Mercury in Pancreatic Cells of People with and without Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Sample Collection
2.3. Mercury (Autometallography) Staining
2.4. Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS)
2.5. Statistical Analyses
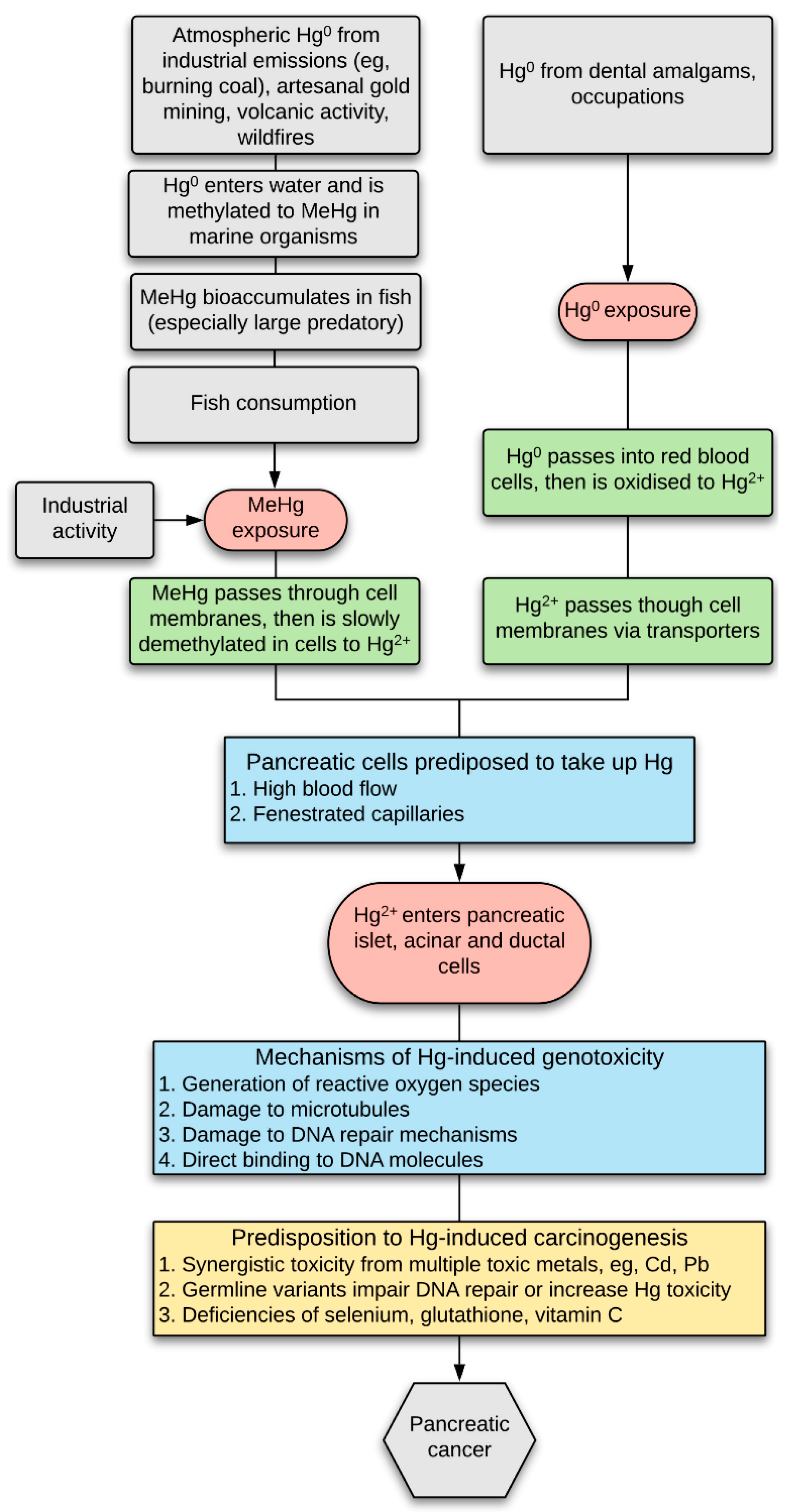
3. Results
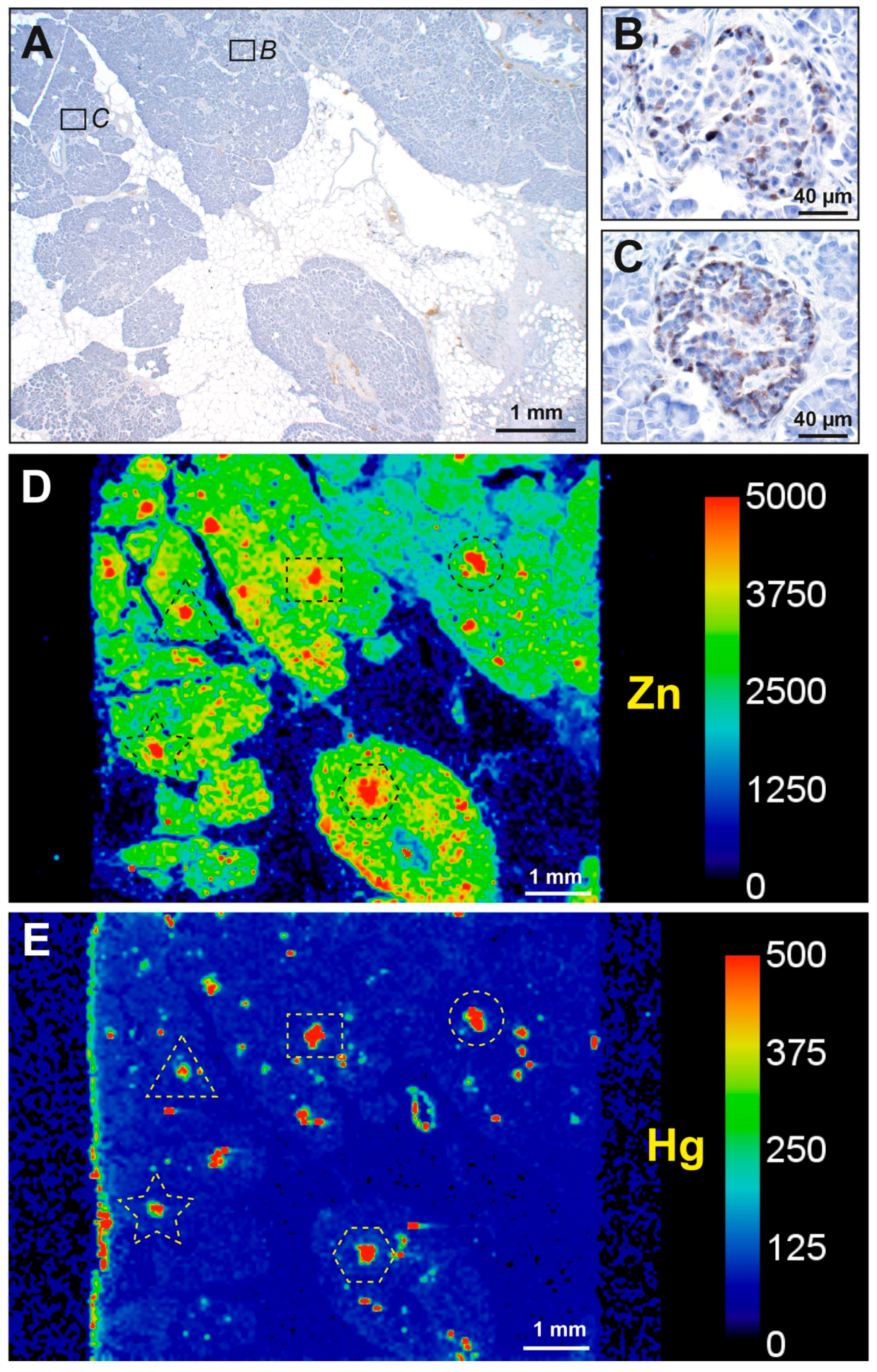
3.1. Mercury (Autometallography) Staining
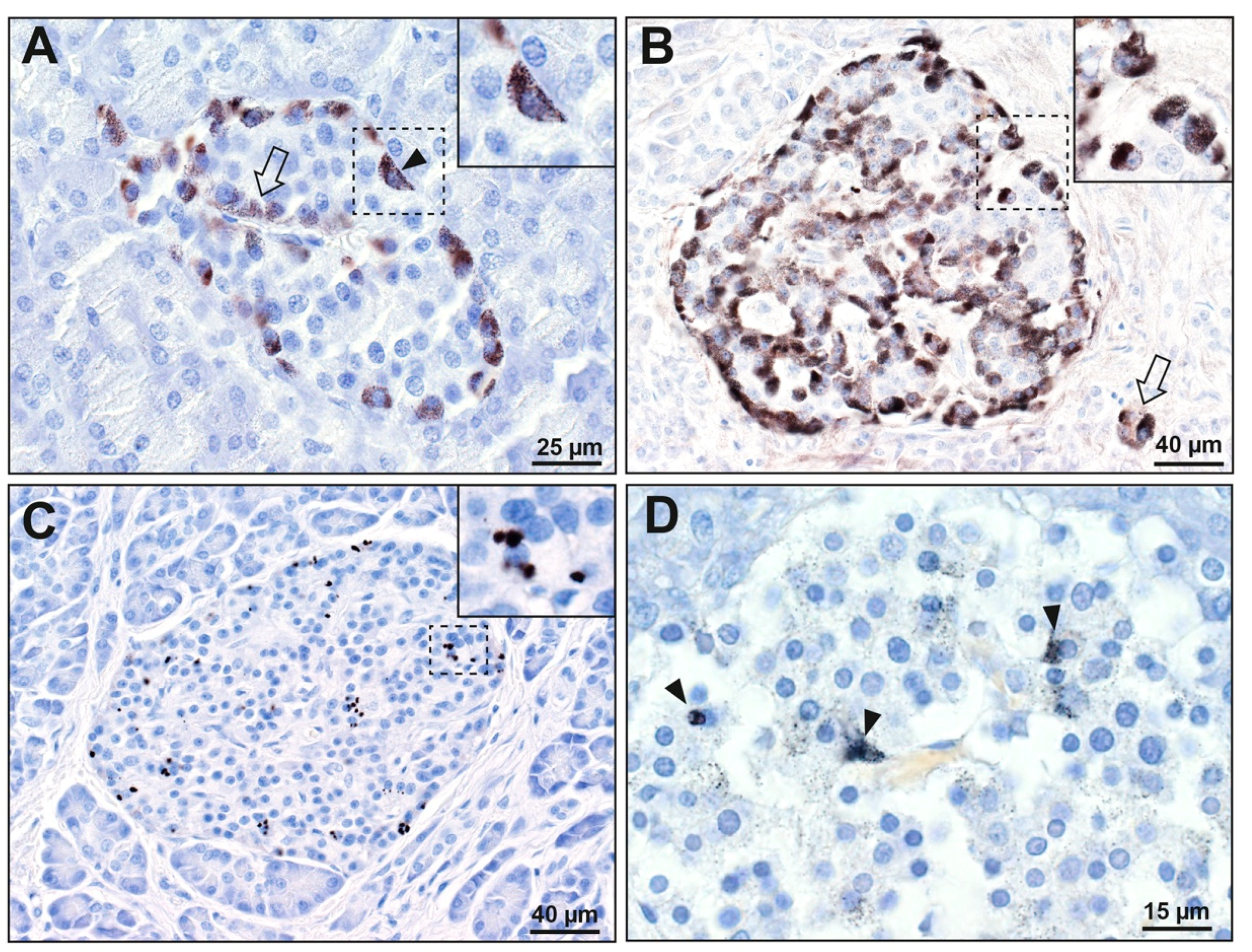
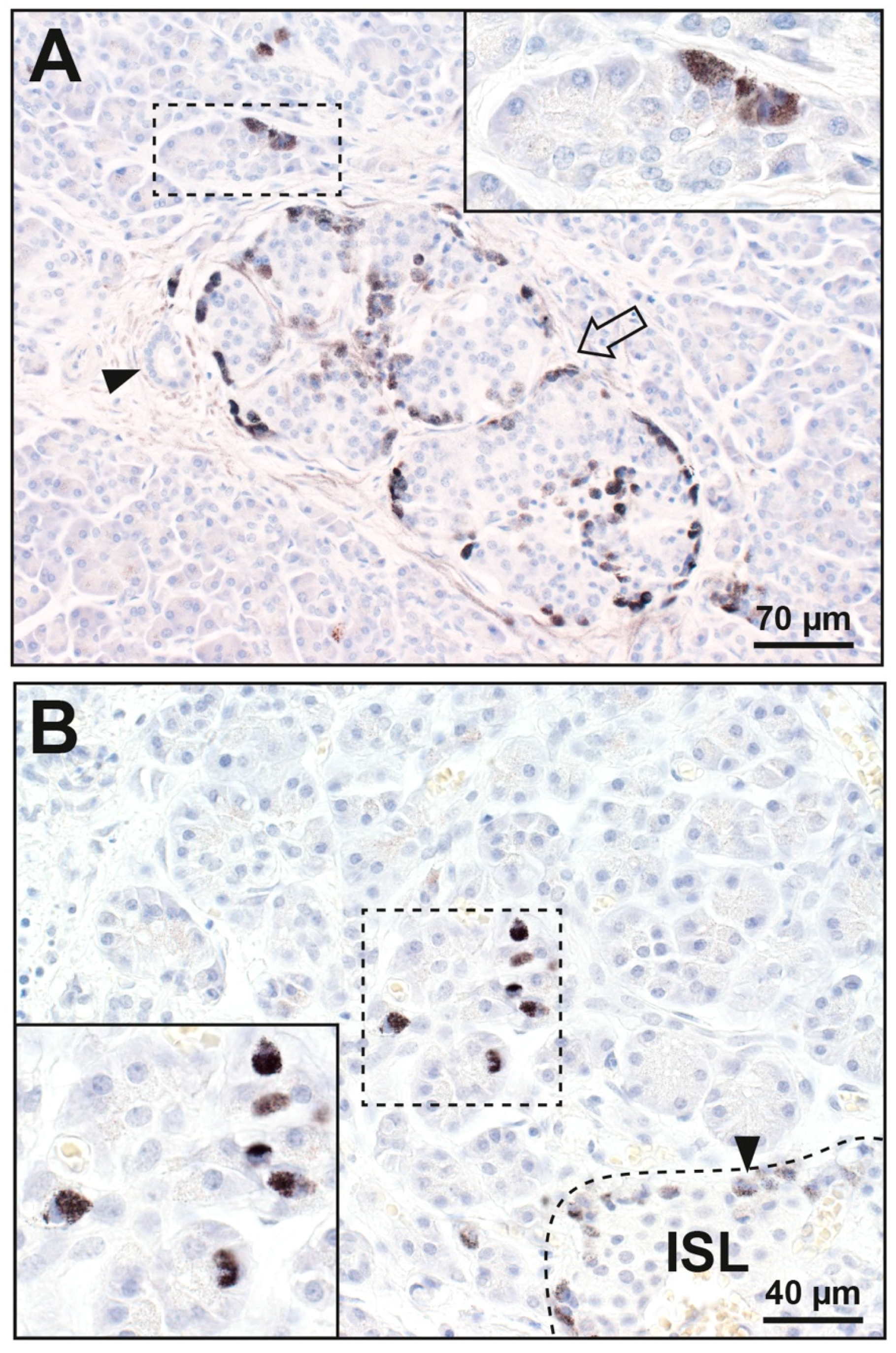
3.1.1. Islet Cells
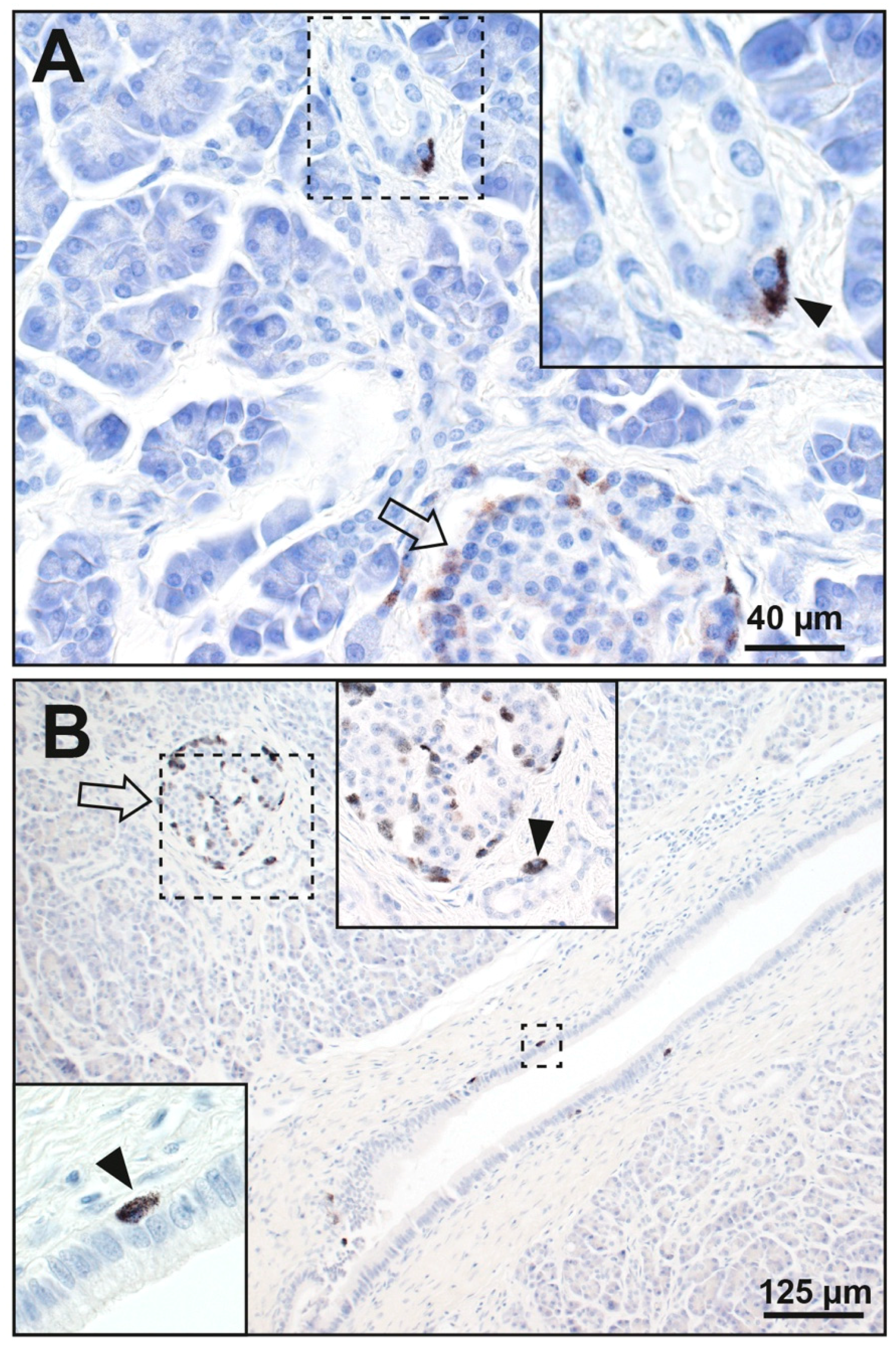
3.1.2. Acinar Cells
3.1.3. Periductal Cells
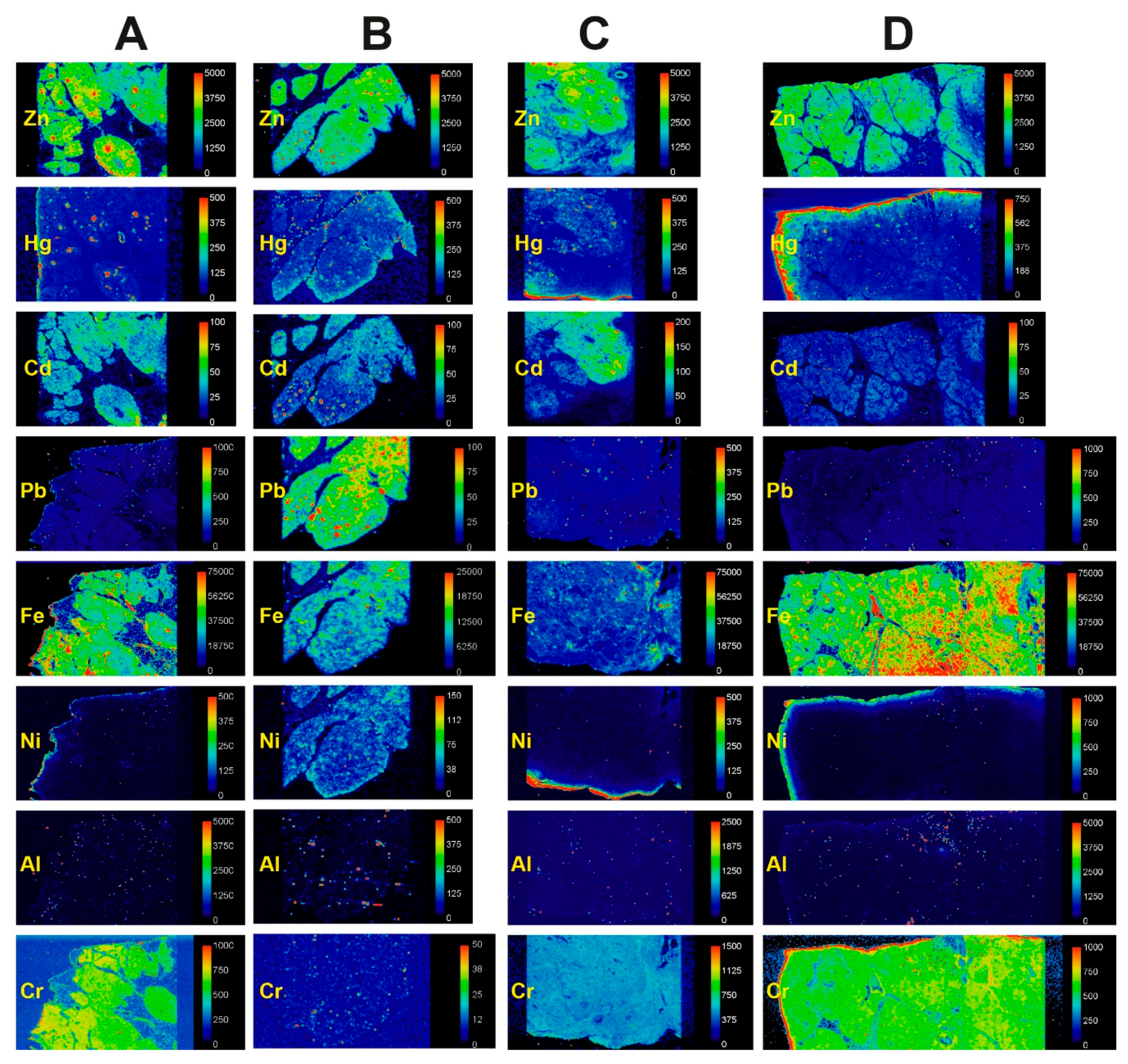
3.2. LA-ICP-MS
3.2.1. Correlation of Mercury Detected on Autometallography with LA-ICP-MS
3.2.2. Mercury and Other Toxic Metals Detected with LA-ICP-MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef]
- Klein, A.P. Genetic susceptibility to pancreatic cancer. Mol. Carcinog. 2012, 51, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Chang, J.S. Environmental Risk Factors of Pancreatic Cancer. J. Clin. Med. 2019, 8, 1427. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Reis, I.M. Is cadmium a cause of human pancreatic cancer? Cancer Epidemiol. Biomark. Prev. 2000, 9, 139–145. [Google Scholar]
- Amaral, A.F.; Porta, M.; Silverman, D.T.; Milne, R.L.; Kogevinas, M.; Rothman, N.; Cantor, K.P.; Jackson, B.P.; Pumarega, J.A.; Lopez, T.; et al. Pancreatic cancer risk and levels of trace elements. Gut 2012, 61, 1583–1588. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Nishijo, M.; Sekikawa, A.; He, K. Cadmium exposure and risk of pancreatic cancer: A meta-analysis of prospective cohort studies and case-control studies among individuals without occupational exposure history. Environ. Sci. Pollut. Res. Int. 2015, 22, 17465–17474. [Google Scholar] [CrossRef]
