Human Health Risk Assessment by Dietary Intake and Spatial Distribution Pattern of Polybrominated Diphenyl Ethers and Dechloran Plus from Selected Cities of Pakistan
Abstract
1. Introduction
2. Material and Methods
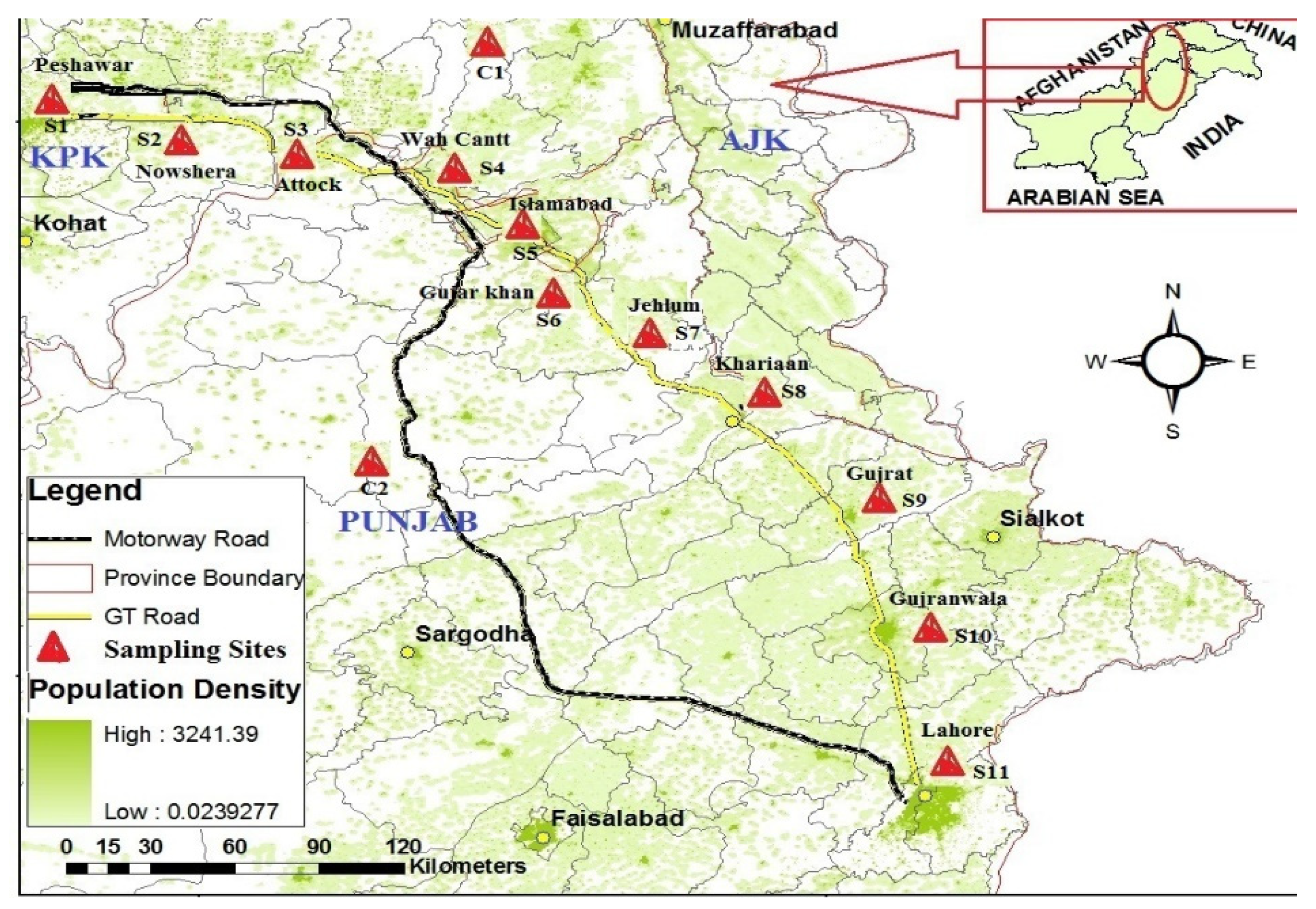
2.1. Study Area and Sampling Strategy
2.2. Field Sampling
2.2.1. Agricultural-Soil and Wheat-Grain Sampling
2.2.2. Dust Sampling
2.2.3. Extraction and Clean-Up Procedure
2.3. Chromatographic Analysis
2.4. Quality Control and Quality Assurance (QC/QA)
3. Results and Discussion
3.1. PBDE and DP Profile
3.1.1. Agricultural Soil
3.1.2. Dust
3.1.3. Cereal Crop (Wheat)
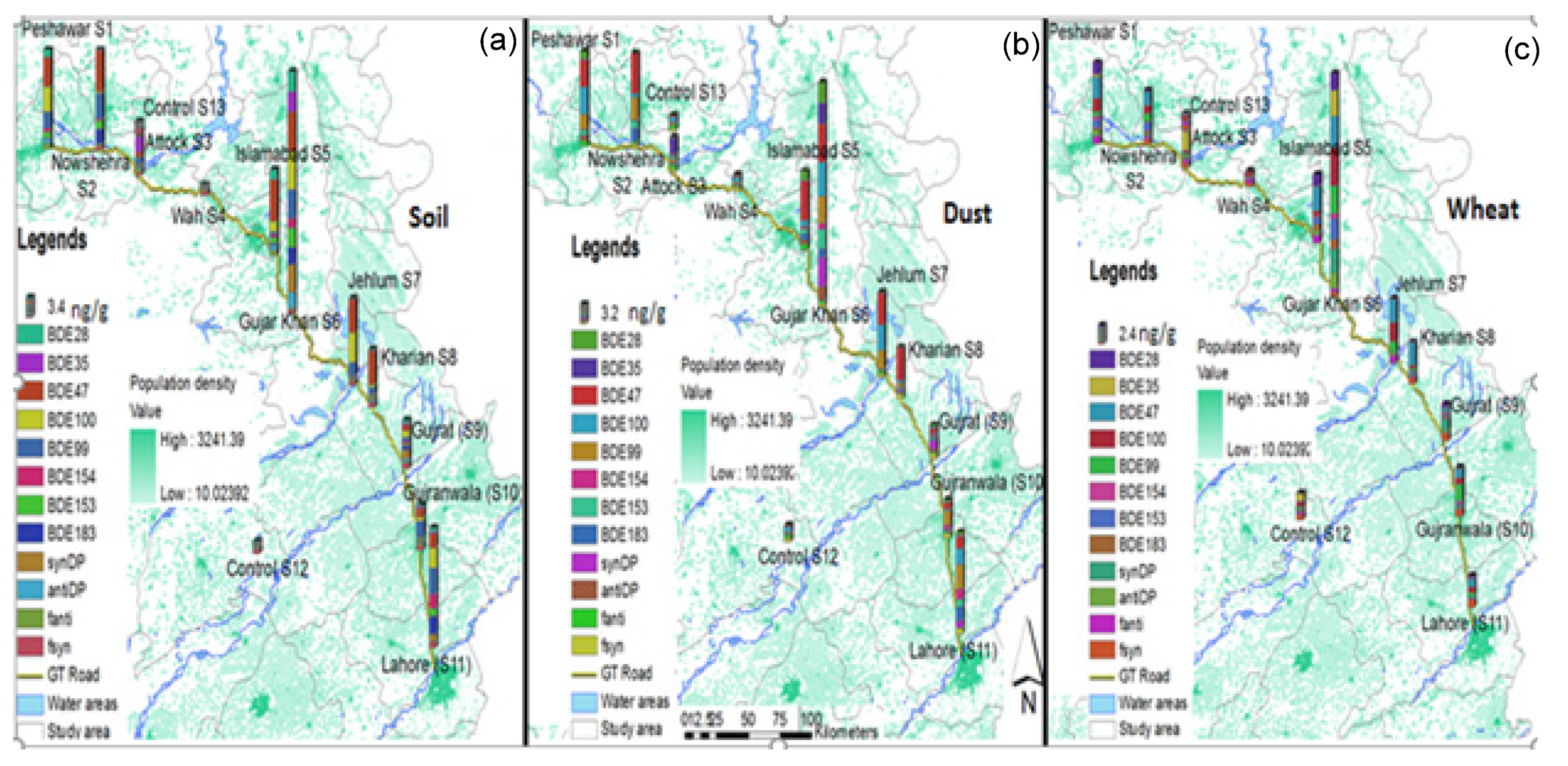
3.2. Spatial Distribution and Source Apportionment
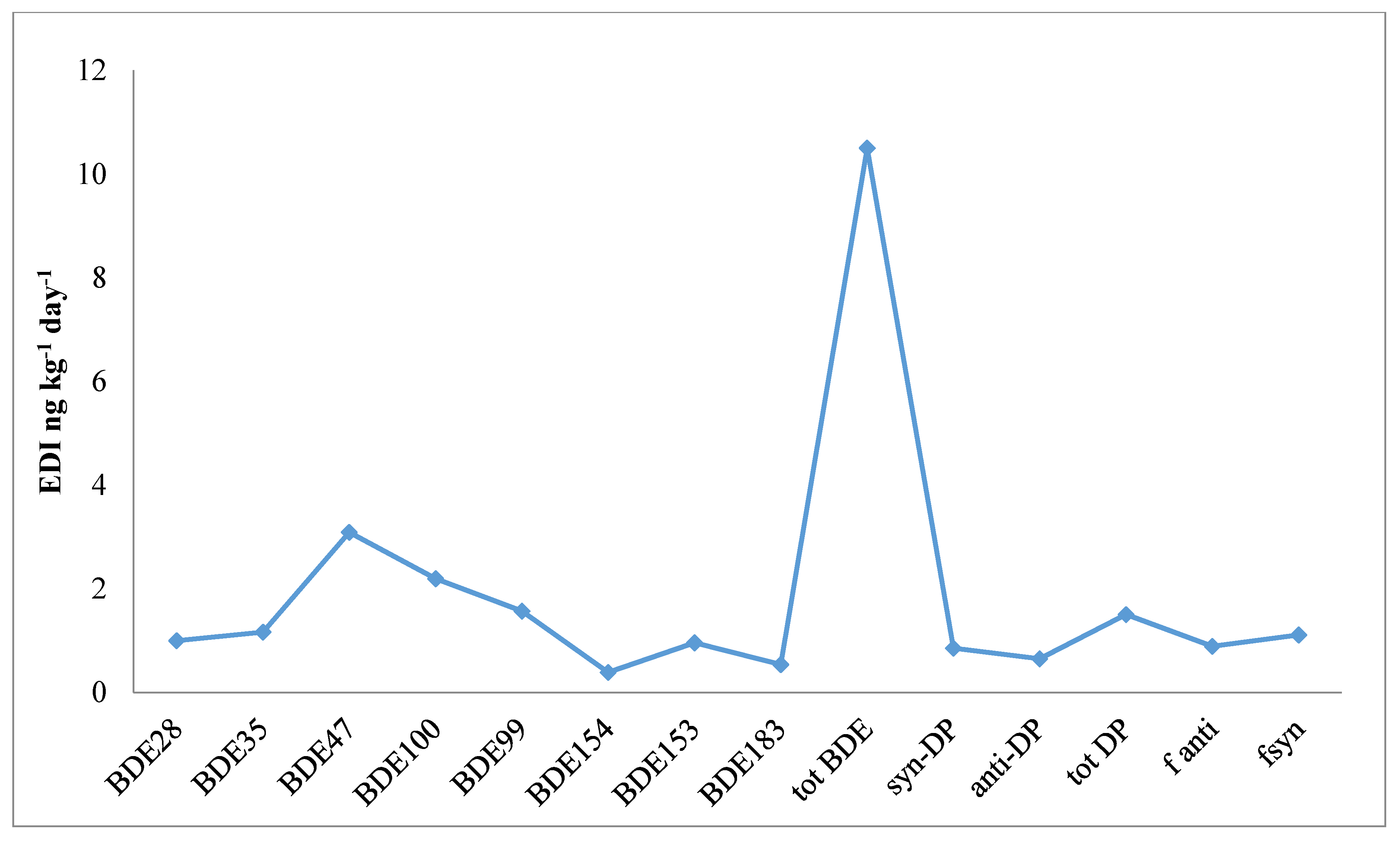
3.3. Dietary Intake
3.4. Probable Health Risks by PBDEs and DPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect 2004, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lenters, V.; Iszatt, N.; Forns, J.; Čechová, E.; Kočan, A.; Legler, J.; Leonards, P.; Stigum, H.; Eggesbø, M. Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a Norwegian birth cohort. Environ. Int. 2019, 125, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Alaee, M.; Wenning, R.J. The significance of brominated flame retardants in the environment: Current understanding, issues and challenges. Chemosphere 2002, 46, 579–582. [Google Scholar] [CrossRef]
- Baqar, M.; Arslan, M.; Sadef, Y.; Mahmood, A.; Qadir, A.; Ahmad, S.R. Persistent organic pollutants in Pakistan: Potential threat to ecological integrities in terms of genotoxicity and oxidative stress. Hum. Ecol. Risk Assess. An Int. J. 2017, 23, 1249–1271. [Google Scholar] [CrossRef]
- Syed, J.H.; Malik, R.N.; Li, J.; Wang, Y.; Xu, Y.; Zhang, G.; Jones, K.C. Levels, profile and distribution of Dechloran Plus (DP) and Polybrominated Diphenyl Ethers (PBDEs) in the environment of Pakistan. Chemosphere 2013, 93, 1646–1653. [Google Scholar] [CrossRef]
- Mehmood, A.; Mahmood, A.; Eqani, S.A.M.A.S.; Ishtiaq, M.; Ashraf, A.; Bibi, N.; Qadir, A.; Li, J.; Zhang, G. A review on emerging persistent organic pollutants: Current scenario in Pakistan. Hum. Ecol. Risk Assess. An Int. J. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. NeuroToxicology 2007, 28, 1047–1067. [Google Scholar] [CrossRef]
- Khan, M.U.; Li, J.; Zhang, G.; Malik, R.N. First insight into the levels and distribution of flame retardants in potable water in Pakistan: An underestimated problem with an associated health risk diagnosis. Sci. Total Environ. 2016, 565, 346–359. [Google Scholar] [CrossRef]
