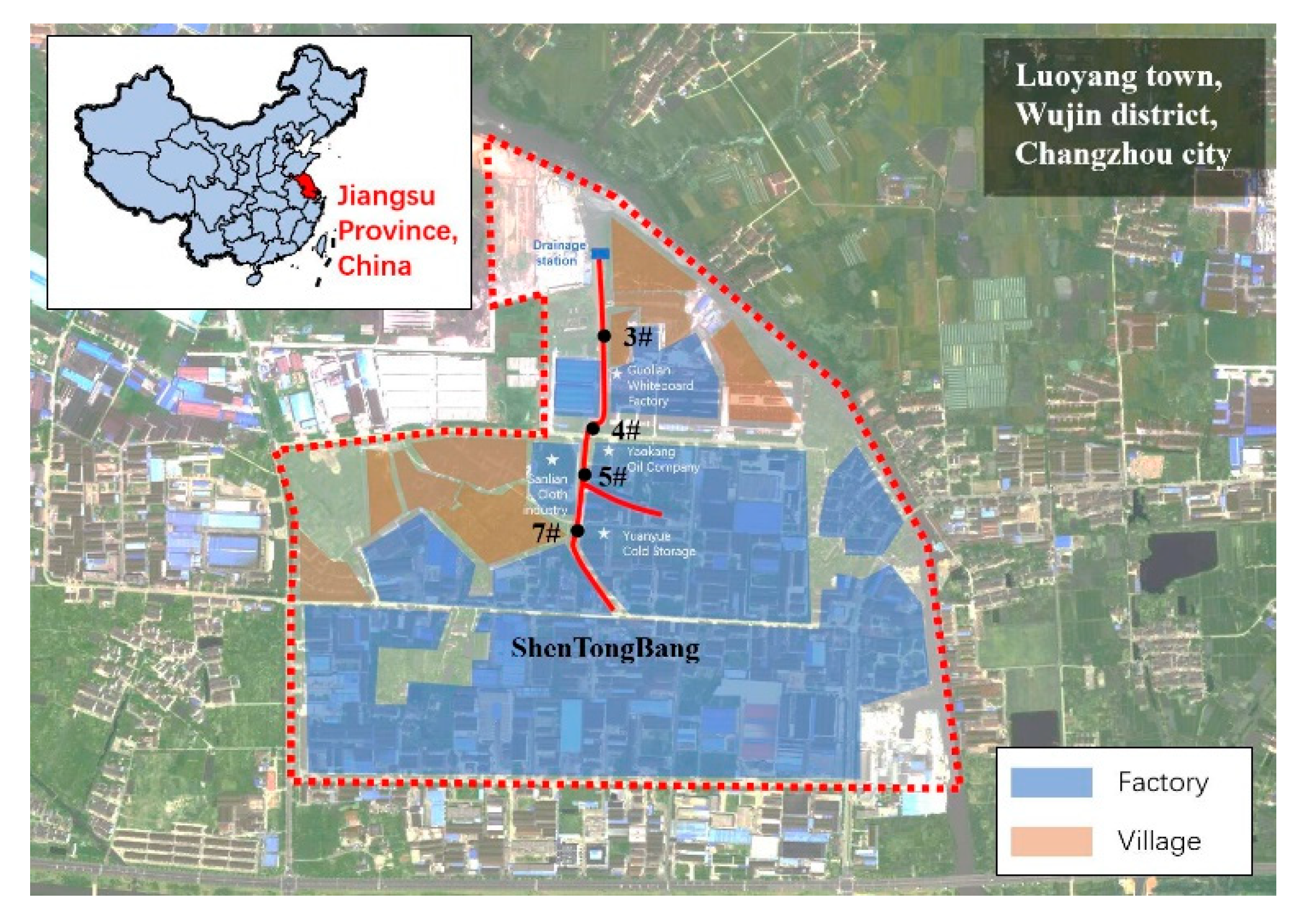
2.1. Sampling Sites and Remediation Process
2.2. Physicochemical Analysis of Water Quality
2.3. Toxicity Tests
2.3.1. Acute Toxicity Experiments with C. vulgaris
2.3.2. Acute Toxicity Experiments with D. magna
2.3.3. Ames Test
2.4. Statistical Analysis
3. Results and Discussion
3.1. Water Quality