- Djordjevic, V.R.; Wallace, D.R.; Schweitzer, A.; Boricic, N.; Knezevic, D.; Matic, S.; Grubor, N.; Kerkez, M.; Radenkovic, D.; Bulat, Z.; et al. Environmental cadmium exposure and pancreatic cancer: Evidence from case control, animal and in vitro studies. Environ. Int. 2019, 128, 353–361. [Google Scholar] [CrossRef]
- Wallace, D.R.; Spandidos, D.A.; Tsatsakis, A.; Schweitzer, A.; Djordjevic, V.; Djordjevic, A.B. Potential interaction of cadmium chloride with pancreatic mitochondria: Implications for pancreatic cancer. Int. J. Mol. Med. 2019, 44, 145–156. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Macedo, G.L.; Pereira, S.I.; Arrifano, G.P.; Picanco-Diniz, D.L.; do Nascimento, J.L.; Herculano, A.M. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef]
- Nersesyan, A.; Kundi, M.; Waldherr, M.; Setayesh, T.; Misik, M.; Wultsch, G.; Filipic, M.; Mazzaron Barcelos, G.R.; Knasmueller, S. Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium. Mutat. Res. 2016, 770, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Christie, N.T.; Cantoni, O.; Zelikoff, J.T.; Xin, W.W.; Rossman, T.G. DNA damage by mercury compounds: An overview. In Advances in Mercury Toxicology; Suzuki, T., Imura, N., Clarkson, T.W., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 255–273. [Google Scholar]
- Clarkson, T.W. The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 1997, 34, 369–403. [Google Scholar] [CrossRef]
- Danscher, G.; Stoltenberg, M.; Juhl, S. How to detect gold, silver and mercury in human brain and other tissues by autometallographic silver amplification. Neuropathol. Appl. Neurobiol. 1994, 20, 454–467. [Google Scholar] [CrossRef]
- Danscher, G.; Stoltenberg, M.; Kemp, K.; Pamphlett, R. Bismuth autometallography: Protocol, specificity, and differentiation. J. Histochem. Cytochem. 2000, 48, 1503–1510. [Google Scholar] [CrossRef]