- Qiu, X.; Marvin, C.H.; Hites, R.A. Dechlorane Plus and Other Flame Retardants in a Sediment Core from Lake Ontario. Environ. Sci. Technol. 2007, 41, 6014–6019. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N.; Syed, J.H.; Li, J.; Zhang, G. Dietary exposure and screening-level risk assessment of polybrominated diphenyl ethers (PBDEs) and dechloran plus (DP) in wheat, rice, soil and air along two tributaries of the River Chenab, Pakistan. Chemosphere 2015, 118, 57–64. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, V.J. Studies on brominated chemicals in the environment. Ann. New York Acad. Sci. 1979, 320, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Andersson, Ö.; Blomkvist, G. Polybrominated aromatic pollutants found in fish in Sweden. Chemosphere 1981, 10, 1051–1060. [Google Scholar] [CrossRef]
- Nylund, K.; Asplund, L.; Jansson, B.; Jonsson, P.; Litzén, K.; Sellström, U. Analysis of some polyhalogenated organic pollutants in sediment and sewage sludge. Chemosphere 1992, 24, 1721–1730. [Google Scholar] [CrossRef]
- de Boer, J.; Wester, P.G.; Klamer, H.J.; Lewis, W.E.; Boon, J.P. Do flame retardants threaten ocean life? Nature 1998, 394, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Alaee, M.; Luross, J.; Sergeant, D.B.; Muir, D.C.G.; Whittle, D.M.; Solomon, K. Distribution of polybrominated diphenyl ethers in the Canadian environment. Organohalogen Compd. 1999, 40, 347–350. [Google Scholar]
- Chiuchiolo, A.L.; Dickhut, R.M.; Cochran, M.A.; Ducklow, H.W. Persistent Organic Pollutants at the Base of the Antarctic Marine Food Web. Environ. Sci. Technol. 2004, 38, 3551–3557. [Google Scholar] [CrossRef]
- de Wit, C.A.; Alaee, M.; Muir, D.C.G. Levels and trends of brominated flame retardants in the Arctic. Chemosphere 2006, 64, 209–233. [Google Scholar] [CrossRef]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Kester, M.H.A.; Andersson, P.L.; Legler, J.; Brouwer, A. In Vitro Profiling of the Endocrine-Disrupting Potency of Brominated Flame Retardants. Toxicol. Sci. 2006, 92, 157–173. [Google Scholar] [CrossRef]
- Khan, M.U.; Li, J.; Zhang, G.; Malik, R.N. New insight into the levels, distribution and health risk diagnosis of indoor and outdoor dust-bound FRs in colder, rural and industrial zones of Pakistan. Environ. Pollut. 2016, 216, 662–674. [Google Scholar] [CrossRef]
