Comparison of Oral Health Impact Profile (OHIP-14) Values in Cancer Survivor Patients Treated Orthodontically with Either Rapid or Standard Duration Protocols of Treatment—A Prospective Case–Control Study
Abstract
1. Introduction
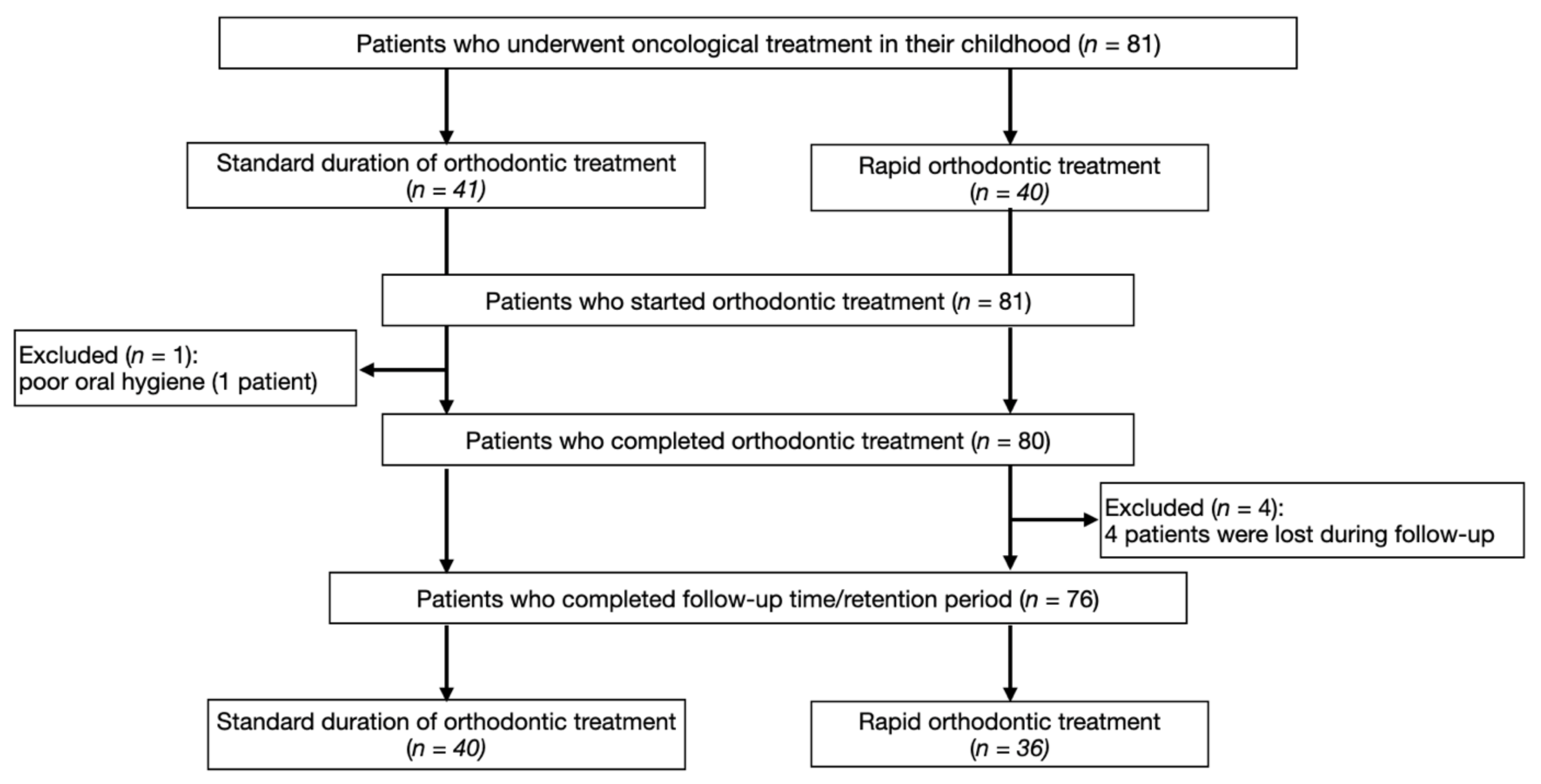
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Statistical Analysis
3. Results
3.1. OHIP-14 Mean Total Score
3.2. OHIP-14 Simple Count
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Heeseling, P.; Shin, H.Y.; Stiller, C.A.; IICC-3 contributors. International incidence of childhood cancer, 2001–10: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Fidler, M.M.; Colombet, M.; Lacour, B.; Kaatsch, P.; Piñeros, M.; Soerjomataram, I.; Bray, F.; Coebergh, J.W.; Peris-Bonet, R.; et al. Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991–2010 (Automated Childhood Cancer Information System): A population-based study. Lancet Oncol. 2018, 19, 1159–1169. [Google Scholar] [CrossRef]
- Knighting, K.; Kirton, J.; Thorp, N.; Hayden, J.; Appleton, L.; Bray, L. A study of childhood cancer survivors’ engagement with long-term follow-up care: ‘To attend or not to attend, that is the question’. Eur. J. Oncol. Nurs. 2020, 45, 101728. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood cancer survival in Europe 1999–2007: Results of EUROCARE-5--a population-based study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2020, 101733. [Google Scholar] [CrossRef]
- Saletta, F.; Seng, M.S.; Lau, L.M.S. Advances in paediatric cancer treatment. Transl. Pediatr. 2014, 3, 156–182. [Google Scholar]
- Neill, C.C.; Migliorati, C.; Trojan, T.; Kaste, S.; Karydis, A.; Rowland, C.; Parris, W. Experience and expertise regarding orthodontic management of childhood and adolescent cancer survivors. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 765–770. [Google Scholar] [CrossRef]
- Busenhart, D.M.; Erb, J.; Rigakos, G.; Eliades, T.; Papageorgiou, S.N. Adverse effects of chemotherapy on the teeth and surrounding tissues of children with cancer: A systematic review with meta-analysis. Oral Oncol. 2018, 83, 64–72. [Google Scholar] [CrossRef]
