Assessment of a Screening Questionnaire to Identify Exposure to Lead in Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Data Collection
2.4. Determining Blood Lead Levels
2.5. Statistical Analyses
2.6. Authorizations
3. Results
4. Discussion
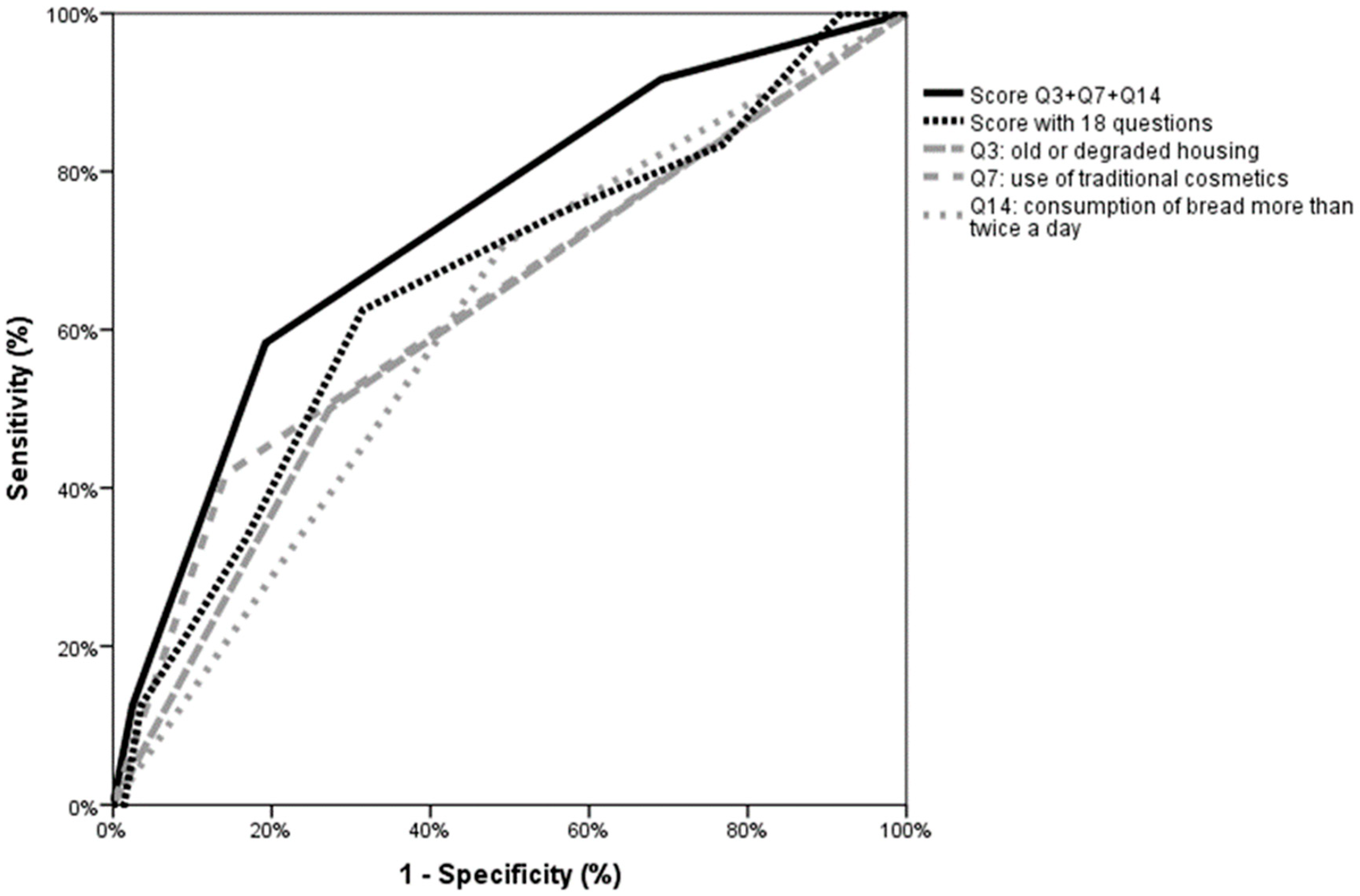
4.1. Towards an Efficient and Simple Screening Lead Poisoning Questionnaire
4.2. A snapshot of Lead Poisoning in a Pregnant Population Consulted at University Hospital in Marseille
4.3. Implications for Health Professionals and Patients
4.4. Strengths and Weaknesses of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Lead Poisoning Screening Questionnaire
| Q1: Have you ever had a personal health history with lead poisoning (acute and chronic absorption of lead into the body)? Or has someone in your surroundings? | YES ❑ NO ❑ |
| Q2: Were you born in a country in Africa (including Comoros, Madagascar), the Middle East, Southeast Asia, the West Indies or Eastern Europe? | YES ❑ NO ❑ |
| Q3: Do you live in an old and degraded apartment (built before 1975)? | YES ❑ NO ❑ |
| Q4: Over the last 6 months, have you been in contact with someone working in construction, in an industrial site, or have you been living in an old apartment or in an apartment in construction (spread of dust, including stripping or sanding old paint)? | YES ❑ NO ❑ |
| Q5: Do you ingest traditional food supplements (clays, medicinal herbs?) | YES ❑ NO ❑ |
| Q6: Have you ever eaten non-food substances such as clay, soil, plaster, paint flakes? | YES ❑ NO ❑ |
| Q7: Do you use traditional cosmetics? (kohl, surma)? | YES ❑ NO ❑ |
| Q8: For the preparation/preservation of food, do you often use traditional containers (tajine dishes), enamelled ceramic pottery, pewter, or crystal dishes? | YES ❑ NO ❑ |
| Q9: Do you drink tap water? | YES ❑ NO ❑ |
| Q10: if yes, are there any lead pipes? | YES ❑ NO ❑ |
| Q11: Do you smoke? | YES ❑ NO ❑ |
