Arthroderma tuberculatum and Arthroderma multifidum Isolated from Soils in Rook (Corvus frugilegus) Colonies as Producers of Keratinolytic Enzymes and Mineral Forms of N and S
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Keratinolytic Fungi
2.2. Identification of Fungal Species
2.3. Determination of Keratinolytic Activity
2.4. Determination of Organic and Mineral Bioproducts of Waste Feather Biodegradation
2.4.1. Mineral Forms of N and S in Post-Culture Fluids
2.4.2. The Amino Acid Composition in Post-Culture Fluids
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification of Fungi
3.2. Degree of Keratin Substrate Utilization
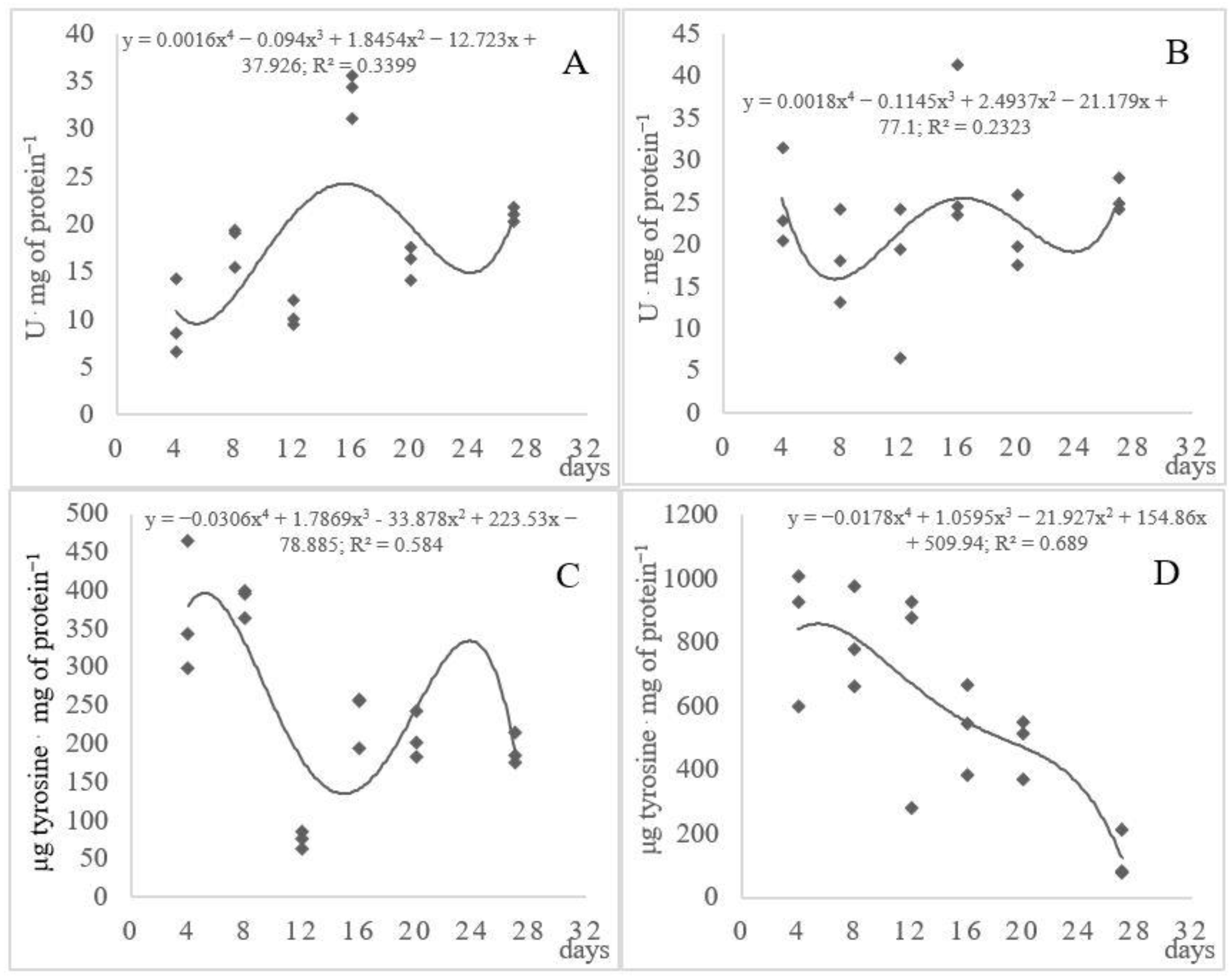
3.3. Proteolytic and Keratinolytic Activity
3.4. Soluble Proteins and Peptides
3.5. Ammonium and Sulfate Ions
3.6. Changes in pH
3.7. Amino Acid Composition
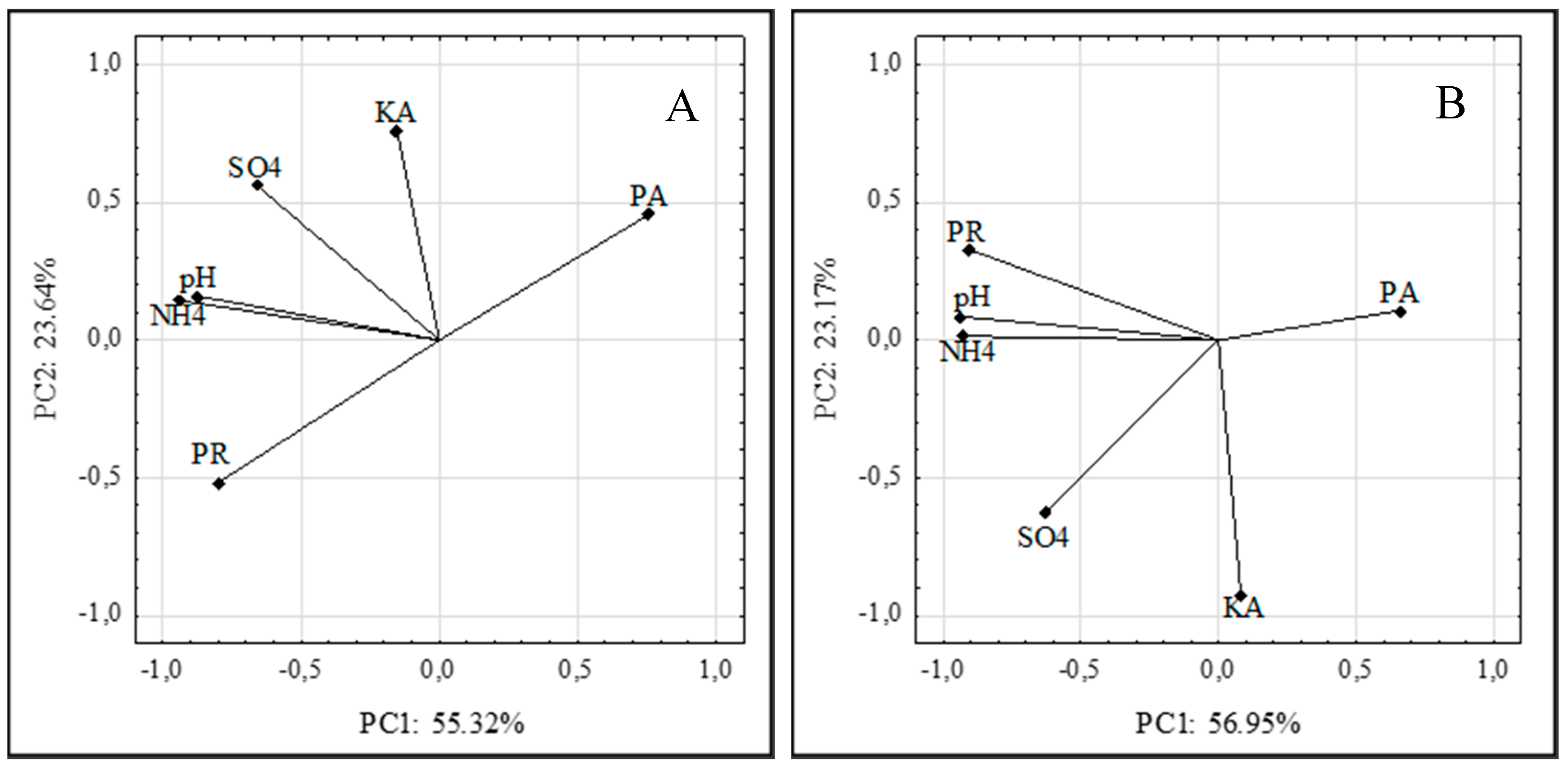
3.8. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mokrejs, P.; Svoboda, P.; Hrncirik, J.; Janacova, D.; Vasek, V. Processing poultry feathers into keratin hydrolysate through alkaline-enzymatic hydrolysis. Waste Manag. Res. 2010, 29, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Staroń, P.; Banach, M.; Kowalski, Z. Keratin–origins, properties, application. Chemik 2011, 65, 1019–1026. [Google Scholar]
- Verma, A.; Singh, H.; Anwar, S.; Chattopadhyay, A.; Tiwari, K.K.; Kaur, S.; Singh Dhilon, G. Microbial keratinases: Industrial enzymes with waste management potential. Crit. Rev. Biotechnol. 2017, 37, 476–491. [Google Scholar] [CrossRef]
- Bohacz, J. Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus Chrysosporium and statistical optimization of feather mass loss. World J. Microb. Biot. 2017, 33, 1–16. [Google Scholar] [CrossRef]
- Forgács, G.; Alinezhad, S.; Mirabdollah, A.; Feuk-Lagerstedt, E.; Horváth, I.S. Biological treatment of chicken feather waste for improved biogas production. J. Environ. Sci. China 2011, 23, 1747–1753. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A. Sustainable Management of Keratin Waste Biomass: Applications and Future Perspectives. Braz. Arch. Biol. Technol. 2016, 59, 1–14. [Google Scholar] [CrossRef]
- Brandelli, A.; Sala, L.; Kalil, S.J. Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res. Int. 2015, 73, 3–12. [Google Scholar] [CrossRef]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef]