- Goodman, W.; Shelton, C.T. Analysis of Polybrominated Diphenyl Ether Flame Retardants by Gas Chromatography/Mass Spectrometry. Available online: https://www.perkinelmer.com.cn/CMSResources/Images/46-173732APP_Analysis-of-Polybrominated-Diphenyl-Ether-Flame-Retardants-by-GC-MS-007876B_01.pdf (accessed on 9 November 2020).
- Duan, Y.P.; Meng, X.Z.; Yang, C.; Pan, Z.Y.; Chen, L.; Yu, R.; Li, F.T. Polybrominated diphenyl ethers in background surface soils from the Yangtze River Delta (YRD), China: Occurrence, sources, and inventory. Environ. Sci. Pollut. Res. Int. 2010, 17, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.; Breivik, K.; Meijer, S.N.; Steinnes, E.; Thomas, G.O.; Jones, K.C. PBDEs in European Background Soils: Levels and Factors Controlling Their Distribution. Environ. Sci. Technol. 2004, 38, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.; Odabasi, M. Particle-Phase Dry Deposition and Air−Soil Gas-Exchange of Polybrominated Diphenyl Ethers (PBDEs) in Izmir, Turkey. Environ. Sci. Technol. 2007, 41, 4986–4992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, C.; Li, J.; Yin, H.; Li, X.; Zhang, G. Characterization of PBDEs in soils and vegetations near an e-waste recycling site in South China. Environ. Pollut. 2011, 159, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-L.; Liu, L.-Y.; Qi, H.; Sun, D.-Z.; Shen, J.-M.; Wang, D.-G.; Li, Y.-F. Dechlorane plus in multimedia in northeastern Chinese urban region. Environ. Int. 2011, 37, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Yang, M.; Qi, H.; Sverko, E.; Ma, W.-L.; Li, Y.-F.; Alaee, M.; Reiner, E.J.; Shen, L. An Asia-Specific Source of Dechlorane Plus: Concentration, Isomer Profiles, and Other Related Compounds. Environ. Sci. Technol. 2010, 44, 6608–6613. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, S.; Gao, S.; Wang, J.; Li, H.; Zeng, X.; Sheng, G.; Fu, J. Levels and isomer profiles of Dechlorane Plus in the surface soils from e-waste recycling areas and industrial areas in South China. Environ. Pollut. 2010, 158, 2920–2925. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N.; Li, J.; Zhang, G. Distribution, Congener Profile, and Risk of Polybrominated Diphenyl Ethers and Dechlorane Plus in Water and Sediment From Two Tributaries of the Chenab River, Pakistan. Arch. Environ. Contam. Toxicol. 2015, 68, 83–91. [Google Scholar] [CrossRef]
- D’Hollander, W.; Roosens, L.; Covaci, A.; Cornelis, C.; Reynders, H.; Campenhout, K.V.; Voogt, P.d.; Bervoets, L. Brominated flame retardants and perfluorinated compounds in indoor dust from homes and offices in Flanders, Belgium. Chemosphere 2010, 81, 478–487. [Google Scholar] [CrossRef]