| Q12 Are you passively exposed to the smoke of cigarettes? | YES ❑ NO ❑ |
| Q13: Have you been drinking alcohol while pregnant? | YES ❑ NO ❑ |
| Q14: Do you eat bread more than twice a day? | YES ❑ NO ❑ |
| Q15: Do you eat vegetables grown close to an industrial site more than twice a day? | YES ❑ NO ❑ |
| Q16: Have you been eating seafood while pregnant? | YES ❑ NO ❑ |
| Q17: Do you live or spend much time in places close to an industrial site releasing lead into the atmosphere? | YES ❑ NO ❑ |
| Q18: Do you practice (or have you practiced) shooting, hunting, fishing, pottery or a risky professional activity (in the industrial sector, construction work, metallurgy, in contact with objects containing lead)? Or is there in your surroundings someone practicing such leisure activities or working in those areas? | YES ❑ NO ❑ |
References
- Klein, M.; Kaminsky, P.; Duc, M.L.; Duc, M. Current diagnosis and treatment of lead poisoning. Rev. Med. Interne 1994, 15, 101. [Google Scholar] [CrossRef]
- Haut Conseil de la Santé Publique. Mise à Jour du Guide Pratique de Dépistage et de Prise en Charge des Expositions au Plomb chez L’enfant Mineur et la Femme Enceinte. 2017. Available online: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=643 (accessed on 9 December 2020).
- Dereumeaux, C.; Saoudi, A.; Oleko, A.; Pecheux, M.; Vandentorren, S.; Fillol, C.; Denys, S. Surveillance biologique de l’exposition des femmes enceintes françaises aux polluants de l’environnement: Résultats du volet périnatal du programme national de biosurveillance mis en œuvre au sein de la cohorte Elfe. Toxicol. Anal. Clin. 2017, 29, 496–516. [Google Scholar] [CrossRef]
- Ministère de la Santé, et des Solidarités L’intoxication par le Plomb de L’enfant et de la Femme Enceinte. Dépistage—Prise en Charge. 2006. Available online: https://solidarites-sante.gouv.fr/IMG/pdf/guide_depistage_saturnisme.pdf (accessed on 9 December 2020).