- Mazotto, A.M.; Coelho, R.R.R.; Cedrola, S.M.L.; de Lima, M.F.; Couri, S.; de Souza, E.P.; Vermelho, A.B. Keratinase Production by Three Bacillus spp. Using Feather Meal and Whole Feather as Substrate in a Submerged Fermentation. Enzym. Res. 2011, 2011, 523780. [Google Scholar] [CrossRef]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Fungal diversity and keratinolytic activity of fungi from lignocellulosic composts with chicken feathers. Process. Biochem. 2019, 80, 119–128. [Google Scholar] [CrossRef]
- Cascarosa, E.; Gea, G.; Arauzo, J. Thermochemical processing of meat and bone meal: A review. Renew. Sustain Energy Rev. 2012, 16, 942–957. [Google Scholar] [CrossRef]
- Tamreihao, K.; Mukherjee, S.; Khunjaayum, R.; Devi, L.J.; Asem, R.S. Feather degradation by keratinophilic bacteria and biofertilizing potentail for sustainalble agricultural production. J. Basic Microbiol. 2019, 59, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, H.M.; Moubasher, A.H.; Maghazy, S.M. Keratinolytic Fungi in Egyptian Soils. Microbiol. Immunol. 1982, 26, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Some correlations between the occurrence frequency of keratinophilic fungi and selected soil properties. Acta Mycol. 2002, 37, 101–116. [Google Scholar] [CrossRef]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Species diversity of keratinophilic fungi in various soil types. Cent. Eur. J. Biol. 2012, 7, 259–266. [Google Scholar] [CrossRef]
- Ulfig, K.; Terakowski, M.; Płaza, G.; Kosarewicz, O. Keratinolytic fungi in sewage sludge. Mycopathologia 1996, 136, 41–46. [Google Scholar] [CrossRef]
- Ulfig, K.; Guarro, J.; Cano, J.; Gené, J.; Vidal, P.; Figueras, M.J.; Łukasik, W. The occurrence of keratinolytic fungi in sediments of the river Tordera (Spain). FEMS Microbiol. Ecol. 1997, 22, 111–117. [Google Scholar] [CrossRef][Green Version]
- Moorthy, K.; Prasanna, I.; Vimalan, S.; Lavanya, V.; Thamarai Selvi, A.; Mekala, T.; Thajuddin, N. Study on Keratinophilic and Keratinolytic Fungi Isolated from Birds’ Feathers and Animal Hairs. Biosci. Biotechnol. Res. Asia 2011, 8, 633–640. [Google Scholar] [CrossRef]
- Hubálek, Z. Keratinophilic fungi associated with free-living mammals and birds. In Biology of Dermatophytes and Other Keratinophilic Fungi, 1st ed.; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; pp. 86–92. [Google Scholar]