- SUAZUKI, G. PBDEs and PBDD/Fs in house and officd dust from Japan. Organohalogen Compd. 2006, 68, 1843–1846. [Google Scholar]
- Stuart, H.; Ibarra, C.; Abdallah, M.A.-E.; Boon, R.; Neels, H.; Covaci, A. Concentrations of brominated flame retardants in dust from United Kingdom cars, homes, and offices: Causes of variability and implications for human exposure. Environ. Int. 2008, 34, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Körner, W.; Shahin, N.; Wanner, A.; Albrecht, M.; Boehmer, S.; Parlar, H.; Mayer, R.; Liebl, B.; Bolte, G. Human exposure to polybrominated diphenyl ethers (PBDE), as evidenced by data from a duplicate diet study, indoor air, house dust, and biomonitoring in Germany. Environ. Int. 2009, 35, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Thuresson, K.; Björklund, J.A.; de Wit, C.A. Tri-decabrominated diphenyl ethers and hexabromocyclododecane in indoor air and dust from Stockholm microenvironments 1: Levels and profiles. Sci. Total Environ. 2012, 414, 713–721. [Google Scholar] [CrossRef] [PubMed]
- de Wit, C.A.; Björklund, J.A.; Thuresson, K. Tri-decabrominated diphenyl ethers and hexabromocyclododecane in indoor air and dust from Stockholm microenvironments 2: Indoor sources and human exposure. Environ. Int. 2012, 39, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Chain, E.P.o.C.i.t.F. Scientific Opinion on Polybrominated Diphenyl Ethers (PBDEs) in Food. EFSA J. 2011, 9, 2156. [Google Scholar] [CrossRef]
- Schecter, A.; Smith, S.; Colacino, J.; Malik, N.; Opel, M.; Paepke, O.; Birnbaum, L. Contamination of US butter with polybrominated diphenyl ethers from wrapping paper. Environ. Health Perspect 2011, 119, 151–154. [Google Scholar] [CrossRef]
- Ryan, J.J.; Patry, B. Body burdens and exposure from food for polybrominated diphenyl ethers (BDEs) in Canada. Available online: https://www.semanticscholar.org/paper/Body-burdens-and-exposure-from-food-for-diphenyl-%28-Ryan-Patry/ea6a6d98c67bdf1301db3a7335c86b88429a5527?p2df#page=3 (accessed on 9 November 2020).
- Wijesekera, R.; Halliwell, C.; Hunter, S.; Harrad, S. A preliminary assessment of UK human exposure to polybrominated diphenyl ethers (PBDEs). Organohalogen Compd. 2002, 55, 239–242. [Google Scholar]
