The Incidence of Intestinal Gastric Cancer among Resettlers in Germany—Do Resettlers Remain at an Elevated Risk in Comparison to the General Population?
Abstract
1. Introduction
2. Methods
2.1. Münster Cohort
Statistical Analyses
2.2. Pooled Data from Münster and Saarland Cohorts
Statistical Analyses
3. Results
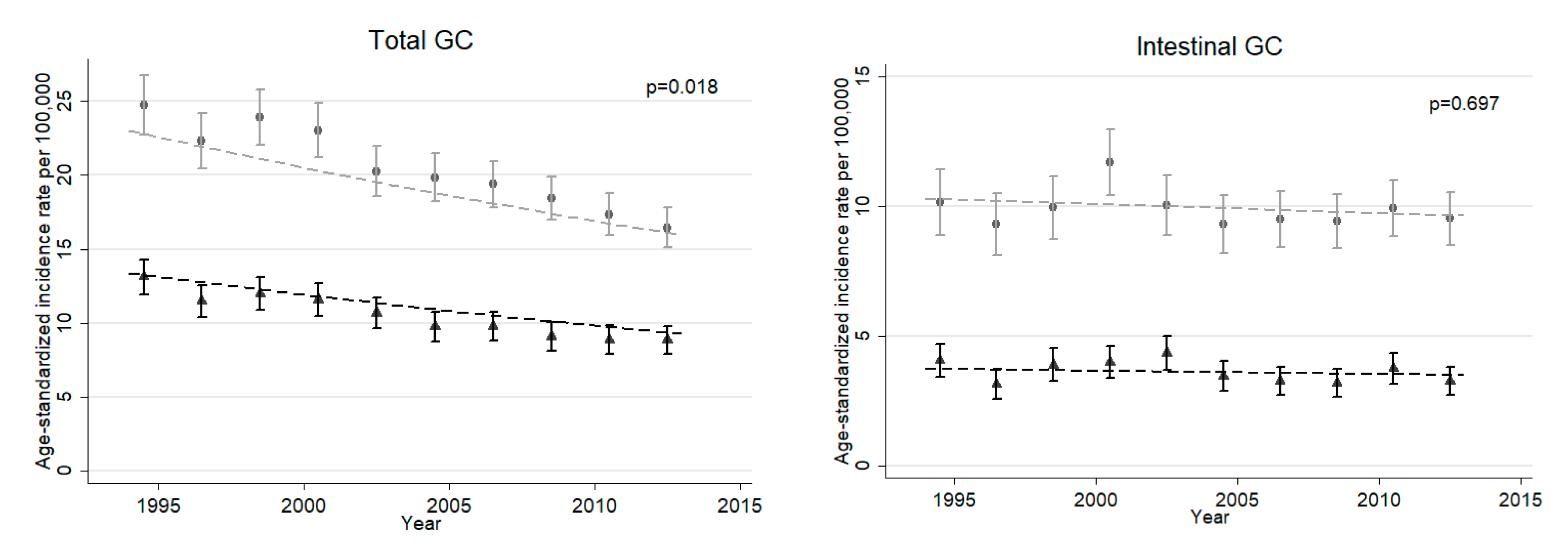
3.1. Descriptive Results
3.2. Münster Population
3.3. Resettlers in Münster
3.4. Pooled Cohorts Analyses
4. Discussion and Conclusions
4.1. Key Findings
4.2. Shortcomings and Limitations
4.3. Implications for Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Münster | 1994–2003 a | 2004–2013 b | ||
| Observed | SIR (95% CI) | Observed | SIR (95% CI) | |
| Total GC | ||||
| Male | 17 | 1.30 (0.76–2.09) | 34 | 1.63 (1.13–2.27) |
| Female | 16 | 1.48 (0.85–2.40) | 20 | 1.22 (0.75–1.88) |
| Intestinal GC | ||||
| Male | 8 | 1.33 (0.58–2.62) | 20 | 1.81 (1.10–2.79) |
| Female | 4 | 1.10 (0.30–2.81) | 15 | 2.37 (1.33–3.92) |
| Diffuse GC | ||||
| Male | 4 | 1.31 (0.36–3.36) | 10 | 1.77 (0.85–3.26) |
| Female | 3 | 0.84 (0.17–2.44) | 3 | 0.52 (0.11–1.51) |
| Other/Missing GC | ||||
| Male | 5 | 1.25 (0.41–2.00) | 4 | 0.95 (0.26–2.44) |
| Female | 9 | 2.51 (1.15–4.77) | 2 | 0.47 (0.06–1.68) |
| Saarland | 1990–1999 c | 2000–2009 d | ||
| Observed | SIR (95% CI) | Observed | SIR (95% CI) | |
| Total GC | ||||
| Male | 15 | 2.88 (1.61–4.75) | 21 | 2.08 (1.29–3.18) |
| Female | 11 | 2.62 (1.31–4.68) | 18 | 2.45 (1.45–3.87) |
| Intestinal GC | ||||
| Male | 10 | 3.90 (1.87–7.18) | 15 | 2.80 (1.57–4.62) |
| Female | 6 | 3.78 (1.39–8.22) | 7 | 2.50 (1.01–5.16) |
| Diffuse GC | ||||
| Male | 2 | 1.32 (0.16–4.77) | 1 | 0.30 (0.01–1.68) |
| Female | 1 | 0.65 (0.02–3.63) | 9 | 2.59 (1.19–4.92) |
| Other/Missing GC | ||||