- Gulson, B.L.; Jameson, C.W.; Mahaffey, K.R.; Mizon, K.J.; Korsch, M.J.; Vimpani, G. Pregnancy increases mobilization of lead from maternal skeleton. J. Lab. Clin. Med. 1997, 130, 51–62. [Google Scholar] [CrossRef]
- Silbergeld, E.K. Lead in bone: Implications for toxicology during pregnancy and lactation. Environ. Health Perspect. 1991, 91, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gardella, C. Lead exposure in pregnancy: A review of the literature and argument for routine prenatal screening. Obstet. Gynecol. Surv. 2001, 56, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, C.; Thiebaugeorges, O.; Moreau, T.; Goua, V.; Debotte, G.; Sahuquillo, J.; Forhan, A.; Foliguet, B.; Magnin, G.; Slama, R.; et al. Maternal blood lead levels and the risk of pregnancy-induced hypertension: The EDEN cohort study. Environ. Health Perspect. 2009, 117, 1526–1530. [Google Scholar] [CrossRef]
- Poropat, A.E.; Laidlaw, M.A.S.; Lanphear, B.; Ball, A.; Mielke, H.W. Blood lead and preeclampsia: A meta-analysis and review of implications. Environ. Res. 2018, 160, 12–19. [Google Scholar] [CrossRef]
- Xie, X.; Ding, G.; Cui, C.; Chen, L.; Gao, Y.; Zhou, Y.; Shi, R.; Tian, Y. The effects of low-level prenatal lead exposure on birth outcomes. Environ. Pollut. 2013, 175, 30–34. [Google Scholar] [CrossRef]
- Rahman, A.; Kumarathasan, P.; Gomes, J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci. Total Environ. 2016, 569, 1022–1031. [Google Scholar] [CrossRef]
- Wasserman, G.A.; Liu, X.; Popovac, D.; Factor-Litvak, P.; Kline, J.; Waternaux, C.; LoIacono, N.; Graziano, J.H. The Yugoslavia prospective lead study: Contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicology Teratol. 2000, 22, 811–818. [Google Scholar] [CrossRef]
- Schnaas, L.; Rothenberg, S.J.; Flores, M.F.; Martinez, S.; Hernandez, C.; Osorio, E.; Velasco, S.R.; Perroni, E. Reduced intellectual development in children with prenatal lead exposure. Environ. Health Perspect. 2006, 114, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Cheymol, J.; Desplanques, L.; Deffontaines, D. Fœtopathie saturnine. Arch. Pédiatrie 2002, 8, 506s–507s. [Google Scholar] [CrossRef]
- Hu, H.; Téllez-Rojo, M.M.; Bellinger, D.; Smith, D.; Ettinger, A.S.; Lamadrid-Figueroa, H.; Schwartz, J.; Schnaas, L.; Mercado-García, A.; Hernández-Avila, M. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ. Health Perspect. 2006, 114, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Kaminsky, P.; Barbe, F.; Duc, M. Lead poisoning in pregnancy [SATURNISME AU COURS DE LA GROSSESSE]. Presse Med. 1994, 23, 576–580. [Google Scholar]
- Ernhart, C.B. A critical review of low-level prenatal lead exposure in the human: 1. Effects on the fetus and newborn. Reprod. Toxicol. 1992, 6, 9–19. [Google Scholar] [CrossRef]