- Ohta, S.; Ishizuka, D.; Nishimura, H.; Nakao, T.; Aozasa, O.; Shimidzu, Y.; Ochiai, F.; Kida, T.; Nishi, M.; Miyata, H. Comparison of polybrominated diphenyl ethers in fish, vegetables, and meats and levels in human milk of nursing women in Japan. Chemosphere 2002, 46, 689–696. [Google Scholar] [CrossRef]
- Bocio, A.; Llobet, J.; Domingo, J.; Corbella, J.; Teixido, A.; Casas, C. Polybrominated diphenyl ethers (PBDEs) in foodstuffs: Human exposure through the diet. J. Agric. Food Chem. 2003, 51, 3191–3195. [Google Scholar] [CrossRef]
- Domingo, J.L.; Martí-Cid, R.; Castell, V.; Llobet, J.M. Human exposure to PBDEs through the diet in Catalonia, Spain: Temporal trend: A review of recent literature on dietary PBDE intake. Toxicology 2008, 248, 25–32. [Google Scholar] [CrossRef]
- Roszko, M.; Jędrzejczak, R.; Szymczyk, K. Polychlorinated biphenyls (PCBs), polychlorinated diphenyl ethers (PBDEs) and organochlorine pesticides in selected cereals available on the Polish retail market. Sci. Total Environ. 2014, 466, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Tian, M.; Wang, J.; Shi, T.; Luo, Y.; Luo, X.-J.; Mai, B.-X. Dechlorane Plus (DP) in air and plants at an electronic waste (e-waste) site in South China. Environ. Pollut. 2011, 159, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Syed, J.H.; Malik, R.N.; Zheng, Q.; Cheng, Z.; Li, J.; Zhang, G. Polychlorinated biphenyls (PCBs) in air, soil, and cereal crops along the two tributaries of River Chenab, Pakistan: Concentrations, distribution, and screening level risk assessment. Sci. Total Environ. 2014, 481, 596–604. [Google Scholar] [CrossRef]
- Syed, J.H.; Malik, R.N. Occurrence and source identification of organochlorine pesticides in the surrounding surface soils of the Ittehad Chemical Industries Kalashah Kaku, Pakistan. Environ. Earth Sci. 2011, 62, 1311–1321. [Google Scholar] [CrossRef]
- Eqani, S.A.-M.-A.-S.; Malik, R.N.; Katsoyiannis, A.; Zhang, G.; Chakraborty, P.; Mohammad, A.; Jones, K.C. Distribution and risk assessment of organochlorine contaminants in surface water from River Chenab, Pakistan. J. Environ. Monit. 2012, 14, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Luo, X.-J.; Mai, B.-X.; Sheng, G.-Y.; Fu, J.-M.; Zeng, E.Y. Distribution and mass inventories of polycyclic aromatic hydrocarbons and organochlorine pesticides in sediments of the Pearl River Estuary and the northern South China Sea. Environ. Sci. Technol. 2006, 40, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-G.; Mai, B.-X.; Bi, X.-H.; Chen, S.-J.; Wang, X.-M.; Ran, Y.; Luo, X.-J.; Sheng, G.-Y.; Fu, J.-M.; Zeng, E.Y. Concentration levels, compositional profiles, and gas-particle partitioning of polybrominated diphenyl ethers in the atmosphere of an urban city in South China. Environ. Sci. Technol. 2006, 40, 1190–1196. [Google Scholar] [CrossRef]