| Male | 3 | 2.65 (0.55–7.73) | 5 | 3.55 (1.15–8.28) |
| Female | 4 | 3.70 (1.01–9.48) | 2 | 1.85 (0.22–6.67) |
References
- International Organization for Migration. Migration and Migrants: A Global Overview. Available online: https://www.un-ilibrary.org/content/component/b8cf9ec2-en (accessed on 5 June 2020).
- European Commission Eurostat. Push and Pull Factors of International Migration: A Comparative Report. Available online: https://op.europa.eu/en/publication-detail/-/publication/90913700-dbbb-40b8-8f85-bec0a6e29e83 (accessed on 5 May 2020).
- Rechel, B.; Mladovsky, P.; Ingleby, D.; Mackenbach, J.P.; McKee, M. Migration and health in an increasingly diverse Europe. Lancet 2013, 381, 1235–1245. [Google Scholar] [CrossRef]
- Arnold, M.; Razum, O.; Coebergh, J.-W. Cancer risk diversity in non-western migrants to Europe: An overview of the literature. Eur. J. Cancer 2010, 46, 2647–2659. [Google Scholar] [CrossRef] [PubMed]
- Spallek, J.; Zeeb, H.; Razum, O. What do we have to know from migrants’ past exposures to understand their health status? A life course approach. Emerg. Themes Epidemiol. 2011, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Federal Office for Migration and Refugees. Migration Report 2018: Key Results; Bundesamt für Migration und Flüchtlinge: Nürnberg, Germany, 2019.
- Kaucher, S.; Deckert, A.; Becher, H.; Winkler, V. Migration pattern and mortality of ethnic German migrants from the former Soviet Union: A cohort study in Germany. BMJ Open 2017, 7, e019213. [Google Scholar] [CrossRef] [PubMed]
- Bundeszentrale für politische Bildung. Zuzug Von (Spät-)Aussiedlern und Ihren Familienangehörigen. Available online: http://www.bpb.de/nachschlagen/zahlen-und-fakten/soziale-situation-in-deutschland/61643/aussiedler (accessed on 5 June 2020).
