Incidence and Survival Trends of Pancreatic Cancer in Girona: Impact of the Change in Patient Care in the Last 25 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Study Outcomes and Statistical Analysis
2.4. Ethics Approval
3. Results
3.1. Baseline Characteristics
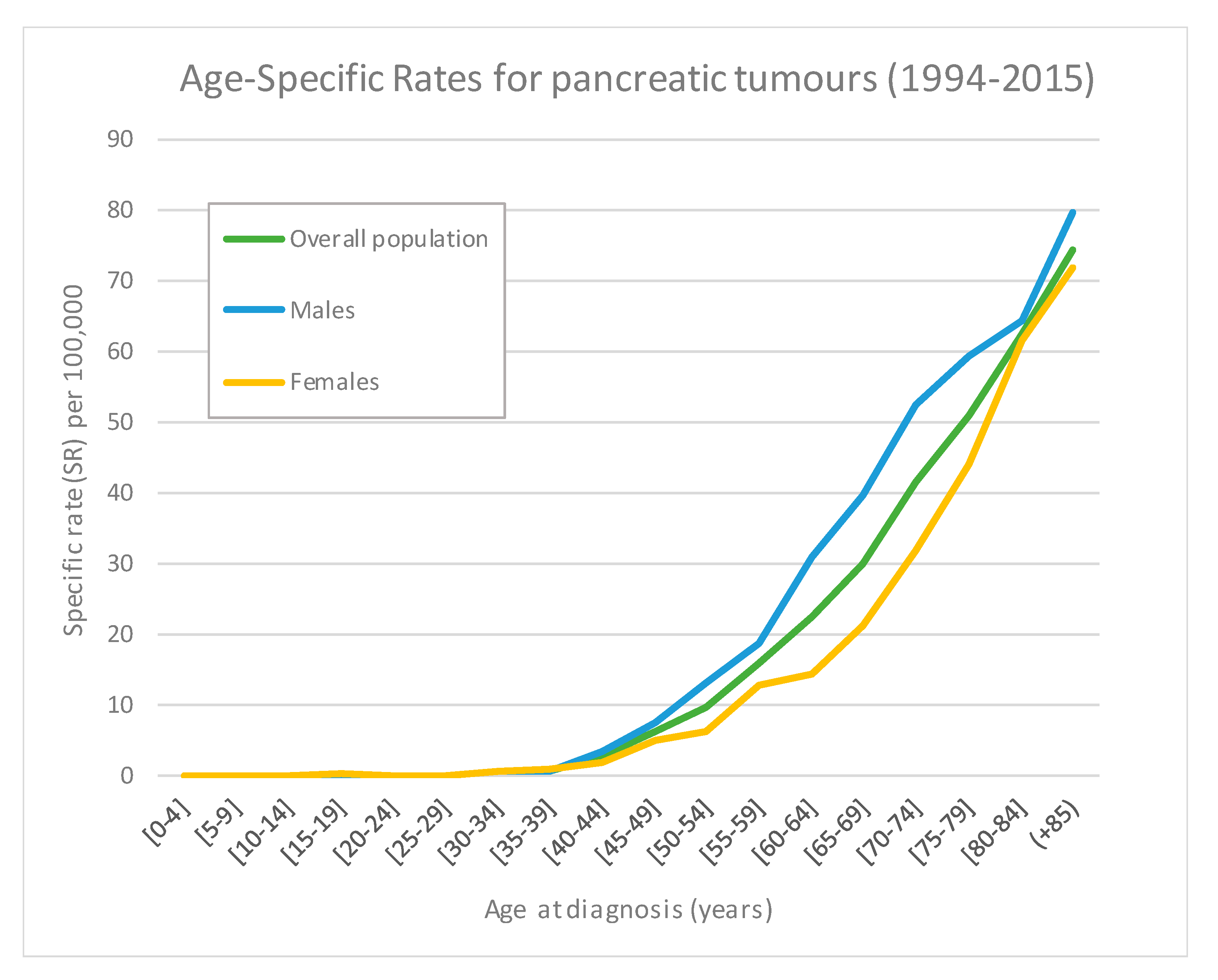
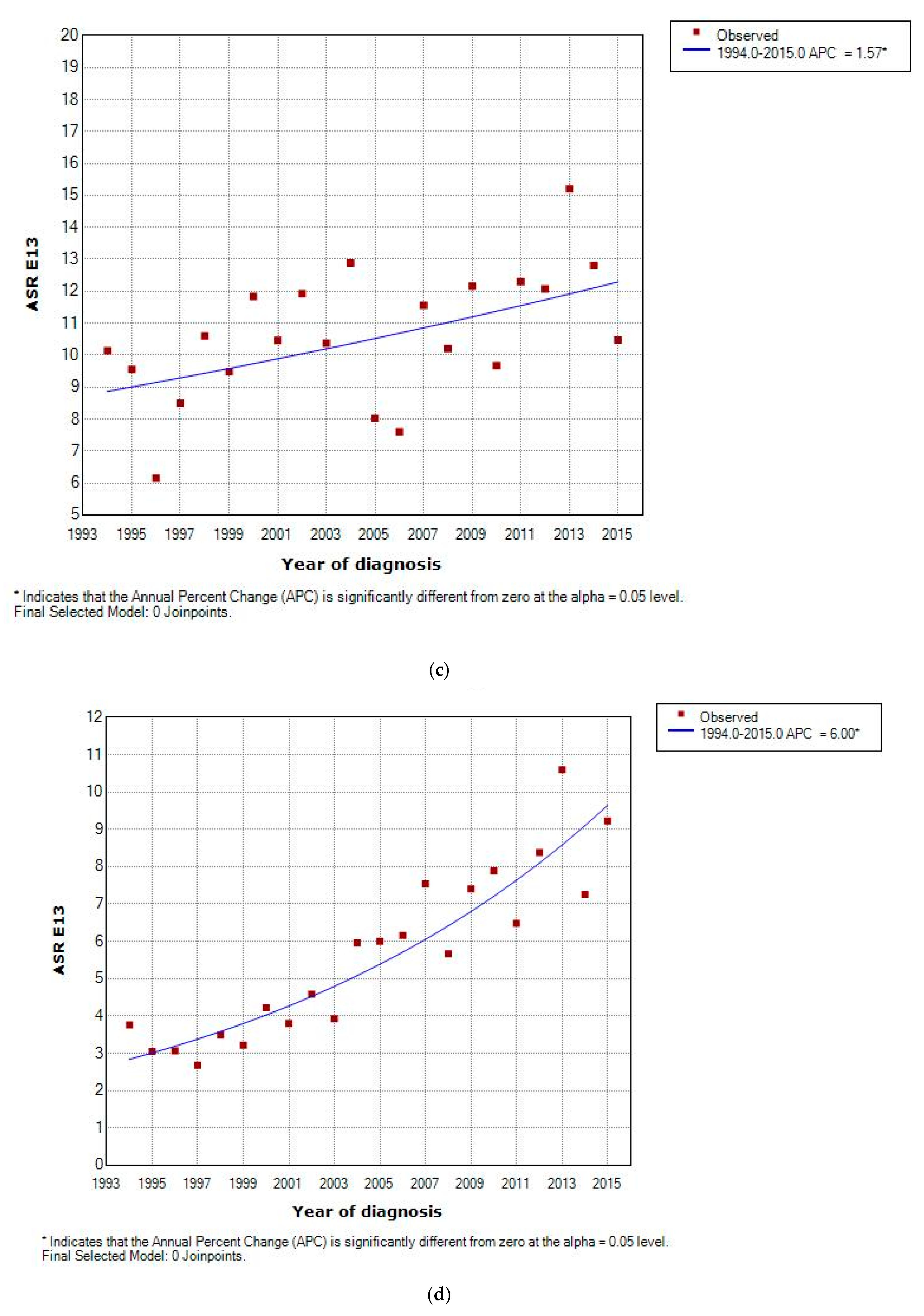
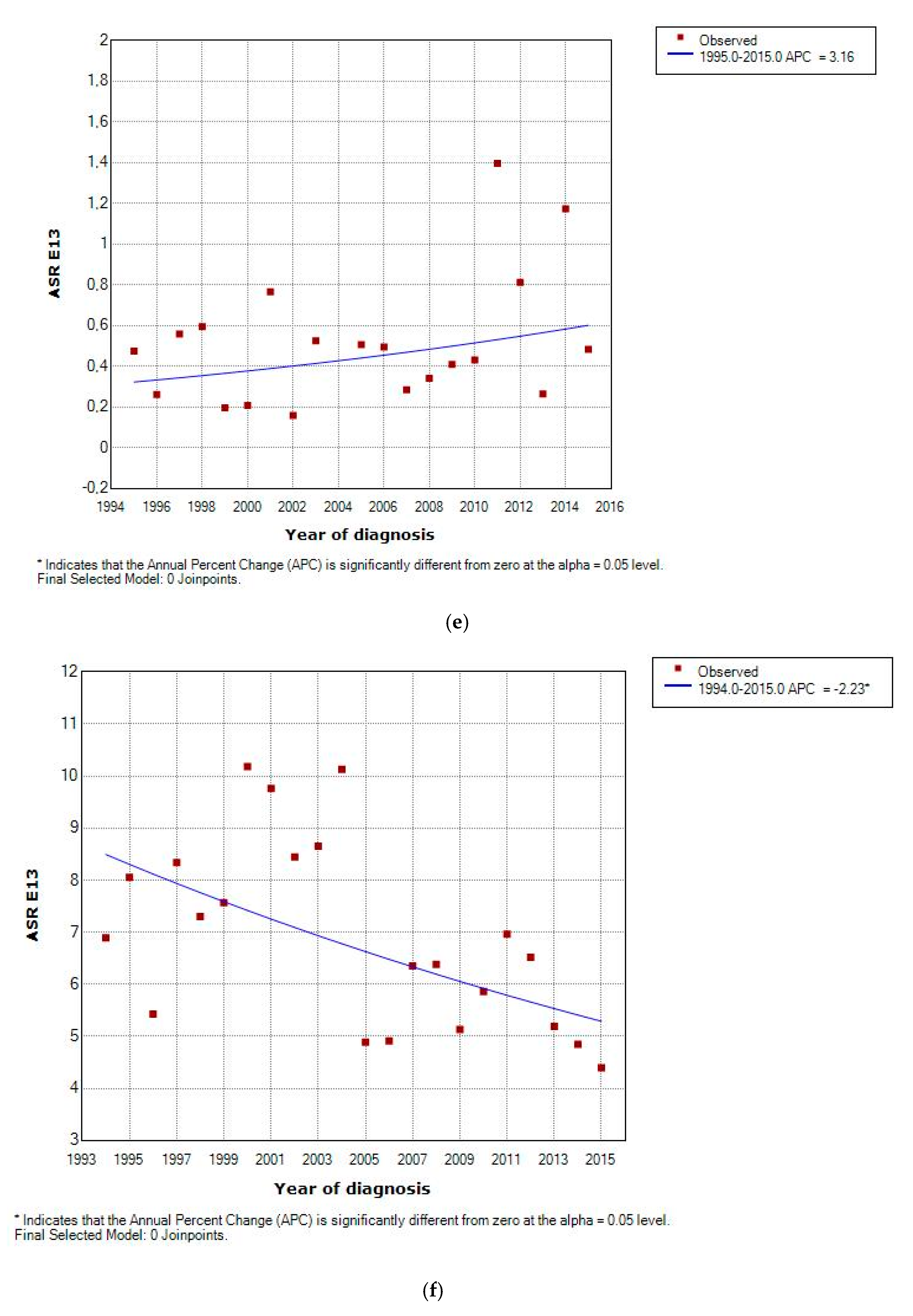
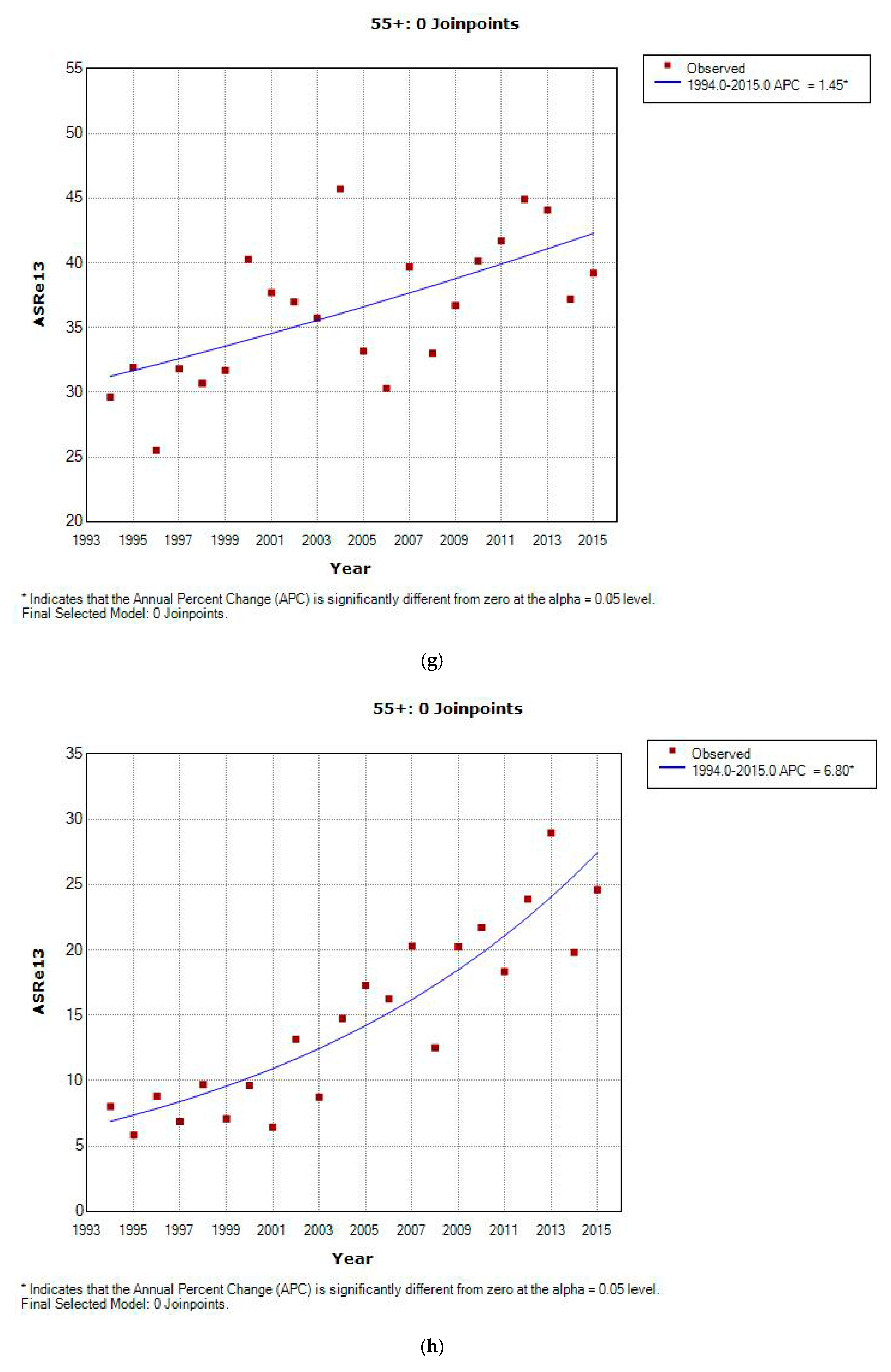
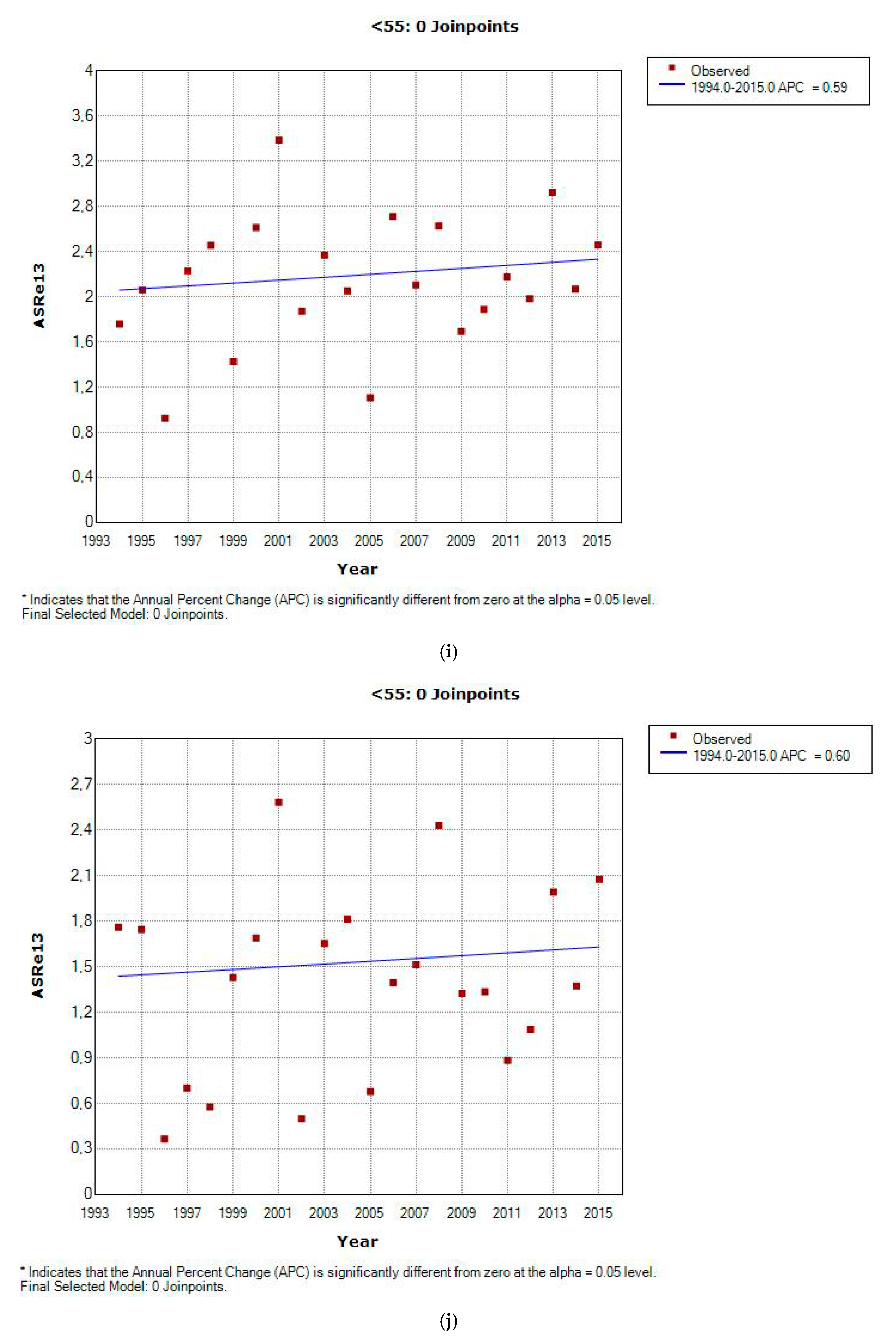
3.2. Incidence and Incidence Temporal Trends
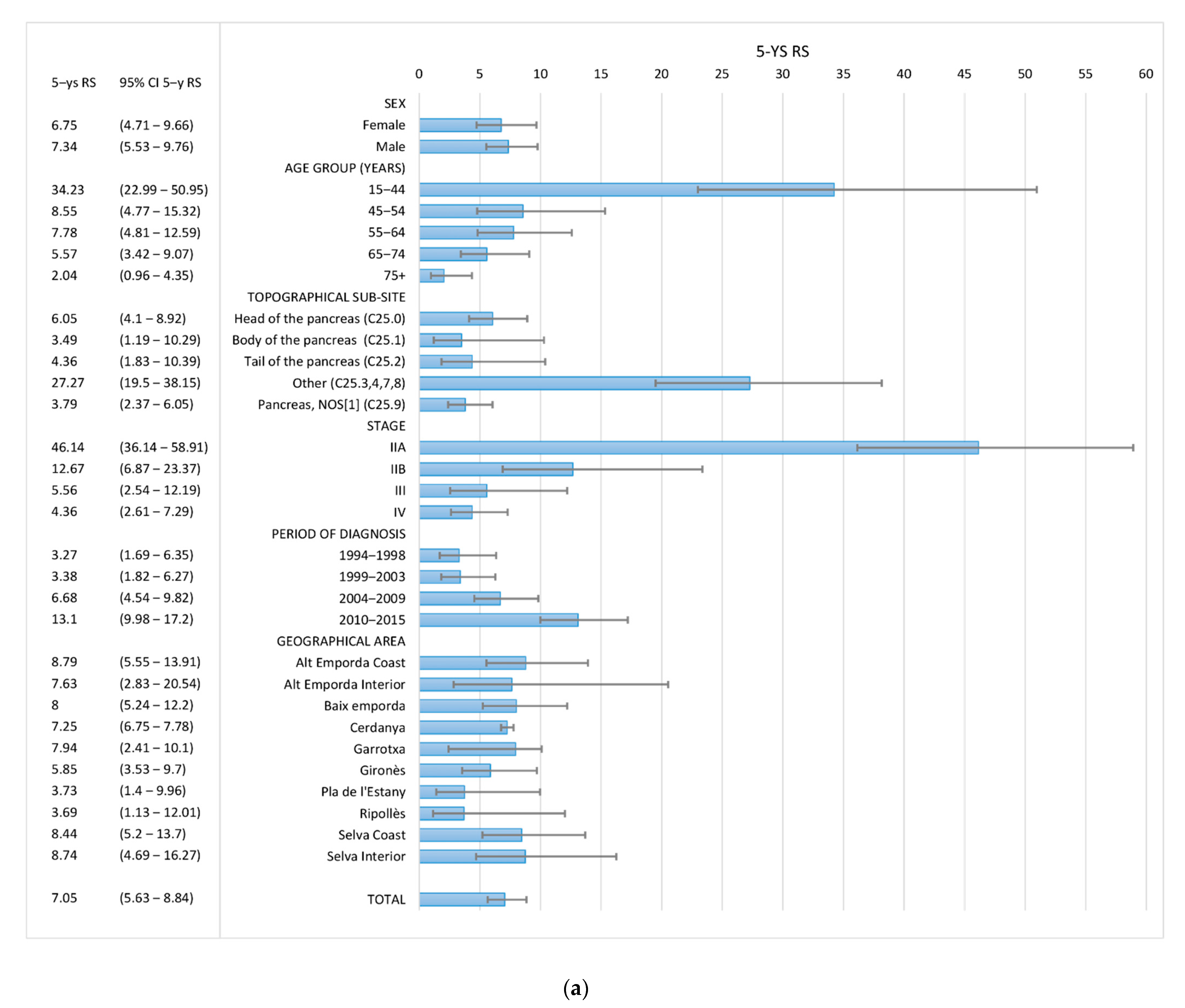
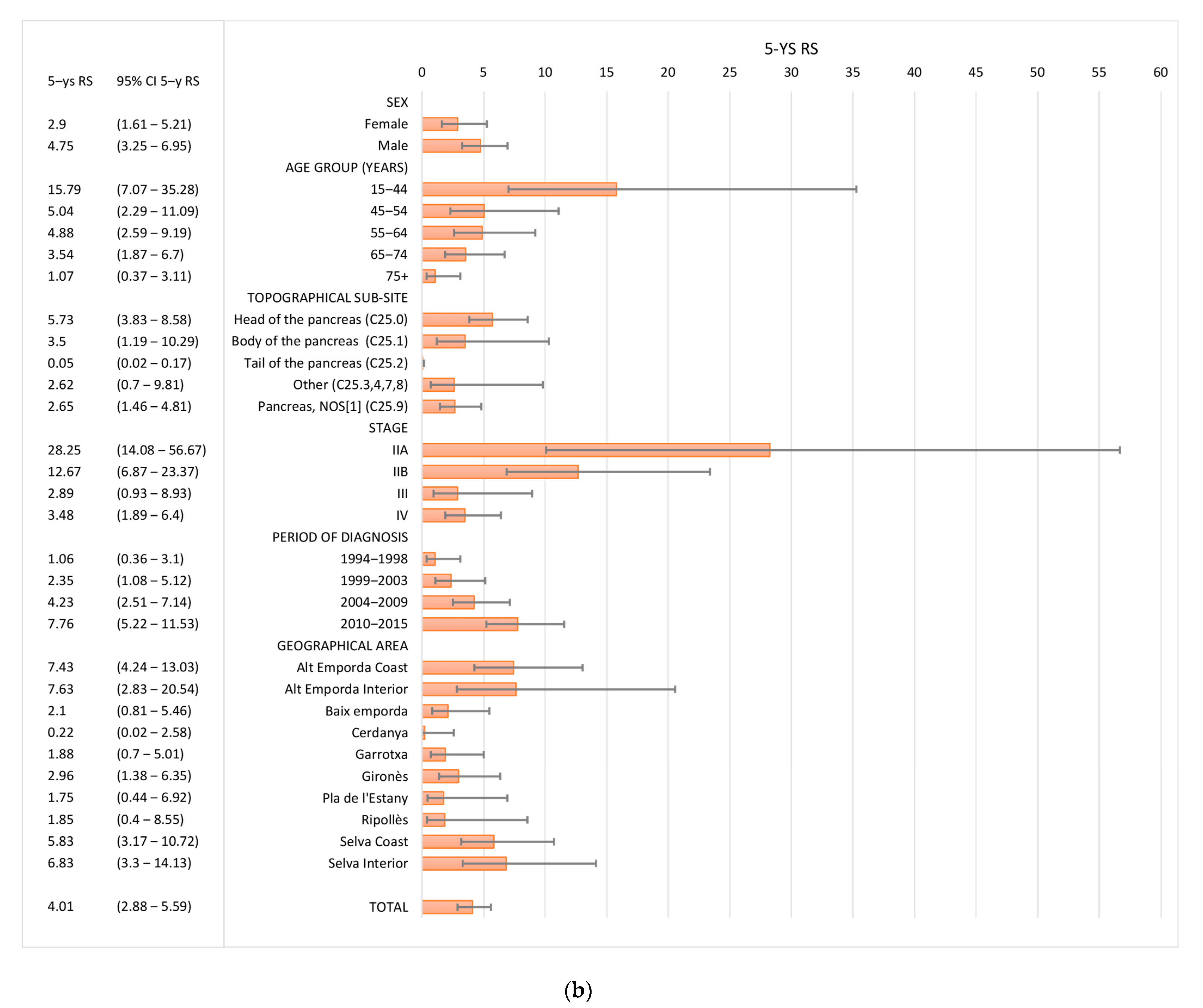
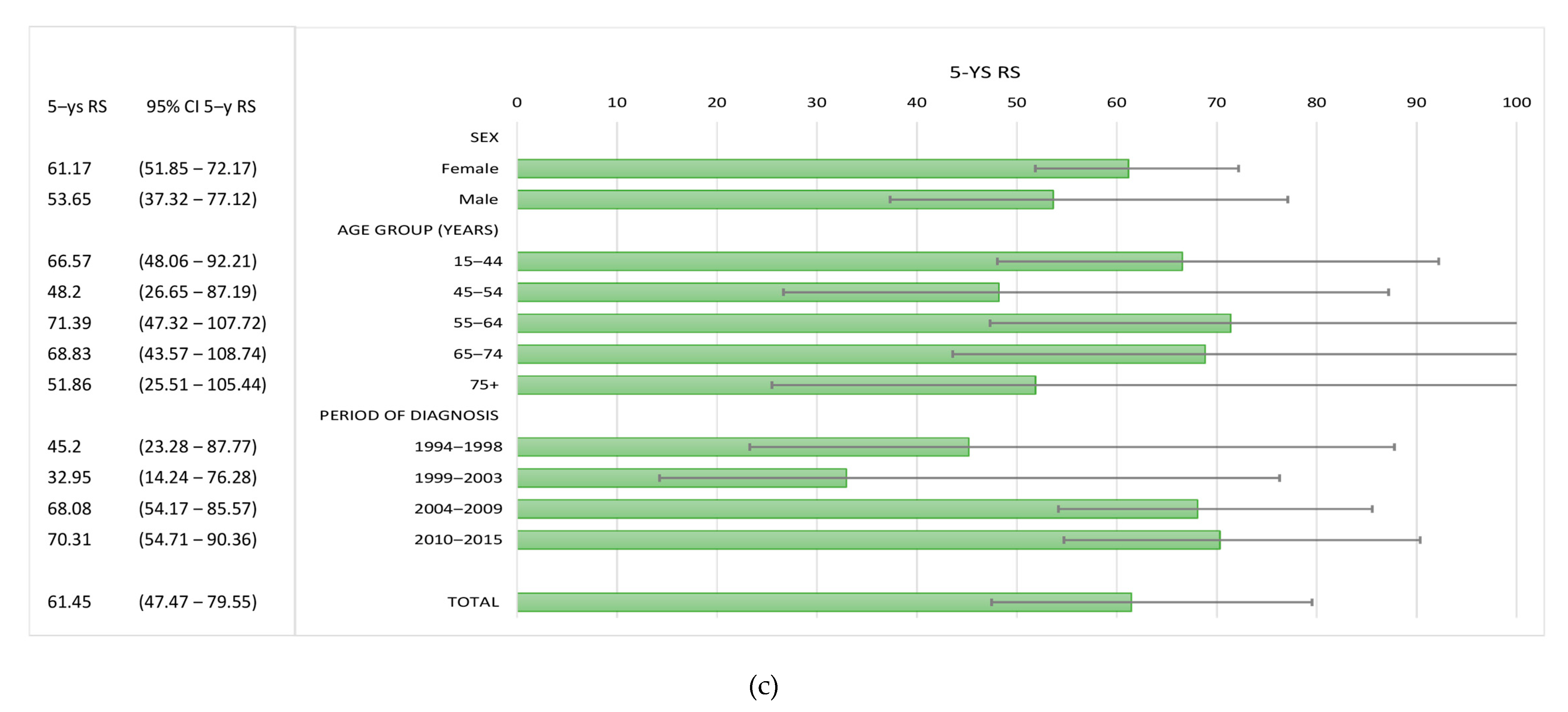
3.3. Survival
4. Discussion
Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PC | Pancreatic cancers |
| PNETS | Pancreatic neuroendocrine tumours |
| Non PNETs | Pancreatic carcinomas non-neuroendocrine |
| Non-HC PC | Pancreatic cancers non-histologically confirmed |
| ASR | Age-standardized rate |
| EAPC | Estimated annual percentage change |
| CR | Crude rate |
| RS | Relative survival |
| GWAS | Genome-wide association study |
References