- Mishra, S. Orthodontic Therapy for Paediatric Cancer Survivors: A Review. J. Clin. Diagn. Res. 2017, 11, ZE01–ZE04. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry (AAPD). Dental management of pediatric patients receiving immunosuppressive therapy and/or radiation. Pediatr. Dent. 2018, 40, 422–430. [Google Scholar]
- Skidmore, K.J.; Brook, K.J.; Thomson, W.M.; Harding, W.J. Factors influencing treatment time in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Assessment, Q.O.L. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- The World Health Organization Quality of Life Assessment (WHOQOL): Development and general psychometric properties. Soc. Sci. Med. 1998, 46, 1569–1585. [CrossRef]
- Locker, D.; Allen, F. What do measures of ‘oral health-related quality of life’ measure? Community Dent. Oral Epidemiol. 2007, 35, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Feu, D.; De Oliveira, B.H.; Almeida, M.A.D.O.; Kiyak, H.A.; Miguel, J.A.M. Oral health-related quality of life and orthodontic treatment seeking. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Andiappan, M.; Gao, W.; Bernabé, E.; Kandala, N.-B.; Donaldson, A.N. Malocclusion, orthodontic treatment, and the Oral Health Impact Profile (OHIP-14): Systematic review and meta-analysis. Angle Orthod. 2014, 85, 493–500. [Google Scholar] [CrossRef]
- Johal, A.; Alyaqoobi, I.; Patel, R.; Cox, S. The impact of orthodontic treatment on quality of life and self-esteem in adult patients. Eur. J. Orthod. 2014, 37, 233–237. [Google Scholar] [CrossRef]
- Mitus-Kenig, M.; Derwich, M.; Czochrowska, E.; Pawlowska, E. Quality of Life in Orthodontic Cancer Survivor Patients—A Prospective Case–Control Study. Int. J. Environ. Res. Public Health 2020, 17, 5824. [Google Scholar] [CrossRef]
- Andrews, L.F. The six keys to normal occlusion. Am. J. Orthod. 1972, 62, 296–309. [Google Scholar] [CrossRef]
- Husain, F.A.; Tatengkeng, F. Oral Health-Related Quality of Life Appraised by OHIP-14 Between Urban and Rural Areas in Kutai Kartanegara Regency, Indonesia: Pilot Pathfinder Survey. Open Dent. J. 2017, 11, 557–564. [Google Scholar] [CrossRef]
- World Health Organization. Process of Translation and Adaptation of Instruments. Available online: https://www.who.int/substance_abuse/research_tools/translation/en/ (accessed on 22 November 2020).