- Jelliffe-Pawlowski, L.L.; Miles, S.Q.; Courtney, J.G.; Materna, B.; Charlton, V. Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J. Perinatol. 2006, 26, 154–162. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Monograph: Health Effects of Low-Level Lead. NTP Monogr. 2012, 1, i. [Google Scholar]
- Yazbeck, C.; Cheymol, J.; Dandres, A.-M.; Barbery-Courcoux, A.-L. Lead exposure in pregnant women and newborns: A screening update. Arch. Pediatr. Organe Off. Soc. Fr. Pediatr. 2007, 14, 15–19. [Google Scholar] [CrossRef]
- Audrey, S.; Takser, L.; André, M.; Martin, S.; Donna, M.; Geneviève, S.A.; Philippe, B.; Georgette, H.; Guy, H. A comparative study of manganese and lead levels in human umbilical cords and maternal blood from two urban centers exposed to different gasoline additives. Sci. Total Environ. 2002, 290, 157–164. [Google Scholar] [CrossRef]
- Saoudi, A.; Dereumeaux, C.; Goria, S.; Berat, B.; Brunel, S.; Pecheux, M.; de Crouy-Chanel, P.; Zeghnoun, A.; Rambaud, L.; Wagnerfr, V.; et al. Prenatal exposure to lead in France: Cord-blood levels and associated factors: Results from the perinatal component of the French Longitudinal Study since Childhood (Elfe). Int. J. Hyg. Environ. Health 2018, 221, 441–450. [Google Scholar] [CrossRef]
- ANSES. Étude de L’alimentation Totale Française 2 (EAT 2) Tome 1—Contaminants Inorganiques, Minéraux, Polluants Organiques Persistants, Mycotoxines et Phyto-Estrogènes. 2011. Available online: https://www.anses.fr/fr/system/files/PASER2006sa0361Ra1.pdf (accessed on 9 December 2020).
- Dereumeaux, C.; Saoudi, A.; Pecheux, M.; Berat, B.; de Crouy-Chanel, P.; Zaros, C.; Brunel, S.; Delamaire, C.; le Tertre, A.; Lefranc, A.; et al. Biomarkers of exposure to environmental contaminants in French pregnant women from the Elfe cohort in 2011. Environ. Int. 2016, 97, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Oleko, A.; Fillol, C.; Balicco, A.; Bidondo, M.L.; Gane, J.; Saoudi, A.; Zeghnoun, A. Imprégnation de la Population Française par le Plomb. Programme National de Biosurveillance, Esteban 2014–2016. Santé Publique France. 2020. Available online: https://www.santepubliquefrance.fr/docs/impregnation-de-la-population-francaise-par-le-plomb.-programme-national-de-biosurveillance-esteban-2014-2016 (accessed on 9 December 2020).
- Bierkens, J.; Smolders, R.; Van Holderbeke, M.; Cornelis, C. Predicting blood lead levels from current and past environmental data in Europe. Sci. Total Environ. 2011, 409, 5101–5110. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, A.; Lecoffre, C.; Le Tertre, A.; Le Strat, Y.; Saturn-Inf, G.I.; De Launay, C.; Bérat, B.; Bidondo, M.L.; Pascal, M.; Fréry, N.; et al. Imprégnation des enfants par le plomb en France en 2008–2009. BEHWeb 2010, 2, 1–8. [Google Scholar]
- EFSA. Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- The 2030 Agenda for Sustainable Development and the SDGs. Available online: https://ec.europa.eu/environment/sustainable-development/SDGs/index_en.htm (accessed on 9 December 2020).