- Bundesverwaltungsamt. Spätaussiedler und ihre Angehörigen, Zeitreihe 1950–2019. Available online: https://www.bva.bund.de/SharedDocs/Downloads/DE/Buerger/Migration-Integration/Spaetaussiedler/Statistik/Zeitreihe_1950_2019.html (accessed on 8 December 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Robert-Koch-Institut-Zentrum für Krebsregisterdaten. Available online: www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Magenkrebs/magenkrebs_node.html (accessed on 3 July 2020).
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Ladeiras-Lopes, R.; Pereira, A.K.; Nogueira, A.; Pinheiro-Torres, T.; Pinto, I.; Santos-Pereira, R.; Lunet, N. Smoking and gastric cancer: Systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008, 19, 689–701. [Google Scholar] [CrossRef]
- Gonzalez, C.A.; Jakszyn, P.; Pera, G.; Agudo, A.; Bingham, S.; Palli, D.; Ferrari, P.; Boeing, H.H.; del Giudice, G.; Plebani, M.; et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2006, 98, 345–8874. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Terry, P.-D.; Yan, H. Review of salt consumption and stomach cancer risk: Epidemiological and biological evidence. World J. Gastroenterol. 2009, 15, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Franceschi, S. Epidemiology of gastric cancer and perspectives for prevention. Salud Publica de Mex. 1997, 39, 318. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Vauhkonen, M.; Vauhkonen, H.; Sipponen, P. Pathology and molecular biology of gastric cancer. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Lunet, N.; Valbuena, C.; Vieira, A.L.; Lopes, C.; David, L.; Carneiro, F.; Barros, H. Fruit and vegetable consumption and gastric cancer by location and histological type: Case-control and meta-analysis. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2007, 16, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.J.; Travier, N.; Lujan-Barroso, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Morois, S.; Palli, D.; Krogh, V.; Panico, S.; Tumino, R.; et al. Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am. J. Clin. Nutr. 2011, 94, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Manish, A.S.; David, P.K. Gastric Cancer: A Primer on the Epidemiology and Biology of the Disease and an Overview of the Medical Management of Advanced Disease. J. Natl. Compr. Cancer Netw. 2010, 8, 437–447. [Google Scholar] [CrossRef]
- Jaehn, P.; Holleczek, B.; Becher, H.; Winkler, V. Histologic types of gastric cancer among migrants from the former Soviet Union and the general population in Germany: What kind of prevention do we need? Eur. J. Gastroenterol. Hepatol. 2016, 28, 863–870. [Google Scholar] [CrossRef]
- Kaucher, S.; Kajüter, H.; Becher, H.; Winkler, V. Cancer Incidence and Mortality Among Ethnic German Migrants From the Former Soviet Union. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Winkler, V.; Holleczek, B.; Stegmaier, C.; Becher, H. Cancer incidence in ethnic German migrants from the Former Soviet Union in comparison to the host population. Cancer Epidemiol. 2014, 38, 22–27. [Google Scholar] [CrossRef]
- Kaucher, S.; Leier, V.; Deckert, A.; Holleczek, B.; Meisinger, C.; Winkler, V.; Becher, H. Time trends of cause-specific mortality among resettlers in Germany, 1990 through 2009. Eur. J. Epidemiol. 2017, 32, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Winkler, V.; Kaucher, S.; Deckert, A.; Leier, V.; Holleczek, B.; Meisinger, C.; Razum, O.; Becher, H. Aussiedler Mortality (AMOR): Cohort studies on ethnic German migrants from the Former Soviet Union. BMJ Open 2019, 9, e024865. [Google Scholar] [CrossRef]
- Becher, H.; Winkler, V. Estimating the standardized incidence ratio (SIR) with incomplete follow-up data. BMC Med. Res. Methodol. 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Kajüter, H.; Batzler, W.U.; Krieg, V.; Heidinger, O.; Hense, H.W. Abgleich von Sekundärdaten mit einem epidemiologischen Krebsregister auf der Basis verschlüsselter Personendaten-Ergebnisse einer Pilotstudie in Nordrhein-Westfalen. Gesundheitswesen 2012, 74, e84–e89. [Google Scholar] [CrossRef]
- National Institutes of Health. N.C.I. ICD-O-2 to ICD-O-3 Neoplasms. Available online: https://seer.cancer.gov/tools/conversion/ICDO2-3manual.pdf (accessed on 20 May 2020).