- Korniłłowicz-Kowalska, T.; Kitowski, I. Nests of Marsh harrier (Circus aeruginosus L.) as refuges of potentially phytopathogenic and zoopathogenic fungi. Saudi J. Biol. Sci. 2018, 25, 136–143. [Google Scholar] [CrossRef]
- Ciesielska, A.; Korniłłowicz-Kowalska, T.; Kitowski, I.; Bohacz, J. The dispersal of rodent-borne strains of Aphanoascus keratinophilus and Chrysosporium tropicum by pellets of predatory birds. Avian Biol. Res. 2017, 10, 218–230. [Google Scholar] [CrossRef]
- Błyskal, B. Fungi utilizing keratinous substrates. Int. Biodeterior. Biodegrad. 2009, 63, 631–653. [Google Scholar] [CrossRef]
- Kunert, J. Physiology of keratinophilic fungi. In Biology of Dermatophytes and Other Keratinophilic Fungi, 1st ed.; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; pp. 77–85. [Google Scholar]
- Mitola, G.; Escalona, F.; Salas, R.; Garcia, E.; Ledesma, A. Morphological characterization of in-vitro human hair keratinolysis, produced by identified wild strains of Chrysosporium species. Mycopathologia 2002, 156, 163–169. [Google Scholar] [CrossRef]
- Călin, M.; Constantinescu-Aruxandei, D.; Alexandrescu, E.; Răut, I.; Badea Doni, M.; Arsene, M.L.; Oancea, F.; Jecu, L.; Lazărb, V. Degradation of keratin substrates by keratinolytic fungi. Electron. J. Biotechnol. 2017, 28, 101–112. [Google Scholar] [CrossRef]
- Sutoyo, S.; Subandi; Ardyati, T.; Suharjono. Screening of Keratinolytic Fungi for Biodegradation Agent of Keratin from Chicken Feather Waste. Annu. Conf. Environ. Sci. Soc. Appl. 2019, 391, 1–8. [Google Scholar] [CrossRef]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Modification of post-industrial lignin by fungal strains of the genus Trichoderma isolated from different composting stages. J. Environ. Manag. 2020, 266, 110573. [Google Scholar] [CrossRef] [PubMed]
- Hordowski, J. Rook Corvus frugilegus in Podkarpacie. A Monograph of the Species and Economic Importance; Arboretum and Physiography Department: Bolestraszyce, Poland, 2009. (In Polish) [Google Scholar]
- Ligęza, S. Ornithogenic diagnostic material. Are there ornithic soils in Poland. Soil Sci. Ann. 2010, 61, 60–66. (In Polish) [Google Scholar]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Dynamics of growth and succession of bacterial and fungal communities during composting of feather waste. Biores. Technol. 2010, 101, 1268–1276. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag Eching: München, Germany, 2007. [Google Scholar]