- Ethers, W.B.D. IPCS Environmental Health Criteria 162; WHO: Geneva, Switzerland, 1994. [Google Scholar]
- Ryan, J.; Patry, B. Body burdens and food exposure in Canada for polybrominated diphenyl ethers (BDEs). Organohalogen Compd. 2001, 51, 226–229. [Google Scholar]
- Sverko, E.; Reiner, E.J.; Tomy, G.T.; McCrindle, R.; Shen, L.; Arsenault, G.; Zaruk, D.; MacPherson, K.A.; Marvin, C.H.; Helm, P.A. Compounds structurally related to Dechlorane Plus in sediment and biota from Lake Ontario (Canada). Environ. Sci. Technol. 2010, 44, 574–579. [Google Scholar] [CrossRef]
- Bocio, A.; Domingo, J.L.; Falcó, G.; Llobet, J.M. Concentrations of PCDD/PCDFs and PCBs in fish and seafood from the Catalan (Spain) market: Estimated human intake. Environ. Int. 2007, 33, 170–175. [Google Scholar] [CrossRef]
- Domingo, J.L. Human exposure to polybrominated diphenyl ethers through the diet. J. Chromatogr. A 2004, 1054, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Bocio, A.; Falcó, G.; Llobet, J.M. Exposure to PBDEs and PCDEs associated with the consumption of edible marine species. Environ. Sci. Technol. 2006, 40, 4394–4399. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Dicks, P.; Mortimer, D.; Gem, M.; Smith, F.; Driffield, M.; White, S.; Rose, M. Brominated and chlorinated dioxins, PCBs and brominated flame retardants in Scottish shellfish: Methodology, occurrence and human dietary exposure. Mol. Nutr. Food Res. 2008, 52, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Mortimer, D.; Gem, M.; Dicks, P.; Smith, F.; White, S.; Rose, M. Brominated dioxins (PBDD/Fs) and PBDEs in marine shellfish in the UK. Food Addit. Contam. 2009, 26, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Tlustos, C.; Smith, F.; Carr, M.; Petch, R.; Rose, M. Polybrominated diphenylethers (PBDEs) and brominated dioxins (PBDD/Fs) in Irish food of animal origin. Food Addit. Contam. Part B 2009, 2, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Food Standards Programme Codex Committee on Food Additives and Contaminants; Thirty-sixth Session Rotterdam, The Netherlands, 22–26 March 2004. Available online: http://www.fao.org/tempref/codex/Meetings/CCFAC/ccfac36/fa36_09e.pdf (accessed on 9 November 2020).