3.2. Acute Toxicity of Overlying Water before and after Remediation
3.2.1. Acute Toxicity on C. vulgaris
3.2.2. Acute Toxicity on D. magna
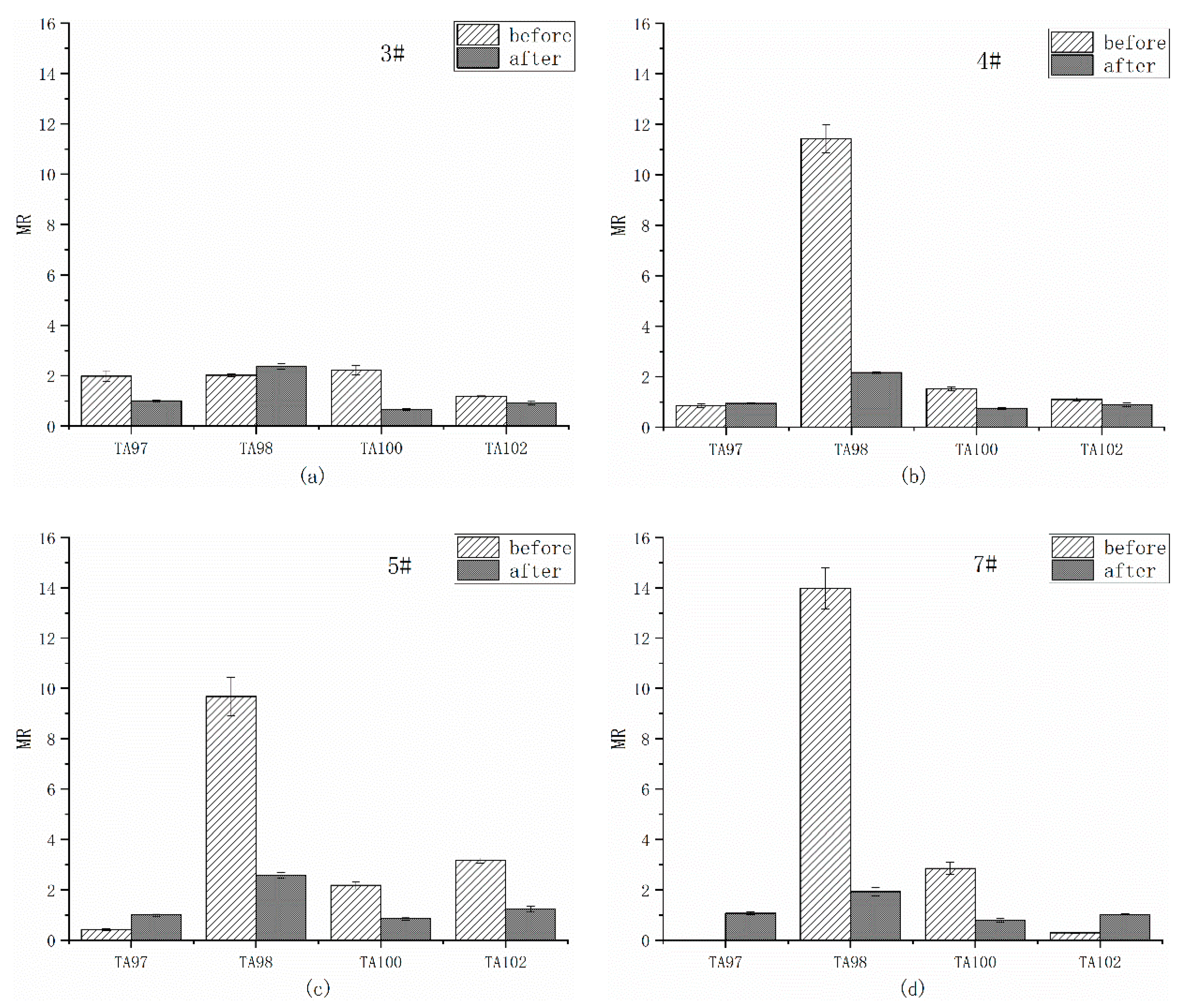
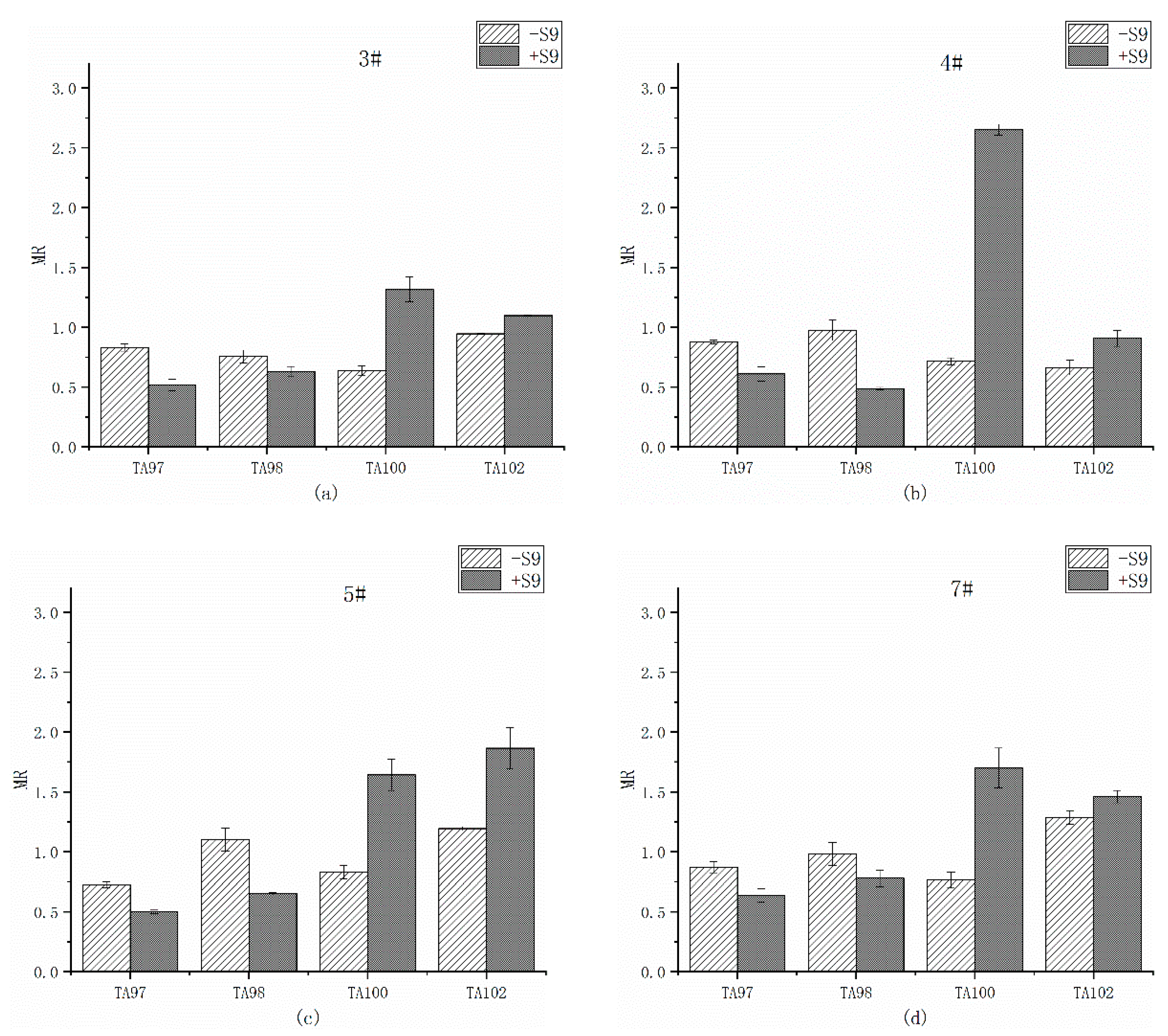
3.3. Mutagenicity of Overlying Water
3.3.1. Ames Test of Overlying Water Extracts Before and After Remediation
3.3.2. Ames Test of Overlying Water after Remediation in the Presence and Absence of Liver Microsomal S9
4. Conclusions
- Acute toxicity of overlying water from Shengtongbang by C. vulgaris tests showed nontoxic before and after remediation, but promoted the growth of C. vulgaris because of the excessive TN and TP, indicated that the restrictions on the TN and TP are still needed.