- Hongxing, L.; List, T.; Nilsson, I.-M.; Johansson, A.; Astrom, A.N. Validity and reliability of OIDP and OHIP-14: A survey of Chinese high school students. BMC Oral Health 2014, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Dahllöf, G.; Huggare, J. Orthodontic considerations in the pediatric cancer patient: A review. Semin. Orthod. 2004, 10, 266–276. [Google Scholar] [CrossRef]
- Lopes, N.N.F.; Petrilli, A.S.; Caran, E.M.M.; França, C.M.; Chilvarquer, I.; Lederman, H. Dental abnormalities in children submitted to antineoplastic therapy. J. Dent. Child. 2007, 73, 140–145. [Google Scholar]
- Kılınç, G.; Bulut, G.; Ertuğrul, F.; Ören, H.; Demirağ, B.; Demiral, A.; Aksoylar, S.; Kamer, E.S.; Ellidokuz, H.; Olgun, N. Long-term Dental Anomalies after Pediatric Cancer Treatment in Children. Turk. J. Haematol. 2019, 36, 155–161. [Google Scholar] [CrossRef]
- Sonis, A.L.; Tarbell, N.; Valachovic, R.W.; Gelber, R.; Schwenn, M.; Sallan, S. Dentofacial development in long-term survivors of acute lymphoblastic leukemia: A comparison of three treatment modalities. Cancer 1990, 66, 2645–2652. [Google Scholar] [CrossRef]
- Roman, J.; Villaizán, C.J.; García-Foncillas, J.; Salvador, J.; Sierrasesúmaga, L. Growth and growth hormone secretion in children with cancer treated with chemotherapy. J. Pediatr. 1997, 131, 105–112. [Google Scholar] [CrossRef]
- Sonis, S.T. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009, 45, 1015–1020. [Google Scholar] [CrossRef]
- Barkokebas, A.; Silva, I.H.M.; De Andrade, S.C.; Carvalho, A.A.T.; Gueiros, L.A.; Paiva, S.M.; Leão, J.C. Impact of oral mucositis on oral-health-related quality of life of patients diagnosed with cancer. J. Oral Pathol. Med. 2014, 44, 746–751. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Hu, S.; Haverman, T.; Stokman, M.; Napeñas, J.J.; Braber, J.B.-D.; Gerber, E.; Geuke, M.; Vardas, E.; Waltimo, T.; et al. A systematic review of dental disease management in cancer patients. Support. Care Cancer 2018, 26, 155–174. [Google Scholar] [CrossRef]
- Pan, H.-T.; Wu, L.-M.; Wen, S.-H. Quality of Life and Its Predictors Among Children and Adolescents With Cancer. Cancer Nurs. 2017, 40, 343–351. [Google Scholar] [CrossRef]
- Nguee, A.-M.A.; Ongkosuwito, E.M.; Jaddoe, V.W.; Wolvius, E.B.; Kragt, L. Impact of orthodontic treatment need and deviant occlusal traits on oral health–related quality of life in children: A cross-sectional study in the Generation R cohort. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Demirovic, K.; Habibovic, J.; Dzemidzic, V.; Tiro, A.; Nakas, E. Comparison of Oral Health-Related Quality of Life in Treated and Non-Treated Orthodontic Patients. Med. Arch. 2019, 73, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Antoun, J.S.; Fowler, P.V.; Jack, H.C.; Farella, M. Oral health–related quality of life changes in standard, cleft, and surgery patients after orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, V.D.C.; Conti, A.C.D.C.F.; Cardoso, M.D.A.; Valarelli, D.P.; De Almeida-Pedrin, R.R. Impact of orthodontic treatment on self-esteem and quality of life of adult patients requiring oral rehabilitation. Angle Orthod. 2016, 86, 839–845. [Google Scholar] [CrossRef] [PubMed]
| Factor | Rapid Group | Standard Group | p-Value a |
|---|---|---|---|