- European Commission Eurostat. Revision of the European Standard Population. Report of Eurostat’s Task Force. 2013. Edition. Available online: https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF/e713fa79-1add-44e8-b23d-5e8fa09b3f8f (accessed on 2 July 2020).
- John, E.M.; Phipps, A.I.; Davis, A.; Koo, J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2905. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 10 June 2020).
- Correa, P. Human gastric carcinogenesis-a multistep and multifactorial process-1st american-cancer-society award lecture on cancer-epidemiology and prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Bornschein, J.; Selgrad, M.; Warnecke, M.; Kuester, D.; Wex, T.; Malfertheiner, P.H. Pylori Infection Is a Key Risk Factor for Proximal Gastric Cancer. Dig. Dis. Sci. 2010, 55, 3124–3131. [Google Scholar] [CrossRef]
- Sipponen, P.; Riihelä, M.; Hyvärinen, H.; Seppälä, K. Chronic Nonatrophic (‘Superficial’) Gastritis Increases the Risk of Gastric Carcinoma: A Case-Control Study. Scand. J. Gastroenterol. 1994, 29, 336–340. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Sridhar, S.; Chen, Y.; Hunt, R.H. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998, 114, 1169–1179. [Google Scholar] [CrossRef]
- Aparicio, M.L.; Döring, A.; Mielck, A.; Holle, R.; KORA Studiengruppe. Unterschiede zwischen Aussiedlern und der übrigen deutschen Bevölkerung bezüglich Gesundheit, Gesundheitsversorgung und Gesundheitsverhalten: Eine vergleichende Analyse anhand des KORA-Surveys 2000. Sozial-und Präventivmedizin/Soc. Prevent. Med. A 2005, 50, 107–118. [Google Scholar] [CrossRef]
- Kuhrs, E.; Winkler, V.; Becher, H. Risk factors for cardiovascular and cerebrovascular diseases among ethnic Germans from the former Soviet Union: Results of a nested case-control study. BMC Public Health 2012, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Ronellenfitsch, U.; Razum, O. Deteriorating health satisfaction among immigrants from Eastern Europe to Germany. Int. J. Equity Health 2004, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.E.; Ostrove, J.M. Socioeconomic Status and Health: What We Know and What We Don’t. Ann. N. Y. Acad. Sci. 1999, 896, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Berlth, F.; Bollschweiler, E.; Drebber, U.; Hoelscher, A.H.; Moenig, S. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World J. Gastroenterol. 2014, 20, 5679–5684. [Google Scholar] [CrossRef]
- Henson, D.E.; Dittus, C.; Younes, M.; Nguyen, H.; Albores-Saavedra, J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: Increase in the signet ring cell type. Arch. Pathol. Lab. Med. 2004, 128, 765. [Google Scholar]
- Becher, H.; Kyobutungi, C.; Laki, J.; Ott, J.J.; Razum, O.; Ronellenfitsch, U.; Winkler, V. Mortality of immigrants from the former Soviet Union: Results of a cohort study. Dtsch. Arztebl. Int. 2007, 104, 1655–1661. [Google Scholar]