- The D. magna tests showed the toxicity of overlying water on site 3# before remediation, and be declined to nontoxic after remediation. The results suggested the remediation effects were effective for D. magna.
- The mutagenicity of organic extracts from overlying water by Ames test at all sampling sites was positive before remediation. After remediation, the mutagenicity of most sites eliminated except of the TA98 strain from 3 of 4 sites. The addition of liver microsomal S9 induced the positive mutagenicity on site 4#, implying that the metabolism of the microorganisms changed the mutagenicity of the organic extracts from overlying water. The remediation significantly improved the water quality, but the potential ecological risk still exists in Shengtongbang.
- The results of this study applied comprehensive toxicity tests to evaluate the remediation results in urban black-odor rivers, and provided biological assessing method for the remediation evaluation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Wang, Y.G.; Sun, C.H.; Pan, T. Formation mechanism and assessment method for urban black and odorous water body: A review. Chin. J. Appl. Ecol. 2016, 27, 1331–1340. [Google Scholar]
- Kerr, J.L.; Baldwin, D.S.; Whitworth, K.L. Options for managing hypoxic blackwater events in river systems: A review. J. Environ. Manag. 2013, 114, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.J.; Gurnell, A.M.; Armitage, P.D. Habitat survey and classification of urban rivers. River Res. Appl. 2004, 20, 687–704. [Google Scholar] [CrossRef]
- Malmaeus, J.; Hakanson, L. Development of a lake eutrophication model. Ecol. Model. 2004, 171, 35–63. [Google Scholar] [CrossRef]
- Sugiura, N.; Utsumi, M.; Wei, B.; Iwami, N.; Okano, K.; Kawauchi, Y.; Maekawa, T. Assessment for the complicated occurrence of nuisance odours from phytoplankton and environmental factors in a eutrophic lake. Lakes Reserv. Res. Manag. 2004, 9, 195–201. [Google Scholar] [CrossRef]
- Shen, Q.; Zhu, L.; Cao, H.Y. Remote sensing monitoring and screening for urban black and odorous water body: A review. Chin. J. Appl. Ecol. 2017, 28, 3433–3439. [Google Scholar]
- Chen, G.; White, P.A. The mutagenic hazards of aquatic sediments: A review. Mutat. Res. 2004, 567, 151–225. [Google Scholar] [CrossRef]
- USEPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms; U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- Fang, Y.X.; Ying, G.G.; Zhang, L.J.; Zhao, J.L.; Su, H.C.; Yang, B.; Liu, S. Use of TIE techniques to characterize industrial effluents in the Pearl River Delta region. Ecotoxicol. Environ. Saf. 2011, 76, 143–152. [Google Scholar] [CrossRef]
- Ames, B.N.; Mccann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975, 31, 347–363. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, B.J.; Lee, S.K.; Kim, K.; Lee, K.H.; Che, J.H.; Kang, K.S.; Lee, Y.S. Genotoxicity of drinking water from three Korean cities. Mutat. Res. 2000, 466, 173–178. [Google Scholar] [CrossRef]
- Kutlu, M.; Aydoğan, G.; Susuz, F.; Özata, A. The Salmonella mutagenicity of water and sediments from the Porsuk River in Turkey. Environ. Toxicol. Phar. 2004, 17, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Environmental Quality Standards for Surface Water, GB3838-2002. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Available online: http://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/shjzlbz/200206/W020061027509896672057.pdf (accessed on 1 June 2002).