- Van Oorschot, C.A.N. A revision of Chrysosporium and allied genera. Stud. Mycol. 1980, 20, 1–89. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Anson, M.L. The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Korniłłowicz, T. Methods for determining keratinolytic activity of saprophyticfungi. Acta Mycol. 1994, 29, 169–178. [Google Scholar] [CrossRef]
- Anbu, P.; Hilda, A.; Sur, H.W.; Hur, B.K.; Jayanthi, S. Extracellular keratinase from Trichophyton sp. HA-2 isolated from feather dumping soil. Int. Biodeterior. Biodegrad. 2008, 62, 287–292. [Google Scholar] [CrossRef]
- Lowry, O.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951, 93, 265–275. [Google Scholar]
- Li, Z.W.; Liang, S.; Ke, Y.; Deng, J.J.; Zhang, M.S.; Lu, D.L.; Li, J.Z.; Luo, X.C. The feather degradation mechanisms of a new Streptomyces sp. isolate SCUT-3. Commun. Biol. 2020, 3, 1–13. [Google Scholar] [CrossRef]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Effective biodegradation of chicken feather waste by co-cultivation of keratinase producing strains. Microb. Cell Factories 2019, 18, 84. [Google Scholar] [CrossRef]
- Jin, H.-S.; Park, S.-Y.; Kim, K.; Lee, Y.-J.; Nam, G.-W.; Kang, N.-J.; Lee, D.-W. Development of a keratinase activity assay using recombinanat chicken feather keratin substrates. PLoS ONE 2017, 12, e0172712. [Google Scholar] [CrossRef]
- Anitha, T.S.; Palanivelu, P. Purification and characterization of an extracellular keratinolytic protease from a new isolate of Aspergillus parasiticus. Protein Expr. Purif. 2013, 88, 214–220. [Google Scholar] [CrossRef]
- Singh, I.; Kumar, R.; Kushwaha, R.K.S.; Parihar, P. Keratinophilic fungi in soil of potted plants of indoor environments in Kanpur, India, and their proteolytic ability. Mycoscience 2009, 50, 303–307. [Google Scholar] [CrossRef]
- Cavello, I.A.; Crespo, J.M.; García, S.S.; Zapiola, J.M.; Luna, M.F.; Cavalitto, S.F. Plant Growth Promotion Activity of Keratinolytic Fungi Growing on a Recalcitrant Waste Known as “Hair Waste”. Biotechnol. Res. Int. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Godheja, J.; Shekhar, S.K. Biodegradation of Keratin from Chicken Feathers by Fungal Species as a Means of Sustainable Development. J. Bioremediat. Biodegrad. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Gradišar, H.; Friedrich, J.; Križaj, I.; Jerala, R. Similarities and Specificities of Fungal Keratinolytic Proteases: Comparison of Keratinases of Paecilomyces marquandii and Doratomyces microsporus to Some Known Proteases. Appl. Environ. Microbiol. 2005, 71, 3420–3426. [Google Scholar] [CrossRef] [PubMed]