- Maisonneuve, P. Epidemiology and burden of pancreatic cancer. Presse Med. 2019, 48, e113–e123. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Red Española de Registros de Cáncer (REDECAN). Estimaciones de la Incidencia del Cáncer en España, 2020. Red Española de Registros de Cáncer (REDECAN). 2020. Available online: http://redecan.org/es/index.cfm (accessed on 21 April 2020).
- Carrato, A.; Falcone, A.; Ducreux, M.; Valle, J.W.; Parnaby, A.; Djazouli, K.; Alnwick-Allu, K.; Hutchings, A.; Palaska, C.; Parthenaki, I. A Systematic Review of the Burden of Pancreatic Cancer in Europe: Real-World Impact on Survival, Quality of Life and Costs. J. Gastrointest. Cancer 2015, 46, 201–211. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- NIH National Cancer Institute. Pancreatic Cancer-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 11 May 2019).
- Lepage, C.; Capocaccia, R.; Hackl, M.; Lemmens, V.; Molina, E.; Pierannunzio, D.; Sant, M.; Trama, A.; Faivre, J.; Zielonke, N.; et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: Results of EUROCARE-5. Eur. J. Cancer 2015, 51, 2169–2178. [Google Scholar] [CrossRef]
- Chirlaque, M.-D.; Salmerón, D.; Galceran, J.; Ameijide, A.; Mateos, A.; Torrella, A.; Jiménez, R.; Larrañaga, N.; Marcos-Gragera, R.; Ardanaz, E.; et al. Cancer survival in adult patients in Spain. Results from nine population-based cancer registries. Clin. Transl. Oncol. 