- The State Environmental Protection Administration. Water and Wastewater Monitoring and Analysis Method, 4th ed.; China Environmental Science Press: Beijing, China, 2002.
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard methods for the examination of water and wastewater. Am. J. Public Health Nations Health 1995, 56, 387–388. [Google Scholar]
- Chemicals-Alga Growth Inhibition Test, GB/T 21805-2008. General Administration of Quality Supervision, Inspection and Quarantine, People’s Republic of China; Standardization Administration of China. Available online: https://www.spc.org.cn/online/GB%252FT%252021805-2008/ (accessed on 12 May 2008).
- Tonkes, M.; De Graaf, P.J.; Graansma, J. Assessment of complex industrial effluents in the Netherlands using a whole effluent toxicity (or WET) approach. Water Sci. Technol. 1999, 39, 55–61. [Google Scholar] [CrossRef]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. Int. J. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Method for Acute Toxicity Test of Daphnia Magna Straus, GB/T 16125-2012. Ministry of Health, People’s Republic of China, Standardization Administration of China. Available online: http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=E86B0B717CF7AF44C15AA64118D14E85 (accessed on 20 September 2012).
- Ra, J.S.; Lee, B.C.; Chang, N.I.; Kim, S.D. Comparative whole effluent toxicity assessment of wastewater treatment plant effluents using Daphnia magna. Bull. Environ. Contam. Toxicol. 2008, 80, 196–200. [Google Scholar] [CrossRef]
- Safety and Technical Standards for Cosmetics. China Food and Drug Administration. Available online: http://samr.cfda.gov.cn/directory/web/WS01/images/MjAxNcTqtdoyNji6xbmruOa4vbz+LnBkZg==.pdf (accessed on 23 December 2015).
- Saskia, R.; Werner, K.; Christiane, Z. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 2016, 153, 91–99. [Google Scholar]
- Kluwe, W.M.; McConnell, E.E.; Huff, J.E.; Haseman, J.K.; Douglas, J.F.; Hartwell, W.V. Carcinogenicity testing of phthalate esters and related compounds by the National Toxicology Program and the National Cancer Institute. Environ. Health Perspect. 1982, 45, 129–133. [Google Scholar] [CrossRef]
- Alabi, O.A.; Sorungbe, A.A.; Adeoluwa, Y.M. In vitro mutagenicity and genotoxicity of raw and simulated leachates from plastic waste dumpsite. Toxicol. Mechan. Method. 2019, 29, 1–23. [Google Scholar] [CrossRef]
- Li, G.L.; Lang, Y.H.; Gao, M.S.; Wei, Y.; Peng, P.; Wang, X.M. Carcinogenic and mutagenic potencies for different PAHs sources in coastal sediments of Shandong Peninsula. Marine Pollut. Bull. 2014, 84, 418–423. [Google Scholar] [CrossRef]