- Näsholm, T.; Killand, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Cardoso, M.; Godoy, A.C.; Oxford, J.H.; dos Santos Cardoso, R.M.; Bittencourt, F.; Signor, A.; Boscolo, W.R.; Feiden, A. Apparent digestibility of protein hydrolysates from chicken and swine slaughter residues for Nile tilapia. Aquaculture 2020, 735720. [Google Scholar] [CrossRef]
- Kumari, M.; Kumar, J. Chicken feather waste degradation by Alternaria tenuissima and its application on plant growth. J. Appl. Nat. Sci. 2020, 12, 411–414. [Google Scholar] [CrossRef]


| No. | Strains | Similarity (%) | Highest Sequence Similarity (%) |
|---|---|---|---|
| III | Arthroderma tuberculatum | 99 | 100 |
| IV | Arthroderma tuberculatum | 99 | 100 |
| V | Arthroderma tuberculatum | 99 | 100 |
| VIII | Arthroderma multifdum | 98 | 100 |
| XVIII | Arthroderma multifdum | 98 | 100 |
| XIX | Arthroderma multifdum | 98 | 100 |
| Strains | Loss of Waste Feather Weight (%) |
|---|---|
| III | 39.83 b ± 0.025 |
| IV | 39.30 b ± 0.007 |
| V | 43.00 b ± 0.036 |
| VIII | 30.03 a ± 0.077 |
| XVIII | 24.00 a ± 0.006 |
| XIX | 27.00 a ± 0.033 |
| Strains | Protease | Keratinase | Soluble Proteins and Peptides | pH | N-NH4+ | S-SO42− |
|---|---|---|---|---|---|---|
| (μg tyrosine mg−1 of protein) | (U mg−1 of protein) | (mg proteins mL−1) | (μg NH4+ mL−1) | (mg SO42− mL−1) | ||
| III | 254.34 a ± 105.963 | 18.84 a ± 8.248 | 238.21 ab ± 60.620 | 8.55 a ± 0.197 | 461.13 b ± 160.125 | 0.71 abc ± 0.236 |
| IV | 236.97 a ± 100.289 | 16.65 a ± 7.972 | 245.46 ab ± 68.769 | 8.47 a ± 0.343 | 413.23 ab ± 135.437 | 0.75 bc ± 0.286 |
| V | 240.39 a ± 134.957 | 19.05 a ± 8.558 | 259.06 b ± 70.818 | 8.51 a ± 0.217 | 431.15 ab ± 105.577 | 0.86 c ± 0.314 |
| VIII | 470.18 ab ± 263.830 | 23.24 a ± 10.314 | 173.48 a ± 50.876 | 8.09 a ± 0.659 | 285.07 a ± 120.855 | 0.60 abc ± 0.324 |
| XVIII | 620.53 b ± 288.580 | 23.60 a ± 6.228 | 187.02 ab ± 40.337 | 8.36 a ± 0.535 | 321.41 ab ± 97.693 | 0.43 a ± 0.183 |
| XIX | 647.57 b ± 306.959 | 21.47 a ± 3.332 | 189.64 ab ± 49.001 | 8.35 a ± 0.546 | 301.29 a ± 96.615 | 0.46 ab ± 0.162 |