- Pamphlett, R.; Satgunaseelan, L.; Kum Jew, S.; Doble, P.A.; Bishop, D.P. Elemental bioimaging shows mercury and other toxic metals in normal breast tissue and in breast cancers. PLoS ONE 2020, 15, e0228226. [Google Scholar] [CrossRef]
- Danscher, G.; Moller-Madsen, B. Silver amplification of mercury sulfide and selenide: A histochemical method for light and electron microscopic localization of mercury in tissue. J. Histochem. Cytochem. 1985, 33, 219–228. [Google Scholar] [CrossRef]
- Danscher, G.; Rungby, J. Differentiation of histochemically visualized mercury and silver. Histochem. J. 1986, 18, 109–114. [Google Scholar] [CrossRef]
- Pamphlett, R.; Png, F.Y. Shrinkage of motor axons following systemic exposure to inorganic mercury. J. Neuropathol. Exp. Neurol. 1998, 57, 360–366. [Google Scholar] [CrossRef]
- Wijesekara, N.; Chimienti, F.; Wheeler, M.B. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes. Metab. 2009, 11 (Suppl. 4), 202–214. [Google Scholar] [CrossRef]
- Herbert, R.D. Research Note: Causal inference. J. Physiother. 2020, 66, 273–277. [Google Scholar] [CrossRef]
- Liu, Y.M.; Guth, P.H.; Kaneko, K.; Livingston, E.H.; Brunicardi, F.C. Dynamic in vivo observation of rat islet microcirculation. Pancreas 1993, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jansson, L.; Barbu, A.; Bodin, B.; Drott, C.J.; Espes, D.; Gao, X.; Grapensparr, L.; Kallskog, O.; Lau, J.; Liljeback, H.; et al. Pancreatic islet blood flow and its measurement. Ups. J. Med. Sci. 2016, 121, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G.; Carati, C.J.; Toouli, J.; Saccone, G.T. The islet-acinar axis of the pancreas: More than just insulin. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G10–G22. [Google Scholar] [CrossRef] [PubMed]