2018, 20, 201–211. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Adarnaz, E.; et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE--5-a population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic Adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.W.S. International Classification of Diseases for Oncology (ICD-O)–3rd Edition, 1st Revision; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. (Eds.) WHO Classification of Tumours of the Digestive System, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Clèries, R.; Ameijide, A.; Buxó, M.; Vilardell, M.; Martínez, J.M.; Alarcón, F.; Cordero, D.; Díez-Villanueva, A.; Yasui, Y.; Marcos-Gragera, R.; et al. WebSurvCa: Estimación vía web de las probabilidades de fallecimiento y de supervivencia de una cohorte. Gac. Sanit. 2018, 32, 492–495. [Google Scholar] [CrossRef]
- Perme, M.P.; Stare, J.; Estève, J. On estimation in relative survival. Biometrics 2012, 68, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dickman, P.W.; Coviello, E. Estimating and modeling relative survival. Stata J. 2015, 15, 186–215. [Google Scholar] [CrossRef]
- Huang, L.; Jansen, L.; Balavarca, Y.; Babaei, M.; Van Der Geest, L.G.M.; Lemmens, V.; Van Eycken, L.; De Schutter, H.; Johannesen, T.B.; Žakelj, M.P.; et al. Stratified survival of resected and overall pancreatic cancer patients in Europe and the USA in the early twenty-first century: A large, international population-based study. BMC Med. 2018, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Mancini, S. Pancreatic cancer incidence rises also in Italy. Int. J. Epidemiol. 2017, 46, 2090. [Google Scholar] [CrossRef]
- Bouvier, A.-M.; Uhry, Z.; Jooste, V.; Drouillard, A.; Remontet, L.; Launoy, G.; Leone, N.; French Network of Cancer Registries (Francim). Focus on an unusual rise in pancreatic cancer incidence in France. Int. J. Epidemiol. 2017, 46, 1764–1772. [Google Scholar] [CrossRef]
- Vajdic, C.M.; Laaksonen, M.A. Commentary: Unusual pancreatic cancer incidence and mortality patterns. Int. J. Epidemiol. 2017, 46, 1772–1773. [Google Scholar] [CrossRef]
- Food, Nutrition, Physical Activity, and the Prevention of Pancreatic Cancer. Continuous Update Project Report: World Cancer Research Fund/American Institute for Cancer Research. 2018. Available online: https://www.wcrf.org/dietandcancer (accessed on 10 May 2020).