| Number of patients (n) (female/male ratio) | 36 (26/10) | 40 (22/18) | 0.746 |
| Median age (range) (years) | 19.4 (13–28) | 19.2 (14–28) | 0.811 |
| Orthodontic assessment (number of patients) | Skeletal class I: 10 Skeletal class II: 20 Skeletal class III: 6 | Skeletal class I: 14 Skeletal class II: 22 Skeletal class III: 4 | 0.640 |
| Diagnosis | Number of Cases | Mean Age at Diagnosis [Years] | Follow-Up Time [Years] | Treatment Modality | |
|---|---|---|---|---|---|
| Chemotherapy | Radiotherapy | ||||
| Rapid Group | 36 | ||||
| Leukemia | 22 | 3.9 ± 1.5 | 8.8 ± 4.2 | 22 | 0 |
| Neuroblastoma | 3 | 0.8 ± 0.5 | 4.1 ± 1.2 | 3 | 0 |
| Soft tissue sarcoma | 2 | 2.5 ± 1.1 | 6.4 ± 2.4 | 2 | 1 |
| Non-Hodgkin’s lymphoma | 6 | 4.9 ± 2.4 | 7.3 ± 3.3 | 6 | 1 |
| Wilms’ tumor | 3 | 3.2 ± 2.2 | 6.8 ± 3.1 | 3 | 0 |
| Standard Group | 40 | ||||
| Leukemia | 25 | 3.2 ± 1.2 | 9.8 ± 3.7 | 25 | 0 |
| Neuroblastoma | 4 | 0.8 ± 0.5 | 5.4 ± 1.2 | 4 | 0 |
| Soft tissue sarcoma | 3 | 2.2 ± 0.8 | 7.5 ± 2.2 | 3 | 1 |
| Non-Hodgkin’s lymphoma | 2 | 4.6 ± 2.4 | 7.6 ± 3.9 | 2 | 1 |
| Wilms’ tumor | 6 | 3.6 ± 2.3 | 6.6 ± 2.9 | 6 | 0 |
| The List of Questions in the OHIP-14 Questionnaire |
|---|
| Functional Limitation |
| 1. Have you had trouble pronouncing any words because of problems with your teeth or mouth? 2. Have you felt that your sense of taste has worsened because of problems with your teeth or mouth? |
| Physical Pain |
| 3. Have you had painful aching in your mouth? 4. Have you found it uncomfortable to eat any foods because of problems with your teeth or mouth? |
| Psychological Discomfort |
| 5. Have you been self-conscious because of your teeth or mouth? 6. Have you felt tense because of problems with your teeth or mouth? |
| Physical Disability |
| 7. Has been your diet been unsatisfactory because of problems with your teeth of mouth? 8. Have you had to interrupt meals because of problems with your teeth or mouth? |
| Psychological Disability |
| 9. Have you found it difficult to relax because of problems with your teeth or mouth? 10. Have you been a bit embarrassed because of problems with your teeth or mouth? |
| Social Disability |
| 11. Have you been a bit irritable with other people because of problems with your teeth or mouth? 12. Have you had difficulty doing your usual jobs because of problems with your teeth or mouth? |
| Handicap |
| 13. Have you felt that life in general was less satisfying because of problems with your teeth or mouth? 14. Have you been totally unable to function because of problems with your teeth or mouth? |
| Time of Orthodontic Treatment (TX) | Rapid Group (Mean ± SD) (Range) | Standard Group (Mean ± SD) (Range) | p-Value a |
|---|---|---|---|
| Before TX | 4.1 ± 4.2 1,2,3 (0–14) | 3.8 ± 3.0 4,5,6 (0–9) | 0.311 |
| 2 weeks after the onset of TX | 9.8 ± 8.2 1,7,8 (0–32) | 10.2 ± 6.8 4,10,11 (0–20) | 0.189 |
| 3 months after the onset of TX | 7.8 ± 7.5 2,7,9 (0-28) | 8.5 ± 4.3 5,10,12 (0–15) | 0.324 |
| After TX | 1.1 ± 2.8 3,8,9 (0–14) | 1.3 ± 2.3 6,11,12 (0–12) | 0.108 |
| OHIP-14 Domains | Functional Limitation | Physical Pain | Psychological Discomfort | Physical Disability | Psychological Disability | Social Disability | Handicap |
|---|---|---|---|---|---|---|---|
| Before | 0.3 ± 0.6 | 0.1 ± 0.4 | 0.4 ± 0.7 | 0.7 ± 1.1 | 0.7 ± 1.2 | 0.4 ± 0.5 | 0.8 ± 1.3 |