- Pour, P.M.; Pandey, K.K.; Batra, S.K. What is the origin of pancreatic adenocarcinoma? Mol. Cancer 2003, 2, 13. [Google Scholar] [CrossRef]
- Zefferino, R.; Piccoli, C.; Ricciardi, N.; Scrima, R.; Capitanio, N. Possible Mechanisms of Mercury Toxicity and Cancer Promotion: Involvement of Gap Junction Intercellular Communications and Inflammatory Cytokines. Oxid. Med. Cell Longev. 2017, 2017, 7028583. [Google Scholar] [CrossRef]
- Kresovich, J.K.; Erdal, S.; Chen, H.Y.; Gann, P.H.; Argos, M.; Rauscher, G.H. Metallic air pollutants and breast cancer heterogeneity. Environ. Res. 2019, 177, 108639. [Google Scholar] [CrossRef]
- Ku, H.T. Minireview: Pancreatic progenitor cells—Recent studies. Endocrinology 2008, 149, 4312–4316. [Google Scholar] [CrossRef][Green Version]
- Noguchi, H. Pancreatic stem/progenitor cells for the treatment of diabetes. Rev. Diabet. Stud. 2010, 7, 105–111. [Google Scholar] [CrossRef][Green Version]
- Carpino, G.; Renzi, A.; Cardinale, V.; Franchitto, A.; Onori, P.; Overi, D.; Rossi, M.; Berloco, P.B.; Alvaro, D.; Reid, L.M.; et al. Progenitor cell niches in the human pancreatic duct system and associated pancreatic duct glands: An anatomical and immunophenotyping study. J. Anat. 2016, 228, 474–486. [Google Scholar] [CrossRef]
- Huising, M.O.; Lee, S.; van der Meulen, T. Evidence for a Neogenic Niche at the Periphery of Pancreatic Islets. Bioessays 2018, 40, e1800119. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Nipper, M.; Wang, P. Ductal vs. acinar? Recent insights into identifying cell lineage of pancreatic ductal adenocarcinoma. Ann. Pancreat. Cancer 2019, 2. [Google Scholar] [CrossRef]
