Small Subcutaneous Soft Tissue Tumors (<5 cm) Can Be Sarcomas and Contrast-Enhanced Ultrasound (CEUS) Is Useful to Identify Potentially Malignant Masses
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging
2.2.1. US and Power Doppler Sonography (PD)
2.2.2. CEUS
2.2.3. MRI
2.3. Histology
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enneking, W.F. Clinical Musculoskeletal Pathology, 3rd rev. ed.; University of Florida Press: Gainesville, FL, USA, 1990; ISBN 978-0-8130-1016-8. [Google Scholar]
- Beaman, F.D.; Kransdorf, M.J.; Andrews, T.R.; Murphey, M.D.; Arcara, L.K.; Keeling, J.H. Superficial Soft-Tissue Masses: Analysis, Diagnosis, and Differential Considerations. RadioGraphics 2007, 27, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Cany, L.; Stoeckle, E.; Coindre, J.M.; Kantor, G.; Bonichon, F.; Bui, B.N. Prognostic factors in superficial adult soft tissue sarcomas: Analysis of a series of 105 patients. J. Surg. Oncol. 1999, 71, 4–9. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Yang, Q.; Eisenberger, C.F.; Boelke, E.; Poremba, C.; Heinecke, A.; Ohmann, C.; Knoefel, W.T.; Peiper, M. Superficial Soft Tissue Sarcomas of the Extremities and Trunk. World J. Surg. 2009, 33, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.; Stoeckle, E.; Collin, F.; Bui, B.; Terrier, P.; Guillou, L.; Trassard, M.; Ranchere-Vince, D.; Gregoire, F.; Coindre, J.-M. Superficial soft tissue sarcomas (S-STS): A study of 367 patients from the French Sarcoma Group (FSG) database. Eur. J. Cancer 2009, 45, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Calleja, M.; Dimigen, M.; Saifuddin, A. MRI of superficial soft tissue masses: Analysis of features useful in distinguishing between benign and malignant lesions. Skelet. Radiol. 2012, 41, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.; Smith, M. Soft tissue sarcomas: Are current referral guidelines sufficient? Ann. R. Coll. Surg. Engl. 2005, 87, 171–173. [Google Scholar] [CrossRef]
- Grimer, R.; Judson, I.; Peake, D.; Seddon, B. Guidelines for the Management of Soft Tissue Sarcomas. Sarcoma 2010, 2010, 506182. [Google Scholar] [CrossRef]
- National Institute for Clinical Excellence. Improving Outcomes for People with Sarcoma: The Manual; National Institute for Clinical Excellence: London, UK, 2006; ISBN 978-1-84629-158-6. [Google Scholar]
- Chiou, H.-J.; Chou, Y.-H.; Chiu, S.-Y.; Wang, H.-K.; Chen, W.-M.; Chen, T.-H.; Chang, C.-Y. Differentiation of Benign and Malignant Superficial Soft-tissue Masses Using Grayscale and Color Doppler Ultrasonography. J. Chin. Med. Assoc. 2009, 72, 307–315. [Google Scholar] [CrossRef]
- De Schepper, A.M.; Bloem, J.L. Soft Tissue Tumors: Grading, Staging, and Tissue-Specific Diagnosis. Top. Magn. Reson. Imaging 2007, 18, 431–444. [Google Scholar] [CrossRef]
- Hung, E.H.Y.; Griffith, J.F.; Hung Ng, A.W.; Lee, R.K.L.; Lau, D.T.Y.; Leung, J.C.S. Ultrasound of Musculoskeletal Soft-Tissue Tumors Superficial to the Investing Fascia. Am. J. Roentgenol. 2014, 202, W532–W540. [Google Scholar] [CrossRef]
- Morel, M.; Taïeb, S.; Penel, N.; Mortier, L.; Vanseymortier, L.; Robin, Y.M.; Gosset, P.; Cotten, A.; Ceugnart, L. Imaging of the most frequent superficial soft-tissue sarcomas. Skelet. Radiol. 2011, 40, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.M.; Lee, K.S.; Rosas, H.; Kliewer, M.A. Accuracy of Sonographic Diagnosis of Superficial Masses. J. Ultrasound Med. 2013, 32, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Wu, H.-T.; Chiou, H.-J.; Wei, C.-J.; Yen, C.-H.; Chang, C.-Y.; Chen, W.-M. Differentiating Benign and Malignant Soft Tissue Masses by Magnetic Resonance Imaging: Role of Tissue Component Analysis. J. Chin. Med. Assoc. 2009, 72, 194–201. [Google Scholar] [CrossRef]
- De Schepper, A.M.; de Beuckeleer, L.; Vandevenne, J.; Somville, J. Magnetic resonance imaging of soft tissue tumors. Eur. Radiol. 2000, 10, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Doganay, S.; Altinok, T.; Alkan, A.; Kahraman, B.; Karakas, H.M. The role of MRS in the differentiation of benign and malignant soft tissue and bone tumors. Eur. J. Radiol. 2011, 79, e33–e37. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.W.; O’Donnell, P.; Saifuddin, A. MRI appearances of common benign soft-tissue tumours. Clin. Radiol. 2007, 62, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Juan, Y.-H.; Saboo, S.S.; Tirumani, S.H.; Khandelwal, A.; Shinagare, A.B.; Ramaiya, N.; Krajewski, K.M. Malignant Skin and Subcutaneous Neoplasms in Adults: Multimodality Imaging With CT, MRI, and 18F-FDG PET/CT. Am. J. Roentgenol. 2014, 202, W422–W438. [Google Scholar] [CrossRef]