- Gu, Y.G.; Lin, Q.; Lu, T.T.; Ke, C.L.; Sun, R.X.; Du, F.Y. Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in surface sediments from Nan’ao Island, a representative mariculture base in South China. Marine Pollut. Bull. 2013, 75, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Chen, C.F.; Chen, C.W. Vertical profile, sources, and equivalent toxicity of polycyclic aromatic hydrocarbons in sediment cores from the river mouths of Kaohsiung Harbor, Taiwan. Marine Pollut. Bull. 2014, 85, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Vanhulle, S.; Trovaslet, M.; Enaud, E.; Lucas, M.; Taghavi, S.; Van, d.L.D.; Van, A.B.; Foret, M.; Onderwater, R.C.; Wesenberg, D. Decolorization, cytotoxicity, and genotoxicity reduction during a combined ozonation/fungal treatment of dye-contaminated wastewater. Environ. Sci. Technol. 2008, 42, 584–589. [Google Scholar] [CrossRef]
- Bryer, P.J.; Elliott, J.N.; Willingham, E.J. The effects of coal tar based pavement sealer on amphibian development and metamorphosis. Ecotoxicology 2006, 15, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, B.A.; Struve, M.F.; Gao, P.; Sharma, S.; Allison, N.; Roberts, K.C.; Letinski, D.J.; Nicolich, M.J.; Bird, M.G.; Dorman, D.C. Genotoxicity of intermittent co-exposure to benzene and toluene in male CD-1 mice. Chem. Biol. Interact. 2008, 173, 166–178. [Google Scholar] [CrossRef]
- Hladik, M.L.; Smalling, K.L.; Kuivila, K.M. A Multi-residue Method for the Analysis of Pesticides and Pesticide Degradates in Water Using HLB Solid-phase Extraction and Gas Chromatography–Ion Trap Mass Spectrometry. Bull. Environ. Contam. Toxicol. 2008, 80, 139–144. [Google Scholar] [CrossRef]
- Hughes, T.J. Genotoxicity of industrial wastes and effluents. Mutat. Res. 1998, 410, 237. [Google Scholar]
| TU | Classification | Toxicity |
|---|---|---|
| TU < 0.4 | I | no acute toxicity (NT) |
| 0.4 ≤ TU < 1 | II | slight acute toxicity (ST) |
| 1 ≤ TU < 10 | III | acute toxicity (AT) |
| 10 ≤ TU < 100 | IV | high acute toxicity (HT) |
| TU ≥ 100 | V | very high acute toxicity (VT) |
| Parameters (mg/L) | COD | TN | TP | DO | pH (Non-Dimensional) | ||
|---|---|---|---|---|---|---|---|
| Before remediation | 3# | 55.68 * | 13.05 * | 0.723 * | 5.007 * | 0.58 | 7.32 |
| 4# | 54.18 * | 13.97 * | 0.832 * | 6.085 * | 0.49 | 7.51 | |
| 5# | 49.66 * | 11.51 * | 0.604 * | 7.533 * | 0.63 | 7.46 | |
| 7# | 91.80 * | 15.85 * | 1.624 * | 9.694 * | 0.77 | 7.29 | |
| After remediation | 3# | 34.55 | 9.55 * | 0.355 | 1.908 | 6.970 | 7.71 |
| 4# | 28.73 | 10.15 * | 0.406 * | 1.426 | 3.570 | 7.94 | |
| 5# | 36.15 | 9.89 * | 0.447 * | 1.828 | 4.940 | 8.12 | |
| 7# | 46.64 * | 14.41 * | 1.071 * | 2.015 * | 1.64 * | 8.17 | |
| Time | Before Remediation | After Remediation |
|---|---|---|
| Sites | Specific Growth Rate (96 h) | |
| 3# | 89.11% ± 6.95% * | 91.04% ± 4.44% |
| 4# | 93.80% ± 1.34% ** | 94.01% ± 2.36% * |
| 5# | 88.55% ± 2.69% | 92.62% ± 3.71% * |
| 7# | 86.32% ± 0.73% | 88.47% ± 5.82% |
| NC | 81.47% ± 1.85% | 85.27% ± 1.44% |
| Sites | 3# | 4# | 5# | 7# | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| 48-h LC50 a | 218.67% | 299.94% | 629.80% | / | 519.70% | 500% | 311.64% | 1515.15% |