- De Angelis, R.; Francisci, S.; Baili, P.; Marchesi, F.; Roazzi, P.; Belot, A.; Crocetti, E.; Pury, P.; Knijn, A.; Coleman, M.; et al. The EUROCARE-4 database on cancer survival in Europe: Data standardisation, quality control and methods of statistical analysis. Eur. J. Cancer 2009, 45, 909–930. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Sauer, A.G.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef]
- Aranceta-Bartrina, J.; Serra-Majem, L.; Foz-Salac, M.; Moreno-Esteban, B. Grupo Colaborativo SEEDO. Prevalencia de obesidad en España. Med. Clin. (Barc.) 2005, 125, 460–466. [Google Scholar] [CrossRef]
- Hidalgo, M.; Cascinu, S.S.; Kleeff, J.J.; Labianca, R.; Löhr, J.M.J.; Neoptolemos, J.; Real, F.F.; Van Laethem, J.-L.; Heinemann, V. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 2015, 15, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.-L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and centralised treatment in Europe of rare tumours: Results of RARECAREnet—a population-based study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Rabe, K.G.; Rubin, J.; Petersen, G.M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef]
- Lykoudis, P.M.; Partelli, S.; Muffatti, F.; Caplin, M.E.; Falconi, M.; Fusai, G.K.; Hackl, M.; Van Eycken, E.; Henau, K.; Dimitrova, N.; et al. Treatment challenges in and outside a specialist network setting: Pancreatic neuroendocrine tumours. Eur. J. Surg. Oncol. 2019, 45, 46–51. [Google Scholar] [CrossRef]
- Cancer Research UK. 2020. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/pancreatic-cancer (accessed on 29 April 2020).
- Akhtar-Danesh, G.G.; Finley, C.; Akhtar-Danesh, N. Long-term trends in the incidence and relative survival of pancreatic cancer in Canada: A population-based study. Pancreatology 2016, 16, 259–265. [Google Scholar] [CrossRef]

| Total | Males | Females | p-Value | |
|---|---|---|---|---|
| n (%) (% *) | n (%) (% *) | n (%) (% *) | ||
| AGE AT DIAGNOSIS ^ | <0.001 | |||
| Mean | 70.57 | 68.49 | 73.01 | |
| Median | 72 | 70 | 75 | |
| Min-max | 19–101 | 28–96 | 19–101 | |
| AGE GROUP (YEARS) + | <0.001 | |||
| 15–44 | 52 (3.25) | 29 (3.35) | 23 (3.12) | |
| 45–54 | 150 (9.36) | 98 (11.33) | 52 (7.06) | |
| 55–64 | 281 (17.54) | 180 (20.81) | 101 (13.7) | |
| 65–74 | 432 (26.97) | 262 (30.29) | 170 (23.07) | |
| 75+ | 687 (42.88) | 296 (34.22) | 391 (53.05) | |
| BASIS OF DIAGNOSIS + | <0.001 | |||
| Death certificate only (DCO) | 119 (7.43) | 65 (7.51) | 54 (7.33) | |
| Clinical only | 4 (0.25) | 1 (0.12) | 3 (0.41) | |
| Clinical investigation | 642 (40.07) | 304 (35.14) | 338 (45.86) | |
| Cytology | 251 (15.67) | 148 (17.11) | 103 (13.98) | |
| Histology of a metastasis | 155 (9.68) | 91 (10.52) | 64 (8.68) | |
| Histology of a primary tumour | 412 (25.72) | 246 (28.44) | 166 (22.52) | |
| Unknown | 19 (1.19) | 10 (1.16) | 9 (1.22) | |
| TOPOGRAPHICAL SUB-SITE + | 0.604 | |||
| Head of the pancreas (C25.0) | 658 (41.07) | 357 (41.27) | 301 (40.84) | |
| Body of the pancreas (C25.1) | 109 (6.8) | 56 (6.47) | 53 (7.19) | |
| Tail of the pancreas (C25.2) | 89 (5.55) | 51 (5.89) | 38 (5.15) | |
| Other specified parts of the pancreas (C25.3,4,7,8) | 61 (3.8) | 28 (3.23) | 33 (7.47) | |
| Pancreas, NOS (C25.9) | 685 (42.75) | 373 (43.12) | 312 (42.33) | |
| HISTOLOGICAL GROUPS + | <0.001 | |||
| PNETs | 65 (4.06) | 37 (4.28) | 28 (3.8) | |
| Pancreatic carcinomas (non-neuroendocrine) | 712 (44.44) | 424 (49.02) | 288 (39.08) | |
| Pancreatic tumours non-histologically confirmed | 825 (51.5) | 404 (46.71) | 421 (57.12) | |
| TNM STAGE + | 0.556 | |||
| I | 21 (1.31) (3.42) | 10 (1.15) (3.04) | 11 (1.49) (3.84) | |
| IIA | 37 (2.31) (6.03) | 21 (2.43) (6.40) | 16 (2.17) (5.61) | |
| IIB | 70 (4.37) (11.41) | 41 (4.74) (12.5) | 29 (3.93) (10.17) | |
| III | 70 (4.37) (11.41) | 43 (4.97) (13.1) | 27 (3.66) (9.47) | |
| IV | 415 (25.90) (67.69) | 213 (24.62) (64.93) | 202 (27.41) (70.87) | |
| Unknown | 989 (61.73) | 537 (62.08) | 452 (61.32) | |
| PERIOD OF DIAGNOSIS + | 0.993 | |||
| 1994–1998 | 239 (14.91) | 128 (14.79) | 111 (15.06) | |
| 1999–2003 | 327 (20.41) | 177 (20.46) | 150 (20.35) | |
| 2004–2009 | 453 (28.27) | 246 (28.43) | 207 (28.08) | |
| 2010–2015 | 583 (36.39) | 314 (36.30) | 269 (36.49) | |
| TOTAL | 1602 (100.0) | 865(54.2) | 737 (45.8) |
| All PC | Non-PNETs | PNETs | PC Non-HC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % Total | ASRE13 | 95% CI | n | % Total | ASRE13 | 95% CI | n | % Total | ASRE13 | 95% CI | n | % Total | ASRE13 | 95% CI | |
| Sex | ||||||||||||||||
| Female | 737 | 46 | 10.82 | (10.05–11.65) | 288 | 40.45 | 4.46 | (3.95–5.01) | 28 | 43.08 | 0.41 | (0.27–0.6) | 421 | 51.03 | 5.96 | (5.4–6.57) |
| Male | 865 | 54 | 15.8 | (14.75–16.91) | 424 | 59.55 | 7.35 | (6.66–8.1) | 37 | 56.92 | 0.6 | (0.42–0.84) | 404 | 48.97 | 7.85 | (7.09–8.68) |
| Age Group (years) | ||||||||||||||||
| 15–44 | 52 | 3.25 | 0.85 | (0.63–1.12) | 27 | 3.79 | 0.44 | (0.29–0.65) | 19 | 29.23 | 0.3 | (0.18–0.48) | 6 | 0.73 | 0.1 | (0.04–0.22) |
| 45–54 | 150 | 9.36 | 8.16 | (6.9–9.58) | 106 | 14.89 | 5.75 | (4.7–6.96) | 13 | 20 | 0.7 | (0.37–1.2) | 31 | 3.76 | 1.71 | (1.16–2.43) |