| (rapid vs. standard) | 0.3 ± 0.5 | 0.1 ± 0.2 | 0.2 ± 0.5 | 0.6 ± 0.7 | 0.8 ± 1.1 | 0.3 ± 0.6 | 0.5 ± 1.2 |
| 2 weeks | 0.8 ± 0.8 | 2.1 ± 2.5 | 1.3 ± 1.9 | 1.6 ± 1.4 | 1.2 ± 1.4 | 0.4 ± 0.7 | 0.7 ± 1.2 |
| (rapid vs. standard) | 0.9 ± 1.3 | 1.8 ± 2.1 | 1.1 ± 0.5 | 1.4 ± 1.1 | 1.0 ± 1.2 | 0.4 ± 0.5 | 0.6 ± 1.4 |
| 3 months | 0.3 ± 0.8 | 1.3 ± 2.3 | 1.0 ± 1.5 | 1.4 ± 1.7 | 1.0 ± 1.3 | 0.4 ± 0.5 | 0.4 ± 1.2 |
| (rapid vs. standard) | 0.5 ± 0.8 | 1.1 ± 0.8 | 0.9 ± 1.3 | 1.2 ± 1.1 | 1.2 ± 0.9 | 0.2 ± 0.5 | 0.5 ± 1.8 |
| After | 0.1 ± 0.4 | 0.2 ± 0.5 | 0.2 ± 0.6 | 0.3 ± 0.6 | 0.1 ± 0.5 | 0.1 ± 0.4 | 0.1 ± 0.2 |
| (rapid vs. standard) | 0.1 ± 1.1 | 0.1 ± 0.3 | 0.1 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.4 | 0.2 ± 0.3 | 0.1 ± 0.6 |
| OHIP-14 Domains | Functional Limitation | Physical Pain | Psychological Discomfort | Physical Disability | Psychological Disability | Social Disability | Handicap |
|---|---|---|---|---|---|---|---|
| Before | 2 | 0 | 6 | 8 | 8 | 7 | 8 |
| (rapid vs. standard) | 3 | 0 | 4 | 6 | 6 | 6 | 7 |
| 2 weeks | 4 | 11 | 18 | 12 | 13 | 5 | 6 |
| (rapid vs. standard) | 4 | 12 | 16 | 11 | 11 | 4 | 5 |
| 3 months | 7 | 10 | 7 | 9 | 9 | 5 | 5 |
| (rapid vs. standard) | 7 | 8 | 8 | 8 | 7 | 3 | 3 |
| After | 1 | 0 | 2 | 2 | 1 | 1 | 1 |
| (rapid vs. standard) | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| Time of Orthodontic Treatment (TX) | Rapid Group | Standard Group | p-Value a |
|---|---|---|---|
| Before | 3 (8.3%) | 3 (7.5%) | 0.965 |
| 2 weeks | 17 (47.2%) | 18 (45.0%) | 0.312 |
| 3 months | 12 (33.3%) | 12 (30.0%) | 0.845 |
| After the treatment | 2 (5.6%) | 1 (2.5%) | 0.514 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitus-Kenig, M.; Derwich, M.; Czochrowska, E.; Pawlowska, E. Comparison of Oral Health Impact Profile (OHIP-14) Values in Cancer Survivor Patients Treated Orthodontically with Either Rapid or Standard Duration Protocols of Treatment—A Prospective Case–Control Study. Int. J. Environ. Res. Public Health 2020, 17, 9068. https://doi.org/10.3390/ijerph17239068
Mitus-Kenig M, Derwich M, Czochrowska E, Pawlowska E. Comparison of Oral Health Impact Profile (OHIP-14) Values in Cancer Survivor Patients Treated Orthodontically with Either Rapid or Standard Duration Protocols of Treatment—A Prospective Case–Control Study. International Journal of Environmental Research and Public Health. 2020; 17(23):9068. https://doi.org/10.3390/ijerph17239068
Chicago/Turabian StyleMitus-Kenig, Maria, Marcin Derwich, Ewa Czochrowska, and Elzbieta Pawlowska. 2020. "Comparison of Oral Health Impact Profile (OHIP-14) Values in Cancer Survivor Patients Treated Orthodontically with Either Rapid or Standard Duration Protocols of Treatment—A Prospective Case–Control Study" International Journal of Environmental Research and Public Health 17, no. 23: 9068. https://doi.org/10.3390/ijerph17239068
APA StyleMitus-Kenig, M., Derwich, M., Czochrowska, E., & Pawlowska, E. (2020). Comparison of Oral Health Impact Profile (OHIP-14) Values in Cancer Survivor Patients Treated Orthodontically with Either Rapid or Standard Duration Protocols of Treatment—A Prospective Case–Control Study. International Journal of Environmental Research and Public Health, 17(23), 9068. https://doi.org/10.3390/ijerph17239068