- Streets, D.G.; Devane, M.K.; Lu, Z.; Bond, T.C.; Sunderland, E.M.; Jacob, D.J. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011, 45, 10485–10491. [Google Scholar] [CrossRef]
- Schartup, A.T.; Thackray, C.P.; Qureshi, A.; Dassuncao, C.; Gillespie, K.; Hanke, A.; Sunderland, E.M. Climate change and overfishing increase neurotoxicant in marine predators. Nature 2019, 572, 648–650. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury—Current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef]
- Karimi, R.; Silbernagel, S.; Fisher, N.S.; Meliker, J.R. Elevated blood Hg at recommended seafood consumption rates in adult seafood consumers. Int. J. Hyg. Environ. Health 2014, 217, 758–764. [Google Scholar] [CrossRef]
- Karimi, R.; Vacchi-Suzzi, C.; Meliker, J.R. Mercury exposure and a shift toward oxidative stress in avid seafood consumers. Environ. Res. 2016, 146, 100–107. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Pamphlett, R.; Bishop, D.P.; Kum Jew, S.; Doble, P.A. Age-related accumulation of toxic metals in the human locus ceruleus. PLoS ONE 2018, 13, e0203627. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S.; Doble, P.A.; Bishop, D.P. Elemental Analysis of Aging Human Pituitary Glands Implicates Mercury as a Contributor to the Somatopause. Front. Endocrinol. (Lausanne) 2019, 10, 419. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017, 91, 63–81. [Google Scholar] [CrossRef]
- Parkin Kullmann, J.A.; Pamphlett, R. A Comparison of Mercury Exposure from Seafood Consumption and Dental Amalgam Fillings in People with and without Amyotrophic Lateral Sclerosis (ALS): An International Online Case-Control Study. Int. J. Environ. Res. Public Health 2018, 15, 2874. [Google Scholar] [CrossRef]
- Ekstrand, J.; Nielsen, J.B.; Havarinasab, S.; Zalups, R.K.; Soderkvist, P.; Hultman, P. Mercury toxicokinetics—Dependency on strain and gender. Toxicol. Appl. Pharmacol. 2010, 243, 283–291. [Google Scholar] [CrossRef]