| YES Number of Patients (n) Frequency (%) | NO Number of Patients (n) Frequency (%) | I Don’t Know Number of Patients (n) Frequency (%) | |
|---|---|---|---|
| Tap water consumption (Q9) a | 598 75.5 | 193 24.4 | 1 0.1 |
| Bread consumption > 2 times/day (Q14) | 406 51.3 | 384 48.5 | 2 0.3 |
| Birth in a high-risk country b (Q2) | 344 43.4 | 448 56.6 | 0 0 |
| Active or passive smoking (Q12) | 310 39.1 | 486 61.4 | 28 3.5 |
| Vegetables consumption > 2 times/day (Q15) | 305 38.5 | 470 59.3 | 17 2.1 |
| Living in an old and deteriorated dwelling (Q3) | 217 27.4 | 571 72.1 | 4 0.5 |
| Use of traditional containers (tagine dish), handmade ceramic, tin or crystal containers (Q8) | 177 22.3 | 609 76.9 | 6 0.8 |
| Exercise of a hobby or profession at risk c (Q18) | 155 19.6 | 634 80.1 | 3 0.4 |
| Consumption of shellfish and crustaceans (Q16) | 152 19.2 | 639 80.7 | 1 0.1 |
| Use of traditional cosmetics (kohl) (Q7) | 115 14.5 | 676 85.4 | 1 0.1 |
| Carrying out recent renovation work in an old home (Q4) | 105 13.3 | 686 86.6 | 1 0.1 |
| Consumption of traditional medicines or food supplements (Q5) | 53 6.7 | 736 92.9 | 3 0.4 |
| Living close to an industrial site (Q17) | 33 4.2 | 740 93.4 | 3 0.4 |
| Alcohol consumption (Q13) | 25 3.2 | 761 96.1 | 1 0.1 |
| Pica behaviour (Q6) | 23 2.9 | 766 96.7 | 3 0.4 |
| Personal or family history of lead poisoning (Q1) | 7 0.9 | 778 98.2 | 7 0.9 |
| Blood Lead Level | pa | ||
|---|---|---|---|
| <25 µg/L (n = 572) | ≥25 µg/L (n = 24) | ||
| n (%) | n (%) | ||
| History of lead poisoning | 5 (0.9) | 1 (4.2) | 0.153 |
| High-risk country of birth b | 250 (43.7) | 15 (62.5) | 0.076 |
| Degraded old housing | 156 (27.3) | 12 (50) | 0.019 |
| Renovation work in an old dwelling | 77 (13.5) | 5 (20.8) | 0.310 |
| Use of traditional medicines or food supplements | 37 (6.5) | 2 (8.3) | 0.718 |
| Pica behaviour | 16 (2.8) | 1 (4.2) | 0.695 |
| Use of traditional cosmetics | 81 (14.2) | 10 (41.7) | 0.001 |
| Use of traditional food containers (tagine dish), handmade ceramic, tin or crystal containers | 123 (21.5) | 5 (20.8) | 0.938 |
| Tap water consumption | 430 (75.2) | 18 (75) | 0.985 |
| Active or passive smoking | 230 (41.6) | 8 (34.8) | 0.530 |
| Alcohol consumption | 20 (3.5) | 1 (4.2) | 0.862 |
| Consumption of shellfish and crustaceans | 103 (18) | 5 (20.8) | 0.725 |
| Bread consumption >2 times/day | 282 (49.3) | 17 (70.8) | 0.045 |
| Vegetable consumption >2 times/day | 218 (38.1) | 9 (37.5) | 0.952 |
| Living close to an industrial site | 14 (2.4) | 2 (8.3) | 0.102 |
| Exercise of a hobby or profession at risk c | 99 (17.3) | 2 (8.3) | 0.264 |
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Crude Odds Ratio | 95% CI | Adjusted Odds Ratio a | 95% CI | p | |
| Living close to an industrial site | 3.62 | [0.78–16.92] | NE b | ||
| High risk country of birth c | 2.15 | [0.92–4.99] | NE | ||
| Consumption of bread more than twice per day | 2.50 | [1.02–6.11] | 2.40 | [0.96–5.99] | 0.060 |
| Use of traditional cosmetics | 4.30 | [1.86–10.08] | 3.90 | [1.65–9.2] | 0.002 |
| Degraded old housing | 2.67 | [1.17–6.06] | 2.76 | [1.19–6.38] | 0.018 |