- Bund, E.; Kohls, M.; Worbs, S. Zuwanderung und Integration von (Spät-)Aussiedlern in Deutschland. Z. Ausländerrecht und Ausländerpolitik (ZAR) 2014, 10, 349–354. [Google Scholar]

| Laurén Classification | ICD-O-3 Codes | N c |
|---|---|---|
| Intestinal GC (44%) | 8140/3 Adenocarcinoma, not otherwise specified | 2486 |
| 8144/3 Adenocarcinoma, intestinal type | 1774 | |
| 8211/3 Tubular adenocarcinoma | 283 | |
| 8260/3 Papillary adenocarcinoma, not otherwise specified | 66 | |
| 8480/3 Mucinous adenocarcinoma | 169 | |
| Diffuse GC (26%) | 8490/3 Signet ring cell carcinoma | 1540 |
| 8142/3 Linitis plastica | 21 | |
| 8145/3 Carcinoma, diffuse type | 1321 | |
| Other/Missing GC (30%) | Sections 802–857: Other carcinoma | 697 |
| Sections 804 and 824: Endocrine carcinoma | 182 | |
| Sections 880–914: Sarcoma | 159 | |
| Sections 917–971: Lymphoma | 1 | |
| Section 800: Neoplasm | 498 | |
| Section 801: Carcinoma, not otherwise specified | 1676 | |
| Total | 10,873 |
| Münster (1994–2013) | Pooled Data (1990–2013) | |||||||
|---|---|---|---|---|---|---|---|---|
| Male [N (%)] | Female [N (%)] | Male [N (%)] | Female [N (%)] | |||||
| Resettlers | Münster | Resettlers | Münster | Resettlers | Population | Resettlers | Population | |
| Incident gastric cancer cases | 51 | 6161 | 36 | 4712 | 87 | 8814 | 65 | 6914 |
| Median age at diagnosis (Interquartile range) | 66 (52–75) | 70 (61–78) | 72.5 (57–78) | 76 (65–83) | 68 (58–75) | 70 (61–77) | 71 (51–77) | 76 (66–83) |
| Laurén classification | ||||||||
| Intestinal GC | 28 (55) | 3033 (49) | 19 (53) | 1745 (37) | 53 (61) | 4400 (50) | 32 (49) | 2634 (38) |
| Diffuse GC | 14 (27) | 1442 (23) | 6 (17) | 1440 (31) | 17 (19) | 2204 (25) | 16 (25) | 2198 (32) |
| Other/missing GC | 9 (18) | 1686 (28) | 11 (30) | 1527 (32) | 17 (20) | 2210 (25) | 17 (26) | 2082 (30) |
| Male | Females | Both Sexes | ||||
|---|---|---|---|---|---|---|
| Observed | SIR (95% CI) | Observed | SIR (95% CI) | Observed | SIR (95% CI) | |
| Total GC | 51 | 1.50 (1.12–1.98) | 36 | 1.32 (0.93–1.83) | 87 | 1.42 (1.14–1.76) |
| Intestinal GC | 28 | 1.64 (1.09–2.37) | 19 | 1.91 (1.15–2.98) | 47 | 1.73 (1.28–2.31) |
| Diffuse GC | 14 | 1.61 (0.88–2.70) | 6 | 0.64 (0.23–1.39) | 20 | 1.11 (0.68–1.71) |
| Other/Missing GC | 9 | 1.10 (0.50–2.09) | 11 | 1.40 (0.70–2.50) | 20 | 1.25 (0.76–1.92) |
| 1990–2001 a | 2002–2013 b | Total | ||||
|---|---|---|---|---|---|---|
| Observed | SIR (95% CI) | Observed | SIR (95% CI) | Observed | SIR (95% CI) | |
| Total GC | ||||||
| Male | 31 | 1.87 (1.27–2.65) | 56 | 1.72 (1.30–2.23) | 87 | 1.77 (1.42–2.18) |
| Female | 28 | 2.09 (1.39–3.03) | 37 | 1.46 (1.03–2.01) | 65 | 1.68 (1.29–2.14) |
| Total | 59 | 1.97 (1.50–2.54) | 93 | 1.60 (1.29–1.96) | 152 | 1.73 (1.46–2.03) |
| Intestinal GC | ||||||