| TU | 0.46 | 0.33 | 0.16 | 0 | 0.19 | 0.20 | 0.32 | 0.07 |
| Toxicity | ST | NT | NT | NT | NT | NT | NT | NT |
| Samples | TA97 | TA98 | TA100 | TA102 | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| 3# | 373 ± 31.5 | 189 ± 7.0 | 88 ± 2.8 * | 104 ± 4.9 * | 419 ± 35.4 * | 125 ± 7.8 | 365 ± 6.4 | 287 ± 23.4 |
| 4# | 160 ± 15.7 | 165 ± 4.9 | 497 ± 24.0 * | 94 ± 1.4 * | 286 ± 14.1 | 139 ± 5.7 | 340 ± 20.7 | 277 ± 23.3 |
| 5# | 79 ± 5.7 a | 190 ± 11.1 | 421 ± 33.1 * | 112 ± 4.6 * | 412 ± 26.9 * | 162 ± 11.0 | 979 ± 31.1 * | 380 ± 33.9 |
| 7# | 0 a | 201 ± 11.3 | 608 ± 35.4 * | 84 ± 7.0 | 538 ± 45.2 * | 149 ± 12.7 | 90 ± 6.8 a | 316 ± 8.5 |
| Blank | 185 ± 10.6 | 45 ± 2.1 | 181 ± 4.4 | 328 ± 10.6 | ||||
| DMSO | 191 ± 10.8 | 43 ± 3.5 | 196 ± 11.1 | 291 ± 1.4 | ||||
| Samples | TA97 | TA98 | TA100 | TA102 | ||||
|---|---|---|---|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | |
| 3# | 255 ± 9.2 | 159 ± 14.7 | 51 ± 3.5 | 42 ± 2.8 | 125 ± 7.8 | 256 ± 20.2 | 323 ± 1.4 | 375 ± 1.4 |
| 4# | 269 ± 4.9 | 187 ± 18 | 65 ± 5.7 | 31 ± 2.1 | 139 ± 5.7 | 517 ± 9.2 * | 227 ± 21.2 | 310 ± 22.6 |
| 5# | 222 ± 7.1 | 164 ± 16.3 | 74 ± 6.4 | 44 ± 0.7 | 162 ± 11.0 | 320 ± 25.5 | 408 ± 5.7 | 638 ± 59.4 |
| 7# | 267 ± 14.1 | 196 ± 17.7 | 66 ± 6.4 | 52 ± 4.6 | 149 ± 12.7 | 331 ± 32.5 | 440 ± 19.2 | 500 ± 17.7 |
| Blank | 182 ± 12 | 166 ± 11.9 | 53 ± 4.9 | 51 ± 1.4 | 181 ± 4.4 | 205 ± 6 | 321 ± 11.3 | 342 ± 1.4 |
| DMSO | 305 ± 10.6 | 309 ± 19.1 | 62 ± 2.8 | 72 ± 3.5 | 196 ± 11.1 | 194 ± 9.1 | 340 ± 26.9 | 344 ± 11.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.-R.; Pei, Z.-T.; Wang, W.-Q.; Zhang, M.; Zhang, L.-L.; Zhang, J.; Wang, W.-Q.; Sun, L.-W.; Zhang, Y.-M. Assessment of Biological Toxicity and Ecological Safety for Urban Black-Odor River Remediation. Int. J. Environ. Res. Public Health 2020, 17, 1025. https://doi.org/10.3390/ijerph17031025
Xu R-R, Pei Z-T, Wang W-Q, Zhang M, Zhang L-L, Zhang J, Wang W-Q, Sun L-W, Zhang Y-M. Assessment of Biological Toxicity and Ecological Safety for Urban Black-Odor River Remediation. International Journal of Environmental Research and Public Health. 2020; 17(3):1025. https://doi.org/10.3390/ijerph17031025
Chicago/Turabian StyleXu, Rou-Rou, Zhou-Tao Pei, Wen-Qian Wang, Meng Zhang, Li-Ling Zhang, Jing Zhang, Wen-Qiang Wang, Li-Wei Sun, and Yi-Min Zhang. 2020. "Assessment of Biological Toxicity and Ecological Safety for Urban Black-Odor River Remediation" International Journal of Environmental Research and Public Health 17, no. 3: 1025. https://doi.org/10.3390/ijerph17031025
APA StyleXu, R.-R., Pei, Z.-T., Wang, W.-Q., Zhang, M., Zhang, L.-L., Zhang, J., Wang, W.-Q., Sun, L.-W., & Zhang, Y.-M. (2020). Assessment of Biological Toxicity and Ecological Safety for Urban Black-Odor River Remediation. International Journal of Environmental Research and Public Health, 17(3), 1025. https://doi.org/10.3390/ijerph17031025