| 55–64 | 281 | 17.54 | 19.32 | (17.13–21.72) | 191 | 26.83 | 13.14 | (11.34 15.14) | 10 | 15.38 | 0.69 | (0.33–1.26) | 80 | 9.7 | 5.5 | (4.36–6.85) |
| 65–74 | 432 | 26.97 | 35.66 | (32.38–39.19) | 221 | 31.04 | 18,23 | (15.91–20.8) | 11 | 16.92 | 0.9 | (0.45–1.62) | 200 | 24.24 | 16.52 | (14.31–18.98) |
| 75+ | 687 | 42.88 | 61.83 | (57.28–66.66) | 167 | 23.46 | 14.87 | (12.69 17.32) | 12 | 18.46 | 1.06 | (0.55–1.86) | 508 | 61.58 | 45.91 | (41.99–50.1) |
| STAGE | ||||||||||||||||
| I | 21 | 1.31 | 0.15 | (0.07–0.32) | 9 | 1.26 | 0.08 | (0.02–0.21) | 8 | 12.31 | 0.05 | (0.01–0.17) | 4 | 0.48 | 0.03 | (0.01–0.14) |
| IIA | 37 | 2.31 | 0.31 | (0.22–0.43) | 25 | 3.51 | 0.21 | (0.14–0.32) | 5 | 7.69 | 0.04 | (0.01–0.09) | 7 | 0.85 | 0.06 | (0.02–0.12) |
| IIB | 70 | 4.37 | 0.59 | (0.46–0.75) | 62 | 8.71 | 0.53 | (0.4–0.68) | 1 | 1.54 | 0.01 | (0–0.04) | 7 | 0.85 | 0.06 | (0.02–0.12) |
| III | 70 | 4.37 | 0.58 | (0.45–0.74) | 43 | 6.04 | 0.36 | (0.26–0.49) | 2 | 3.08 | 0.01 | (0–0.05) | 25 | 3.03 | 0.21 | (0.13–0.31) |
| IV | 415 | 25.90 | 3.40 | (3.07–3.79) | 251 | 35.25 | 2.06 | (1.8–2.37) | 21 | 32.31 | 0.16 | (0.1–0.28) | 143 | 17.33 | 1.18 | (0.99–1.42) |
| TOTAL | 1602 | 13.19 | (12.55–13.85) | 712 | 5.87 | (5.44–6.32) | 65 | 0.49 | (0.38–0.63) | 825 | 6.83 | (6.37–7.32) | ||||
| Observed Survival (OS) | Relative Survival (RS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n Cases | 1–y OS | 95% CI 1–y OS | 5–y OS | 95 %CI 5–y OS | n Cases | 1–y RS | 95% CI 1–y RS | 5–ys RS | 95% CI 5–y RS | |
| SEX | ||||||||||
| Female | 681 | 20.46 | (17.6–23.79) | 4.18 | (2.76–6.34) | 681 | 26.46 | (23.04–30.38) | 6.75 | (4.71–9.66) |
| Male | 800 | 22.47 | (19.69–25.65) | 5.83 | (4.28–7.95) | 800 | 24.12 | (21.28–27.34) | 7.34 | (5.53–9.76) |
| AGE GROUP (YEARS) | ||||||||||
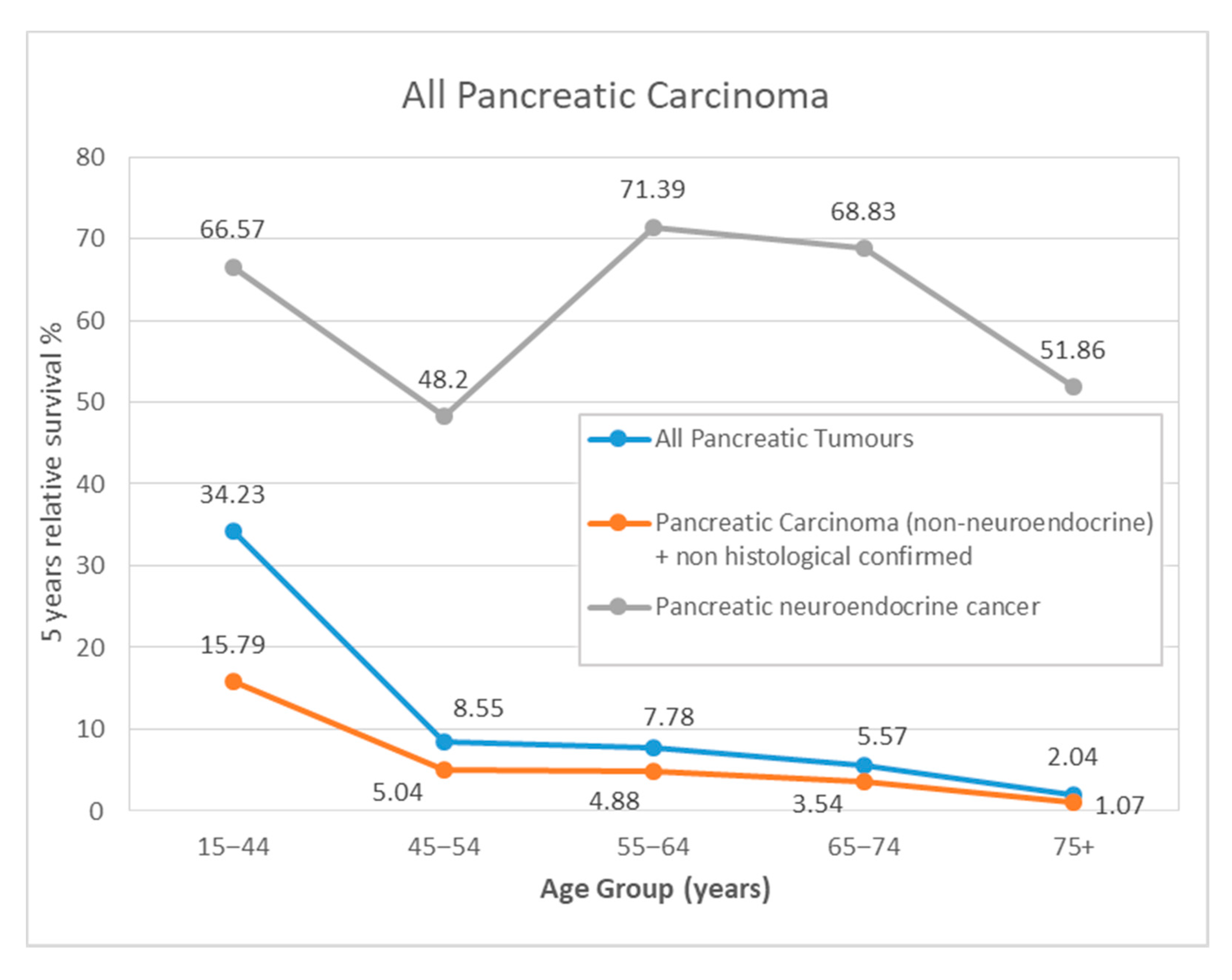
| 15–44 | 51 | 54.65 | (42.51–70.25) | 33.91 | (22.57–50.95) | 51 | 54.73 | (42.72–70.11) | 34.23 | (22.99–50.95) |
| 45–54 | 146 | 37.62 | (30.37–46.61) | 8.39 | (4.6–15.31) | 146 | 37.75 | (30.52–46.68) | 8.55 | (4.77–15.32) |
| 55–64 | 262 | 30.02 | (24.8–36.36) | 7.37 | (4.51–12.05) | 259 | 30.62 | (25.33–37.02) | 7.78 | (4.81–12.59) |
| 65–74 | 400 | 23.98 | (20.05–28.67) | 4.69 | (2.82–7.8) | 398 | 24.64 | (20.62–29.43) | 5.57 | (3.42–9.07) |
| 75+ | 622 | 9.92 | (7.8–12.63) | 1.26 | (0.57–2.77) | 618 | 10.72 | (8.43–13.64) | 2.04 | (0.96–4.35) |
| TOPOGRAPHICAL SUB–SITE | ||||||||||
| Head of the pancreas (C25.0) | 644 | 26.26 | (23–29.99) | 4.26 | (2.77–6.53) | 644 | 30.7 | (27.09–34.79) | 6.05 | (4.1–8.92) |
| Body of the pancreas (C25.1) | 106 | 19.56 | (13.13–29.15) | 2.06 | (0.52–8.11) | 106 | 19.16 | (13.28–27.64) | 3.49 | (1.19–10.29) |
| Tail of the pancreas (C25.2) | 82 | 18.05 | (11.28–28.88) | 4.51 | (1.55–13.1) | 82 | 19.9 | (13.61–29.09) | 4.36 | (1.83–10.39) |
| Other specified parts of the pancreas (C25.3,4,7,8) | 91 | 45.75 | (36.53–57.3) | 30.84 | (22.23–42.79) | 91 | 41.14 | (32.93–51.4) | 27.27 | (19.5–38.15) |
| Pancreas, NOS (C25.9) | 558 | 12.91 | (10.36–16.1) | 2.71 | (1.56–4.7) | 558 | 15.41 | (12.48–19.02) | 3.79 | (2.37–6.05) |
| STAGE | ||||||||||
| I | 21 | – | (NA–NA) | – | (NA–NA) | 21 | – | (NA–NA) | 0.26 | (NA–NA) |
| IIA | 37 | 62.49 | (48.16–81.1) | 36.87 | (22.79–59.63) | 37 | 66 | (53.63–81.23) | 46.14 | (36.14–58.91) |
| IIB | 69 | 60.58 | (49.81–73.68) | 15.4 | (7.58–31.31) | 69 | 56.12 | (45.76–68.81) | 12.67 | (6.87–23.37) |
| III | 70 | 33.34 | (23.88–46.54) | 4.62 | (1.54–13.89) | 70 | 37.55 | (29.73–47.43) | 5.56 | (2.54–12.19) |
| IV | 414 | 19.11 | (15.57–23.45) | 2.78 | (1.37–5.63) | 414 | 21.1 | (17.43–25.53) | 4.36 | (2.61–7.29) |
| PERIOD OF DIAGNOSIS | ||||||||||
| 1994–1998 | 217 | 16.69 | (12.39–22.49) | 2.78 | (1.26–6.12) | 217 | 18.09 | (13.68–23.91) | 3.27 | (1.69–6.35) |
| 1999–2003 | 289 | 14.18 | (10.64–18.89) | 2.48 | (1.19–5.16) | 289 | 16.39 | (12.46–21.55) | 3.38 | (1.82–6.27) |
| 2004–2009 | 415 | 23.04 | (19.3–27.5) | 4.66 | (3–7.22) | 415 | 27.44 | (23.51–32.02) | 6.68 | (4.54–9.82) |
| 2010–2015 | 560 | 26.45 | (22.89–30.55) | 9.65 | (7.09–13.13) | 560 | 31.45 | (27.6–35.83) | 13.1 | (9.98–17.2) |
| GEOGRAPHICAL AREA | ||||||||||
| Alt Empordà Coast | 224 | 21.99 | (17.06–28.35) | 5.98 | (3.42–10.46) | 224 | 26.25 | (21.11–32.66) | 8.79 | (5.55–13.91) |
| Alt Empordà Interior | 38 | 14.75 | (6.25–34.85) | 7.38 | (2–27.16) | 38 | 13.69 | (7.09–26.42) | 7.63 | (2.83–20.54) |
| Baix Empordà | 262 | 23.14 | (18.49–28.95) | 6.03 | (3.5–10.4) | 262 | 24.94 | (20.23–30.74) | 8 | (5.24–12.2) |
| Cerdanya | 24 | 12.5 | (4.34–36.03) | 4.17 | (0.61–28.38) | 24 | 13.87 | (8.82–21.83) | 7.25 | (6.75–7.78) |
| Garrotxa | 129 | 19.19 | (13.42–27.45) | 4.26 | (1.82–9.96) | 129 | 21.69 | (15.77–29.83) | 4.94 | (2.41–10.1) |
| Gironès | 343 | 21.09 | (17.11–26) | 4.02 | (2.21–7.33) | 343 | 25.98 | (21.85–30.88) | 5.85 | (3.53–9.7) |
| Pla De L’Estany | 62 | 26.28 | (17.26–40.02) | 3.5 | (0.9–13.61) | 62 | 34.21 | (25.25–46.36) | 3.73 | (1.4–9.96) |