| History of lead poisoning | 4.93 | [0.55–43.9] | NE | ||
| Renovation work in an old dwelling | 1.70 | [0.61–4.66] | NE | ||
| Use of traditional medicines or food supplements | 1.31 | [0.30–5.81] | NE | ||
| Pica behaviour | 1.51 | [0.19–11.88] | NE | ||
| Use of traditional food containers (tagine dish), handmade ceramic, tin or crystal containers | 0.96 | [0.35–2.62] | NE | ||
| Tap water consumption | 0.99 | [0.38–2.54] | NE | ||
| Active or passive smoking | 0.62 | [0.18–2.12] | NE | ||
| Alcohol consumption | 1.2 | [0.15–9.33] | NE | ||
| Consumption of selfish or crustaceans | 1.20 | [0.43–3.29] | NE | ||
| Vegetable consumption more than twice per day | 0.97 | [0.42–2.27] | NE | ||
| Exercise of a hobby or profession at risk d | 0.43 | [0.10–1.87] | NE |
| Blood Lead Level | pa | ||
|---|---|---|---|
| <25 µg/L (n = 572) | ≥25 µg/L (n = 24) | ||
| n (%) | n (%) | ||
| Age | |||
| >35 years old | 112 (19.6) | 7 (29.2) | 0.294 |
| Obstetrical history | |||
| Spontaneous miscarriage | 134 (26.4) | 5 (21.7) | 0.617 |
| Prematurity (<37 WG) | 24 (10.2) | 1 (4.3) | 0.451 |
| IUGR | 19 (3.8) | 1 (4.3) | 0.596 |
| Pregnancy hypertension | 22 (4.4) | 0 (0.0) | 1 |
| Pregnancy course | |||
| Anemia (Hb < 11 g/L) | 137 (32.6) | 7 (36.1) | 0.701 |
| IUGR | 28 (7.4) | 3 (15.8) | 0.177 |
| Pregnancy hypertension | 15 (4) | 0 (0) | 1 |
| Prematurity (<37 WG) | 37 (9.8) | 1 (5.6) | 1 |
| Low birth weight (<2500 g) | 48 (14) | 2 (14.3) | 1 |
| Blood Lead Level ≥25 µg/L | ||
|---|---|---|
| Score Q3 + Q7 + Q14 a | N | % |
| 0 (n = 179) | 2 | 1.1 |
| 1 (n = 293) | 8 | 2.7 |
| 2 (n = 107) | 11 | 10.3 |
| 3 (n = 17) | 3 | 17.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coiplet, E.; Freuchet, M.; Sunyach, C.; Mancini, J.; Perrin, J.; Courbiere, B.; Heckenroth, H.; Pissier, C.; Hamdaoui, N.; Bretelle, F. Assessment of a Screening Questionnaire to Identify Exposure to Lead in Pregnant Women. Int. J. Environ. Res. Public Health 2020, 17, 9220. https://doi.org/10.3390/ijerph17249220
Coiplet E, Freuchet M, Sunyach C, Mancini J, Perrin J, Courbiere B, Heckenroth H, Pissier C, Hamdaoui N, Bretelle F. Assessment of a Screening Questionnaire to Identify Exposure to Lead in Pregnant Women. International Journal of Environmental Research and Public Health. 2020; 17(24):9220. https://doi.org/10.3390/ijerph17249220
Chicago/Turabian StyleCoiplet, Eléna, Marine Freuchet, Claire Sunyach, Julien Mancini, Jeanne Perrin, Blandine Courbiere, Hélène Heckenroth, Christel Pissier, Naima Hamdaoui, and Florence Bretelle. 2020. "Assessment of a Screening Questionnaire to Identify Exposure to Lead in Pregnant Women" International Journal of Environmental Research and Public Health 17, no. 24: 9220. https://doi.org/10.3390/ijerph17249220
APA StyleCoiplet, E., Freuchet, M., Sunyach, C., Mancini, J., Perrin, J., Courbiere, B., Heckenroth, H., Pissier, C., Hamdaoui, N., & Bretelle, F. (2020). Assessment of a Screening Questionnaire to Identify Exposure to Lead in Pregnant Women. International Journal of Environmental Research and Public Health, 17(24), 9220. https://doi.org/10.3390/ijerph17249220