- Schafer, K.S.; Kegley, S.E. Persistent toxic chemicals in the US food supply. J. Epidemiol. Community Health 2002, 56, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Lee, T.; Chen, K.; Wong, H.; Zheng, J.; Giesy, J.; Lo, K.; Yamashita, N.; Lam, P. Human health risk assessment of organochlorines associated with fish consumption in a coastal city in China. Environ. Pollut. 2005, 136, 155–165. [Google Scholar] [CrossRef]
- Darnerud, P.O.; Eriksen, G.S.; Jóhannesson, T.; Larsen, P.B.; Viluksela, M. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environ. Health Perspect 2001, 109, 49–68. [Google Scholar]
| Compounds | Soil (ng g−1) | Dust (ng g−1) | Wheat (ng g−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | St.Dev | Min | Max | Mean | St.Dev | Min | Max | Mean | St.Dev | |
| BDE28 | 0.04 | 3.28 | 0.65 | 0.93 | 0.03 | 3.17 | 0.57 | 0.91 | 0.02 | 2.13 | 0.50 | 0.71 |
| BDE35 | 0.01 | 3.44 | 0.62 | 1.08 | 0.01 | 3.32 | 0.53 | 1.06 | 0.00 | 3.26 | 0.58 | 1.05 |
| BDE47 | 0.12 | 6.72 | 3.20 | 2.77 | 0.25 | 6.32 | 2.92 | 2.63 | 0.08 | 3.94 | 1.55 | 1.38 |
| BDE100 | 0.07 | 6.22 | 1.79 | 2.12 | 0.17 | 5.37 | 1.64 | 1.78 | 0.12 | 4.75 | 1.10 | 1.25 |
| BDE99 | 0.12 | 4.67 | 1.85 | 1.68 | 0.12 | 4.27 | 1.65 | 1.60 | 0.00 | 6.80 | 1.26 | 2.00 |
| BDE154 | 0.05 | 2.33 | 0.51 | 0.69 | 0.06 | 1.58 | 0.33 | 0.44 | 0.02 | 0.75 | 0.19 | 0.21 |
| BDE153 | 0.04 | 3.16 | 0.74 | 0.86 | 0.00 | 3.04 | 0.64 | 0.84 | 0.01 | 2.54 | 0.48 | 0.71 |
| BDE183 | 0.04 | 2.86 | 0.75 | 1.12 | 0.02 | 2.19 | 0.57 | 0.78 | 0.01 | 1.15 | 0.27 | 0.34 |
| ∑BDE | 0.63 | 31.70 | 10.10 | 8.76 | 1.51 | 27.54 | 8.85 | 7.54 | 0.00 | 3.47 | 0.49 | 0.95 |
| syn-DP | 0.03 | 4.66 | 0.68 | 1.27 | 0.00 | 4.56 | 0.60 | 1.26 | 0.02 | 2.99 | 0.43 | 0.84 |
| anti-DP | 0.04 | 2.39 | 0.41 | 0.64 | 0.01 | 2.30 | 0.34 | 0.63 | 0.00 | 1.96 | 0.32 | 0.54 |
| ∑DP | 0.11 | 7.05 | 1.09 | 1.87 | 0.01 | 6.86 | 0.95 | 1.85 | 0.05 | 4.95 | 0.75 | 1.32 |
| fanti | 0.09 | 0.96 | 0.42 | 0.25 | 0.03 | 0.95 | 0.44 | 0.25 | 0.02 | 0.96 | 0.45 | 0.31 |
| fsyn | 0.04 | 0.91 | 0.58 | 0.25 | 0.05 | 0.97 | 0.56 | 0.25 | 0.04 | 0.98 | 0.55 | 0.31 |