| Male | 19 | 2.45 (1.47–3.82) | 34 | 1.98 (1.37–2.76) | 53 | 2.12 (1.59–2.77) |
| Female | 10 | 2.18 (1.05–4.01) | 22 | 2.25 (1.41–3.41) | 32 | 2.23 (1.53–3.15) |
| Total | 29 | 2.35 (1.57–3.37) | 56 | 2.08 (1.57–2.70) | 85 | 2.16 (1.73–2.67) |
| Diffuse GC | ||||||
| Male | 5 | 1.15 (0.37–2.69) | 12 | 1.31 (0.68–2.28) | 17 | 1.26 (0.73–2.01) |
| Female | 5 | 1.07 (0.35–2.49) | 11 | 1.13 (0.57–2.03) | 16 | 1.11 (0.64–1.81) |
| Total | 10 | 1.11 (0.53–2.04) | 23 | 1.22 (0.77–1.83) | 33 | 1.18 (0.81–1.66) |
| Other/Missing GC | ||||||
| Male | 7 | 1.55 (0.62–3.20) | 10 | 1.61 (0.77–2.96) | 17 | 1.59 (0.92–2.54) |
| Female | 13 | 3.17 (1.69–5.41) | 4 | 0.67 (0.18–1.73) | 17 | 1.69 (0.99–2.71) |
| Total | 20 | 2.32 (1.42–3.59) | 14 | 1.15 (0.63–1.93) | 34 | 1.64 (1.13–2.29) |
| Variable | Coefficient a | 95% CI | p-Value |
|---|---|---|---|
| Year (calendar year–1990) | 0.002 | −0.030, 0.035 | 0.889 |
| Subtype | 0.013 | ||
| Intestinal GC | Ref. | ||
| Diffuse GC | −0.639 | −1.062, −0.215 | |
| Other/Missing GC | −0.224 | −0.648, 0.200 | |
| Sex | 0.886 | ||
| Male | Ref. | ||
| Female | 0.025 | −0.318, 0.368 | |
| Cohort | 0.003 | ||
| Münster | Ref. | ||
| Saarland | 0.558 | 0.195, 0.921 | |
| Constant | 0.535 | −0.053, 1.123 | 0.075 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindblad, A.; Kaucher, S.; Jaehn, P.; Kajüter, H.; Holleczek, B.; Lissner, L.; Becher, H.; Winkler, V. The Incidence of Intestinal Gastric Cancer among Resettlers in Germany—Do Resettlers Remain at an Elevated Risk in Comparison to the General Population? Int. J. Environ. Res. Public Health 2020, 17, 9215. https://doi.org/10.3390/ijerph17249215
Lindblad A, Kaucher S, Jaehn P, Kajüter H, Holleczek B, Lissner L, Becher H, Winkler V. The Incidence of Intestinal Gastric Cancer among Resettlers in Germany—Do Resettlers Remain at an Elevated Risk in Comparison to the General Population? International Journal of Environmental Research and Public Health. 2020; 17(24):9215. https://doi.org/10.3390/ijerph17249215
Chicago/Turabian StyleLindblad, Anna, Simone Kaucher, Philipp Jaehn, Hiltraud Kajüter, Bernd Holleczek, Lauren Lissner, Heiko Becher, and Volker Winkler. 2020. "The Incidence of Intestinal Gastric Cancer among Resettlers in Germany—Do Resettlers Remain at an Elevated Risk in Comparison to the General Population?" International Journal of Environmental Research and Public Health 17, no. 24: 9215. https://doi.org/10.3390/ijerph17249215
APA StyleLindblad, A., Kaucher, S., Jaehn, P., Kajüter, H., Holleczek, B., Lissner, L., Becher, H., & Winkler, V. (2020). The Incidence of Intestinal Gastric Cancer among Resettlers in Germany—Do Resettlers Remain at an Elevated Risk in Comparison to the General Population? International Journal of Environmental Research and Public Health, 17(24), 9215. https://doi.org/10.3390/ijerph17249215