- Joseph, P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Caruso, R.V.; O’Connor, R.J.; Stephens, W.E.; Cummings, K.M.; Fong, G.T. Toxic metal concentrations in cigarettes obtained from U.S. smokers in 2009: Results from the International Tobacco Control (ITC) United States survey cohort. Int. J. Environ. Res. Public Health 2013, 11, 202–217. [Google Scholar] [CrossRef]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. 2012, 23, 1880–1888. [Google Scholar] [CrossRef]
- Tsukahara, T.; Ezaki, T.; Moriguchi, J.; Furuki, K.; Shimbo, S.; Matsuda-Inoguchi, N.; Ikeda, M. Rice as the most influential source of cadmium intake among general Japanese population. Sci. Total Environ. 2003, 305, 41–51. [Google Scholar] [CrossRef]
- Carrigan, P.E.; Hentz, J.G.; Gordon, G.; Morgan, J.L.; Raimondo, M.; Anbar, A.D.; Miller, L.J. Distinctive heavy metal composition of pancreatic juice in patients with pancreatic carcinoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2656–2663. [Google Scholar] [CrossRef]
- Ilychova, S.A.; Zaridze, D.G. Cancer mortality among female and male workers occupationally exposed to inorganic lead in the printing industry. Occup. Environ. Med. 2012, 69, 87–92. [Google Scholar] [CrossRef]
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [CrossRef]
- Klotz, K.; Weistenhofer, W.; Neff, F.; Hartwig, A.; van Thriel, C.; Drexler, H. The Health Effects of Aluminum Exposure. Dtsch. Arztebl. Int. 2017, 114, 653–659. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Fresquez, M.R.; Martone, N.; Watson, C.H. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J. Anal. Toxicol. 2014, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.M.; Cauvi, D.M.; Toomey, C.B.; Hultman, P.; Kono, D.H. Mercury-induced inflammation and autoimmunity. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129299. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharmacol. 2011, 254, 86–99. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Hung, D.Z.; Leung, Y.M.; Liu, S.H. Heavy metals, islet function and diabetes development. Islets 2009, 1, 169–176. [Google Scholar] [CrossRef]
- Roy, C.; Tremblay, P.Y.; Ayotte, P. Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environ. Res. 2017, 156, 747–760. [Google Scholar] [CrossRef]
- Chen, K.L.; Liu, S.H.; Su, C.C.; Yen, C.C.; Yang, C.Y.; Lee, K.I.; Tang, F.C.; Chen, Y.W.; Lu, T.H.; Su, Y.C.; et al. Mercuric compounds induce pancreatic islets dysfunction and apoptosis in vivo. Int. J. Mol. Sci. 2012, 13, 12349–12366. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Shi, P.; Morris, J.S.; Grandjean, P.; Siscovick, D.S.; Spiegelman, D.; Hu, F.B. Methylmercury exposure and incident diabetes in U.S. men and women in two prospective cohorts. Diabetes Care 2013, 36, 3578–3584. [Google Scholar] [CrossRef]
- Rana, S.V. Perspectives in endocrine toxicity of heavy metals—A review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef]
- Gonzalez-Villalva, A.; Colin-Barenque, L.; Bizarro-Nevares, P.; Rojas-Lemus, M.; Rodriguez-Lara, V.; Garcia-Pelaez, I.; Ustarroz-Cano, M.; Lopez-Valdez, N.; Albarran-Alonso, J.C.; Fortoul, T.I. Pollution by metals: Is there a relationship in glycemic control? Environ. Toxicol. Pharmacol. 2016, 46, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, L.; Abbott, L.C. Effects of methyl mercury exposure on pancreatic beta cell development and function. J. Appl. Toxicol. 2017, 37, 4–12. [Google Scholar] [CrossRef] [PubMed]