| PBDE | BDE-28 | BDE-35 | BDE-47 | BDE-100 | BDE-99 | BDE-154 | BDE-153 | BDE-183 | ∑BDE | syn-DP | anti-DP | ∑DP | fanti | fsyn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
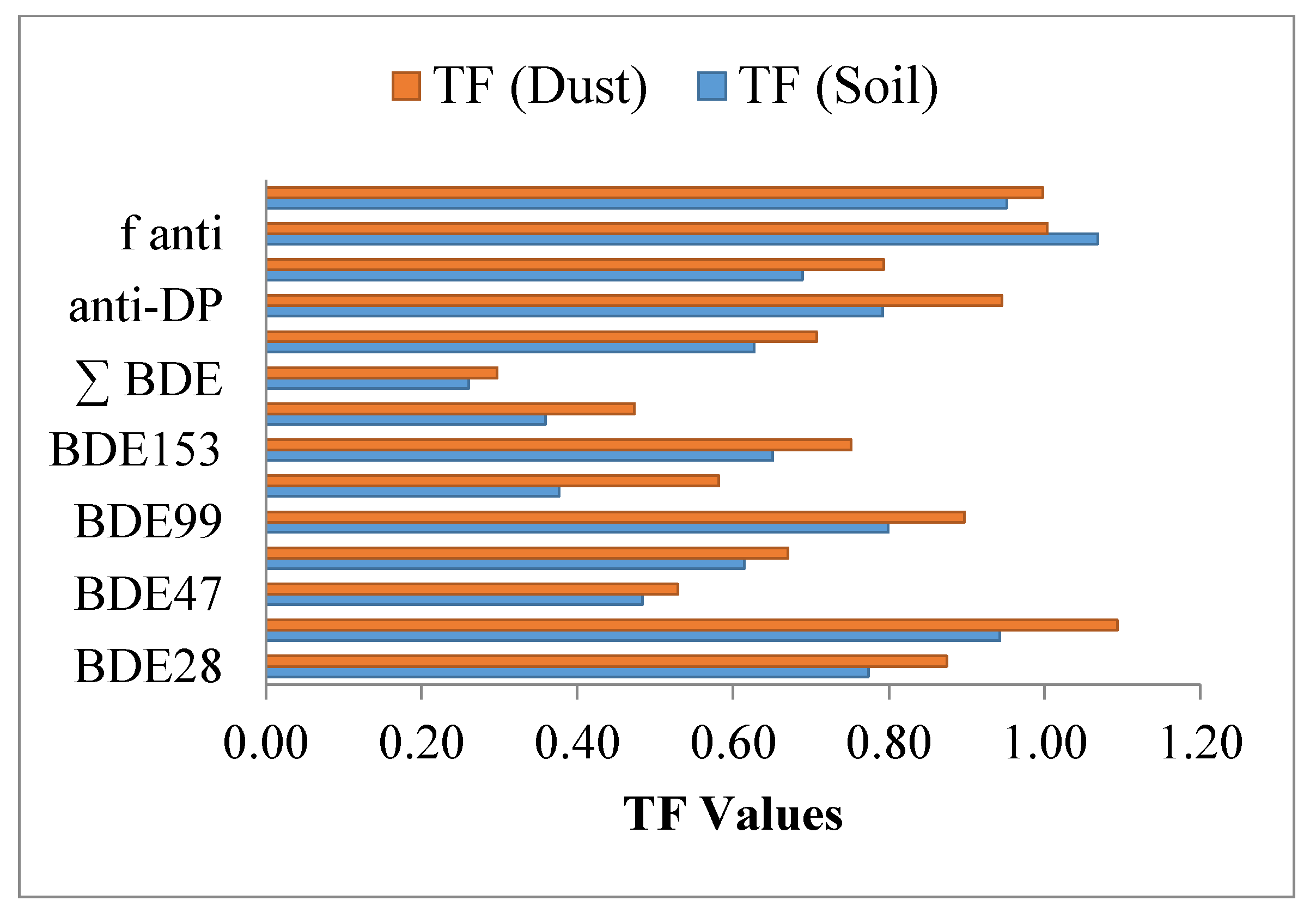
| TF (Soil) | 0.77 | 0.94 | 0.48 | 0.61 | 0.80 | 0.38 | 0.65 | 0.36 | 0.26 | 0.63 | 0.79 | 0.69 | 1.07 | 0.95 |
| TF (Dust) | 0.87 | 1.09 | 0.53 | 0.67 | 0.90 | 0.58 | 0.75 | 0.47 | 0.30 | 0.71 | 0.95 | 0.79 | 1.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, A.; Hussain Syed, J.; Raza, W.; Tabinda, A.B.; Mehmood, A.; Li, J.; Zhang, G.; Azam, M. Human Health Risk Assessment by Dietary Intake and Spatial Distribution Pattern of Polybrominated Diphenyl Ethers and Dechloran Plus from Selected Cities of Pakistan. Int. J. Environ. Res. Public Health 2020, 17, 9543. https://doi.org/10.3390/ijerph17249543
Mahmood A, Hussain Syed J, Raza W, Tabinda AB, Mehmood A, Li J, Zhang G, Azam M. Human Health Risk Assessment by Dietary Intake and Spatial Distribution Pattern of Polybrominated Diphenyl Ethers and Dechloran Plus from Selected Cities of Pakistan. International Journal of Environmental Research and Public Health. 2020; 17(24):9543. https://doi.org/10.3390/ijerph17249543
Chicago/Turabian StyleMahmood, Adeel, Jabir Hussain Syed, Waseem Raza, Amtul Bari Tabinda, Andleeb Mehmood, Jun Li, Gan Zhang, and Mudassar Azam. 2020. "Human Health Risk Assessment by Dietary Intake and Spatial Distribution Pattern of Polybrominated Diphenyl Ethers and Dechloran Plus from Selected Cities of Pakistan" International Journal of Environmental Research and Public Health 17, no. 24: 9543. https://doi.org/10.3390/ijerph17249543
APA StyleMahmood, A., Hussain Syed, J., Raza, W., Tabinda, A. B., Mehmood, A., Li, J., Zhang, G., & Azam, M. (2020). Human Health Risk Assessment by Dietary Intake and Spatial Distribution Pattern of Polybrominated Diphenyl Ethers and Dechloran Plus from Selected Cities of Pakistan. International Journal of Environmental Research and Public Health, 17(24), 9543. https://doi.org/10.3390/ijerph17249543