| Ripollès | 87 | 17.43 | (10.87–27.96) | 3.08 | (0.81–11.71) | 86 | 18.29 | (11.55–28.97) | 3.69 | (1.13–12.01) |
| Selva Coast | 204 | 27.88 | (22.19–35.01) | 5.93 | (3–11.73) | 204 | 30.55 | (24.71–37.78) | 8.44 | (5.2–13.7) |
| Selva Interior | 108 | 14.18 | (8.88–22.64) | 5.88 | (2.61–13.26) | 108 | 17.38 | (11.51–26.24) | 8.74 | (4.69–16.27) |
| TOTAL | 1481 | 21.54 | (19.5–23.79) | 5.07 | (3.95–6.51) | 1481 | 24.94 | (22.72–27.38) | 7.05 | (5.63–8.84) |
| Observed Survival (OS) | Relative Survival (RS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n Cases | 1–y OS | 95% CI 1–y OS | 5–y OS | 95 %CI 5–y OS | n Cases | 1–y RS | 95% CI 1–y RS | 5–ys RS | 95% CI 5–y RS | |
| SEX | ||||||||||
| Female | 653 | 17.79 | (15.04–21.04) | 1.64 | (0.82–3.29) | 653 | 23.24 | (19.75–27.33) | 2.9 | (1.61–5.21) |
| Male | 763 | 20.21 | (17.48–23.37) | 3.62 | (2.4–5.47) | 763 | 21.78 | (18.93–25.07) | 4.75 | (3.25–6.95) |
| AGE GROUP (YEARS) | ||||||||||
| 15–44 | 32 | 37.5 | (23.98–58.65) | 15.62 | (6.59–37.02) | 32 | 37.56 | (24.32–58.02) | 15.79 | (7.07–35.28) |
| 45–54 | 133 | 34.35 | (26.97–43.75) | 4.94 | (2.15–11.36) | 133 | 34.47 | (27.13–43.81) | 5.04 | (2.29–11.09) |
| 55–64 | 252 | 27.88 | (22.68–34.27) | 4.6 | (2.39–8.88) | 249 | 28.45 | (23.19–34.9) | 4.88 | (2.59–9.19) |
| 65–74 | 389 | 22.65 | (18.76–27.33) | 2.89 | (1.46–5.74) | 387 | 23.27 | (19.3–28.06) | 3.54 | (1.87–6.7) |
| 75+ | 610 | 8.99 | (6.96–11.62) | 0.57 | (0.16–1.99) | 606 | 9.73 | (7.53–12.57) | 1.07 | (0.37–3.11) |
| TOPOGRAPHICAL SUB–SITE | ||||||||||
| Head of the pancreas (C25.0) | 638 | 26.19 | (22.92–29.93) | 3.94 | (2.51–6.16) | 638 | 30.74 | (27.11–34.86) | 5.73 | (3.83–8.58) |
| Body of the pancreas (C25.1) | 105 | 19.75 | (13.26–29.42) | 2.08 | (0.53–8.18) | 105 | 19.16 | (13.28–27.64) | 3.5 | (1.19–10.29) |
| Tail of the pancreas (C25.2) | 78 | 13.67 | (7.72–24.19) | 0 | (NA–NA) | 78 | 15.9 | (10.03–25.23) | 0.05 | (0.02–0.17) |
| Other specified parts of the pancreas (C25.3,4,7,8) | 57 | 18.01 | (10.24–31.7) | 2.33 | (0.35–15.74) | 57 | 20.73 | (13.55–31.73) | 2.62 | (0.7–9.81) |
| Pancreas, NOS (C25.9) | 538 | 11.44 | (9–14.55) | 1.83 | (0.94–3.59) | 538 | 13.89 | (11–17.53) | 2.65 | (1.46–4.81) |
| STAGE | ||||||||||
| I | 13 | – | (NA–NA) | – | (NA–NA) | 13 | – | (NA–NA) | – | (NA–NA) |
| IIA | 32 | 55.98 | (40.45–77.47) | 25.02 | (12.29–50.91) | 32 | 58.06 | (42.18–79.92) | 28.25 | (14.08–56.67) |
| IIB | 68 | 61.47 | (50.65–74.61) | 15.63 | (7.69–31.75) | 68 | 56.12 | (45.77–68.81) | 12.67 | (6.87–23.37) |
| III | 68 | 31.35 | (22–44.68) | 3.2 | (0.82–12.44) | 68 | 34.68 | (27.1–44.37) | 2.89 | (0.93–8.93) |
| IV | 393 | 17.27 | (13.81–21.61) | 1.86 | (0.8–4.37) | 393 | 19.61 | (15.92–24.14) | 3.48 | (1.89–6.4) |
| PERIOD OF DIAGNOSIS | ||||||||||
| 1994–1998 | 208 | 14.52 | (10.43–20.21) | 0.97 | (0.24–3.84) | 208 | 16.05 | (11.73–21.97) | 1.06 | (0.36–3.1) |
| 1999–2003 | 279 | 12.5 | (9.13–17.11) | 1.47 | (0.56–3.89) | 279 | 13.95 | (10.3–18.9) | 2.35 | (1.08–5.12) |
| 2004–2009 | 402 | 21.52 | (17.83–25.97) | 2.78 | (1.56–4.99) | 402 | 25.93 | (21.87–30.75) | 4.23 | (2.51–7.14) |
| 2010–2015 | 527 | 22.8 | (19.33–26.89) | 5.42 | (3.44–8.55) | 527 | 27.53 | (23.63–32.08) | 7.76 | (5.22–11.53) |
| GEOGRAPHICAL AREA | ||||||||||
| Alt Empordà Coast | 216 | 20.5 | (15.65–26.87) | 4.53 | (2.34–8.79) | 216 | 25.37 | (19.95–32.26) | 7.43 | (4.24–13.03) |
| Alt Empordà Interior | 38 | 14.75 | (6.25–34.85) | 7.38 | (2–27.16) | 38 | 13.69 | (7.09–26.42) | 7.63 | (2.83–20.54) |
| Baix Empordà | 242 | 18.38 | (14.02–24.08) | 1.25 | (0.34–4.66) | 242 | 19.24 | (14.87–24.88) | 2.1 | (0.81–5.46) |
| Cerdanya | 23 | 8.7 | (2.31–32.69) | 0 | (NA–NA) | 21 | 9.83 | (3.09–31.3) | 0.22 | (0.02–2.58) |
| Garrotxa | 124 | 16.68 | (11.2–24.83) | 1.81 | (0.46–7.07) | 124 | 19.13 | (13.43–27.23) | 1.88 | (0.7–5.01) |
| Gironès | 328 | 18.41 | (14.58–23.25) | 1.69 | (0.62–4.61) | 328 | 23.54 | (19.45–28.48) | 2.96 | (1.38–6.35) |
| Pla De L’Estany | 58 | 24.62 | (15.63–38.79) | 1.89 | (0.27–13.14) | 58 | 36.22 | (26.85–48.84) | 1.75 | (0.44–6.92) |
| Ripollès | 83 | 15.79 | (9.43–26.42) | 1.48 | (0.21–10.22) | 82 | 16.53 | (10.03–27.24) | 1.85 | (0.4–8.55) |
| Selva Coast | 199 | 26.49 | (20.84–33.66) | 4.53 | (2.1–9.78) | 199 | 28.54 | (22.78–35.76) | 5.83 | (3.17–10.72) |
| Selva Interior | 105 | 12.66 | (7.62–21.01) | 4.17 | (1.55–11.24) | 105 | 15.54 | (9.99–24.17) | 6.83 | (3.3–14.13) |
| TOTAL | 1416 | 19.08 | (17.09–21.3) | 2.71 | (1.9–3.87) | 1416 | 22.31 | (20.05–24.83) | 4.01 | (2.88–5.59) |
| Observed Survival (OS) | Relative Survival (RS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n Cases | 1–y OS | 95% CI 1–y OS | 5–y OS | 95 %CI 5–y OS | n Cases | 1–y RS | 95% CI 1–y RS | 5–ys RS | 95% CI 5–y RS | |
| SEX | ||||||||||
| Female | 28 | 81.82 | (68.57–97.62) | 68.33 | (52.12–89.56) | 28 | 63.63 | (55.42–73.06) | 61.17 | (51.85–72.17) |
| Male | 37 | 67.27 | (53.65–84.34) | 51.38 | (36.97–71.42) | 37 | 65.32 | (51.12–83.45) | 53.65 | (37.32–77.12) |
| AGE GROUP (YEARS) | ||||||||||
| 15–44 | 19 | 83.88 | (68.71–100) | 66.09 | (47.14–92.65) | 19 | 84 | (69.21–101.94) | 66.57 | (48.06–92.21) |