- El Muayed, M.; Raja, M.R.; Zhang, X.; MacRenaris, K.W.; Bhatt, S.; Chen, X.; Urbanek, M.; O’Halloran, T.V.; Lowe, W.L., Jr. Accumulation of cadmium in insulin-producing beta cells. Islets 2012, 4, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, V.; Sprovieri, F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef]
- Gregersen, P.K.; Olsson, L.M. Recent advances in the genetics of autoimmune disease. Annu. Rev. Immunol. 2009, 27, 363–391. [Google Scholar] [CrossRef]
- Pamphlett, R.; Kum Jew, S. Different Populations of Human Locus Ceruleus Neurons Contain Heavy Metals or Hyperphosphorylated Tau: Implications for Amyloid-beta and Tau Pathology in Alzheimer’s Disease. J. Alzheimers Dis. 2015, 45, 437–447. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamphlett, R.; Colebatch, A.J.; Doble, P.A.; Bishop, D.P. Mercury in Pancreatic Cells of People with and without Pancreatic Cancer. Int. J. Environ. Res. Public Health 2020, 17, 8990. https://doi.org/10.3390/ijerph17238990
Pamphlett R, Colebatch AJ, Doble PA, Bishop DP. Mercury in Pancreatic Cells of People with and without Pancreatic Cancer. International Journal of Environmental Research and Public Health. 2020; 17(23):8990. https://doi.org/10.3390/ijerph17238990
Chicago/Turabian StylePamphlett, Roger, Andrew J. Colebatch, Philip A. Doble, and David P. Bishop. 2020. "Mercury in Pancreatic Cells of People with and without Pancreatic Cancer" International Journal of Environmental Research and Public Health 17, no. 23: 8990. https://doi.org/10.3390/ijerph17238990
APA StylePamphlett, R., Colebatch, A. J., Doble, P. A., & Bishop, D. P. (2020). Mercury in Pancreatic Cells of People with and without Pancreatic Cancer. International Journal of Environmental Research and Public Health, 17(23), 8990. https://doi.org/10.3390/ijerph17238990