| Strains | Days of Culturing | |||||
|---|---|---|---|---|---|---|
| 4 | 8 | 12 | 16 | 20 | 27 | |
| III | 8.21 ± 0.04 | 8.69 ± 0.08 | 8.79 ± 0.04 | 8.47 ± 0.03 | 8.50 ± 0.07 | 8.63 ± 0.06 |
| IV | 7.76 ± 0.17 | 8.64 ± 0.05 | 8.77 ± 0.03 | 8.51 ± 0.03 | 8.48 ± 0.06 | 8.66 ± 0.04 |
| V | 8.12 ± 0.01 | 8.73 ± 0.04 | 8.76 ± 0.03 | 8.464 ± 0.01 | 8.41 ± 0.03 | 8.58 ± 0.02 |
| VIII | 6.67 ± 0.19 | 8.06 ± 0.13 | 8.47 ± 0.01 | 8.38 ± 0.03 | 8.59 ± 0.02 | 8.56 ± 0.01 |
| XVIII | 7.20 ± 0.01 | 8.35 ± 0.01 | 8.64 ± 0.00 | 8.72 ± 0.01 | 8.68 ± 0.02 | 8.58 ± 0.02 |
| XIX | 7.17 ± 0.03 | 8.26 ± 0.01 | 8.63 ± 0.02 | 8.72 ± 0.01 | 8.71 ± 0.01 | 8.58 ± 0.00 |
| Asp | Thr | Ser | Glu | Pro | Gly | Ala | Cyst Acid | Sulf Met |
| 0.0456 | 0.0113 | 0.0356 | 0.0410 | 0.0027 | 0.0192 | 0.0065 | 0.0648 | 0.0154 |
| Val | Ile | Leu | Tyr | Phe | His | Lys | Arg | Trp |
| 0.0166 | 0.0024 | 0.0074 | 0.0046 | 0.0057 | 0.0156 | 0.0139 | 0.0080 | 0.0764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohacz, J.; Możejko, M.; Kitowski, I. Arthroderma tuberculatum and Arthroderma multifidum Isolated from Soils in Rook (Corvus frugilegus) Colonies as Producers of Keratinolytic Enzymes and Mineral Forms of N and S. Int. J. Environ. Res. Public Health 2020, 17, 9162. https://doi.org/10.3390/ijerph17249162
Bohacz J, Możejko M, Kitowski I. Arthroderma tuberculatum and Arthroderma multifidum Isolated from Soils in Rook (Corvus frugilegus) Colonies as Producers of Keratinolytic Enzymes and Mineral Forms of N and S. International Journal of Environmental Research and Public Health. 2020; 17(24):9162. https://doi.org/10.3390/ijerph17249162
Chicago/Turabian StyleBohacz, Justyna, Michał Możejko, and Ignacy Kitowski. 2020. "Arthroderma tuberculatum and Arthroderma multifidum Isolated from Soils in Rook (Corvus frugilegus) Colonies as Producers of Keratinolytic Enzymes and Mineral Forms of N and S" International Journal of Environmental Research and Public Health 17, no. 24: 9162. https://doi.org/10.3390/ijerph17249162
APA StyleBohacz, J., Możejko, M., & Kitowski, I. (2020). Arthroderma tuberculatum and Arthroderma multifidum Isolated from Soils in Rook (Corvus frugilegus) Colonies as Producers of Keratinolytic Enzymes and Mineral Forms of N and S. International Journal of Environmental Research and Public Health, 17(24), 9162. https://doi.org/10.3390/ijerph17249162