| 45–54 | 13 | 69.23 | (48.19–99.47) | 47.47 | (24.93–90.4) | 13 | 69.39 | (49.09–98.07) | 48.2 | (26.65–87.19) |
| 55–64 | 10 | 80 | (58.68–100) | 68.57 | (44.47–100) | 10 | 80.36 | (59.95–107.72) | 71.39 | (47.32–107.72) |
| 65–74 | 11 | 71.59 | (48.84–100) | 61.36 | (37.69–99.92) | 11 | 72.56 | (50.57–104.1) | 68.83 | (43.57–108.74) |
| 75+ | 12 | 58.33 | (36.16–94.1) | 43.75 | (20.86–91.77) | 12 | 61.42 | (39.06–96.58) | 51.86 | (25.51–105.44) |
| TOPOGRAPHICAL SUB–SITE | ||||||||||
| Head of the pancreas (C25.0) | 6 | 33.33 | (10.75–100) | 33.33 | (10.75–100) | 6 | 33.51 | (12.7–88.41) | 34.11 | (12.93–90) |
| Body of the pancreas (C25.1) | 1 | 0 | (NA–NA) | 0 | (NA–NA) | 1 | 0.03 | (0–0.22) | 0.03 | (0–0.22) |
| Tail of the pancreas (C25.2) | 4 | 100 | (100–100) | 75 | (42.59–100) | 4 | 100 | (100–100) | 82.47 | (50.94–133.54) |
| Other specified parts of the pancreas (C25.3,4,7,8) | 34 | 91.18 | (82.12–100) | 76.55 | (62.56–93.67) | 34 | 87.77 | (75.23–102.4) | 81.77 | (64.89–103.02) |
| Pancreas, NOS (C25.9) | 20 | 52.87 | (34.4–81.24) | 32.9 | (16.24–66.65) | 20 | 60.11 | (46.4–77.86) | 39.95 | (23.52–67.84) |
| STAGE | ||||||||||
| I | 8 | – | (NA–NA) | – | (NA–NA) | 8 | – | (NA–NA) | – | (NA–NA) |
| IIA | 5 | 100 | (100–100) | 100 | (100–100) | 5 | 100 | (100–100) | 106.46 | (106.46–106.46) |
| IIB | 1 | 0 | (NA–NA) | 0 | (NA–NA) | 1 | 0.03 | (0–0.22) | 0.03 | (0–0.22) |
| III | 2 | 100 | (100–100) | 50 | (12.5–100) | 2 | 100 | (100–100) | 57.71 | (18.81–177.01) |
| IV | 21 | 51.95 | (34.31–78.66) | 28.05 | (11.96–65.81) | 21 | 51.45 | (36.42–72.7) | 34.83 | (20.62–58.85) |
| PERIOD OF DIAGNOSIS | ||||||||||
| 1994–1998 | 9 | 66.67 | (42–100) | 44.44 | (21.41–92.27) | 9 | 67.18 | (43.7–103.27) | 45.2 | (23.28–87.77) |
| 1999–2003 | 10 | 60 | (36.17–99.52) | 30 | (11.64–77.32) | 10 | 60.38 | (37.63–96.87) | 32.95 | (14.24–76.28) |
| 2004–2009 | 13 | 69.23 | (48.19–99.47) | 61.54 | (40.04–94.58) | 13 | 69.8 | (56.02–86.96) | 68.08 | (54.17–85.57) |
| 2010–2015 | 33 | 81.38 | (68.98–96.02) | 72.66 | (57.68–91.51) | 33 | 81.2 | (68.9–95.7) | 70.31 | (54.71–90.36) |
| GEOGRAPHICAL AREA | ||||||||||
| Alt Empordà Coast | 8 | 62.5 | (36.54–100) | 46.88 | (21.49–100) | 8 | 63.07 | (38.45–103.46) | 48.1 | (24.06–96.17) |
| Alt Empordà Interior | 20 | 79.69 | (63.73–99.63) | 67.61 | (49.04–93.22) | 20 | 73.82 | (56.89–95.8) | 59.81 | (42.09–84.98) |
| Baix Empordà | 1 | 100 | (100–100) | 100 | (100–100) | 1 | 100 | (100–100) | 100 | (100–100) |
| Cerdanya | 5 | 80 | (51.61–100) | 60 | (29.33–100) | 5 | 80.32 | (54.1–119.23) | 62.04 | (33.12–116.23) |
| Garrotxa | 15 | 80 | (62.12–100) | 54 | (32.22–90.51) | 15 | 78.89 | (64.45–96.56) | 63.59 | (48.16–83.97) |
| Gironès | 4 | 50 | (18.77–100) | 25 | (4.58–100) | 4 | 50.02 | (22.1–113.22) | 25.07 | (7–89.75) |
| Pla De L’estany | 4 | 50 | (18.77–100) | 50 | (18.77–100) | 4 | 52.94 | (23.63–118.62) | 58.18 | (25.97–130.34) |
| Ripollès | 5 | 80 | (51.61–100) | 80 | (51.61–100) | 5 | 80.84 | (54.53–119.86) | 82.27 | (55.49–121.98) |
| Selva Coast | 3 | 66.67 | (29.95–100) | 66.67 | (29.95–100) | 3 | 66.88 | (34.83–128.44) | 68.59 | (35.72–131.71) |
| Selva Interior | 8 | 62.5 | (36.54–100) | 46.88 | (21.49–100) | 8 | 63.07 | (38.45–103.46) | 48.1 | (24.06–96.17) |
| TOTAL | 65 | 73.6 | (63.57–85.21) | 58.69 | (47.28–72.84) | 65 | 70.28 | (58.67–84.19) | 61.45 | (47.47–79.55) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Velasco, A.; Zacarías-Pons, L.; Teixidor, H.; Valeros, M.; Liñan, R.; Carmona-Garcia, M.C.; Puigdemont, M.; Carbajal, W.; Guardeño, R.; Malats, N.; et al. Incidence and Survival Trends of Pancreatic Cancer in Girona: Impact of the Change in Patient Care in the Last 25 Years. Int. J. Environ. Res. Public Health 2020, 17, 9538. https://doi.org/10.3390/ijerph17249538
García-Velasco A, Zacarías-Pons L, Teixidor H, Valeros M, Liñan R, Carmona-Garcia MC, Puigdemont M, Carbajal W, Guardeño R, Malats N, et al. Incidence and Survival Trends of Pancreatic Cancer in Girona: Impact of the Change in Patient Care in the Last 25 Years. International Journal of Environmental Research and Public Health. 2020; 17(24):9538. https://doi.org/10.3390/ijerph17249538
Chicago/Turabian StyleGarcía-Velasco, Adelaida, Lluís Zacarías-Pons, Helena Teixidor, Marc Valeros, Raquel Liñan, M. Carmen Carmona-Garcia, Montse Puigdemont, Walter Carbajal, Raquel Guardeño, Núria Malats, and et al. 2020. "Incidence and Survival Trends of Pancreatic Cancer in Girona: Impact of the Change in Patient Care in the Last 25 Years" International Journal of Environmental Research and Public Health 17, no. 24: 9538. https://doi.org/10.3390/ijerph17249538
APA StyleGarcía-Velasco, A., Zacarías-Pons, L., Teixidor, H., Valeros, M., Liñan, R., Carmona-Garcia, M. C., Puigdemont, M., Carbajal, W., Guardeño, R., Malats, N., Duell, E., & Marcos-Gragera, R. (2020). Incidence and Survival Trends of Pancreatic Cancer in Girona: Impact of the Change in Patient Care in the Last 25 Years. International Journal of Environmental Research and Public Health, 17(24), 9538. https://doi.org/10.3390/ijerph17249538