- Kind, M.; Stock, N.; Coindre, J.M. Histology and imaging of soft tissue sarcomas. Eur. J. Radiol. 2009, 72, 6–15. [Google Scholar] [CrossRef]
- Tuncbilek, N.; Karakas, H.M.; Okten, O.O. Dynamic contrast enhanced MRI in the differential diagnosis of soft tissue tumors. Eur. J. Radiol. 2005, 53, 500–505. [Google Scholar] [CrossRef]
- Vilanova, J.C.; Woertler, K.; Narváez, J.A.; Barceló, J.; Martínez, S.J.; Villalón, M.; Miró, J. Soft-tissue tumors update: MR imaging features according to the WHO classification. Eur. Radiol. 2007, 17, 125–138. [Google Scholar] [CrossRef]
- Berquist, T.H.; Ehman, R.L.; King, B.F.; Hodgman, C.G.; Ilstrup, D.M. Value of MR imaging in differentiating benign from malignant soft-tissue masses: Study of 95 lesions. Am. J. Roentgenol. 1990, 155, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Galant, J.; Martí-Bonmatí, L.; Soler, R.; Saez, F.; Lafuente, J.; Bonmatí, C.; Gonzalez, I. Grading of subcutaneous soft tissue tumors by means of their relationship with the superficial fascia on MR imaging. Skelet. Radiol. 1998, 27, 657–663. [Google Scholar] [CrossRef]
- Chiou, H.-J.; Chou, Y.-H.; Chen, W.-M.; Chen, W.; Wang, H.-K.; Chang, C.-Y. Soft-tissue tumor differentiation using 3D power Doppler ultrasonography with echo-contrast medium injection. J. Chin. Med. Assoc. 2010, 73, 628–633. [Google Scholar] [CrossRef]
- Loizides, A.; Peer, S.; Plaikner, M.; Djurdjevic, T.; Gruber, H. Perfusion pattern of musculoskeletal masses using contrast-enhanced ultrasound: A helpful tool for characterisation? Eur. Radiol. 2012, 22, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, A.; del Prever, E.B.; Cavallo, F.; Pozza, S.; Linari, A.; Lombardo, P.; Comandone, A.; Piana, R.; Faletti, C. Perfusion pattern and time of vascularisation with CEUS increase accuracy in differentiating between benign and malignant tumours in 216 musculoskeletal soft tissue masses. Eur. J. Radiol. 2015, 84, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Imanishi, N.; Minabe, T.; Kishi, K.; Aiso, S. Anatomical study of subcutaneous adipofascial tissue: A concept of the protective adipofascial system (PAFS) and lubricant adipofascial system (LAFS). Scand. J. Plast. Reconstr. Surg. Hand Surg. 2004, 38, 261–266. [Google Scholar] [CrossRef]
- Harish, S.; Lee, J.C.; Ahmad, M.; Saifuddin, A. Soft tissue masses with “cyst-like” appearance on MR imaging: Distinction of benign and malignant lesions. Eur. Radiol. 2006, 16, 2652–2660. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Breitenseher, M.; Amann, G.; Dominkus, M. Are signal intensity and homogeneity useful parameters for distinguishing between benign and malignant soft tissue masses on MR images? Magn. Reson. Imaging 2008, 26, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Hung, E.; Griffith, J. Pitfalls in Ultrasonography of Soft Tissue Tumors. Semin. Musculoskelet. Radiol. 2014, 18, 79–85. [Google Scholar] [CrossRef]
- Jin, W.; Kim, G.Y.; Park, S.Y.; Chun, Y.S.; Rhyu, K.H.; Park, J.S.; Ryu, K.N. The Spectrum of Vascularized Superficial Soft-Tissue Tumors on Sonography with a Histopathologic Correlation: Part 2, Malignant Tumors and Their Look-Alikes. Am. J. Roentgenol. 2010, 195, 446–453. [Google Scholar] [CrossRef]
- Kim, D.G.; Lee, S.J.; Choo, H.J.; Kim, S.K.; Cha, J.G.; Park, H.J.; Kwon, J.W.; Kim, T.E.; Jung, S.-J. Ultrasonographic Findings of Subcutaneous Angioleiomyomas in the Extremities Based on Pathologic Subtypes. Korean J. Radiol. 2018, 19, 752. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chung, H.W. Small Musculoskeletal Soft-Tissue Lesions: US-guided Core Needle Biopsy—Comparative Study of Diagnostic Yields according to Lesion Size. Radiology 2016, 278, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Huang, S.; Wu, Z.; Zheng, J.; Chen, X.; Nie, L. Label-Free Visualization of Early Cancer Hepatic Micrometastasis and Intraoperative Image-Guided Surgery by Photoacoustic Imaging. J. Nucl. Med. 2020, 61, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, F.; Liu, Y.; Nie, L. High-Resolution Photoacoustic Tomography for Early-Stage Cancer Detection and Its Clinical Translation. Radiol. Imaging Cancer 2020, 2, e190030. [Google Scholar] [CrossRef]
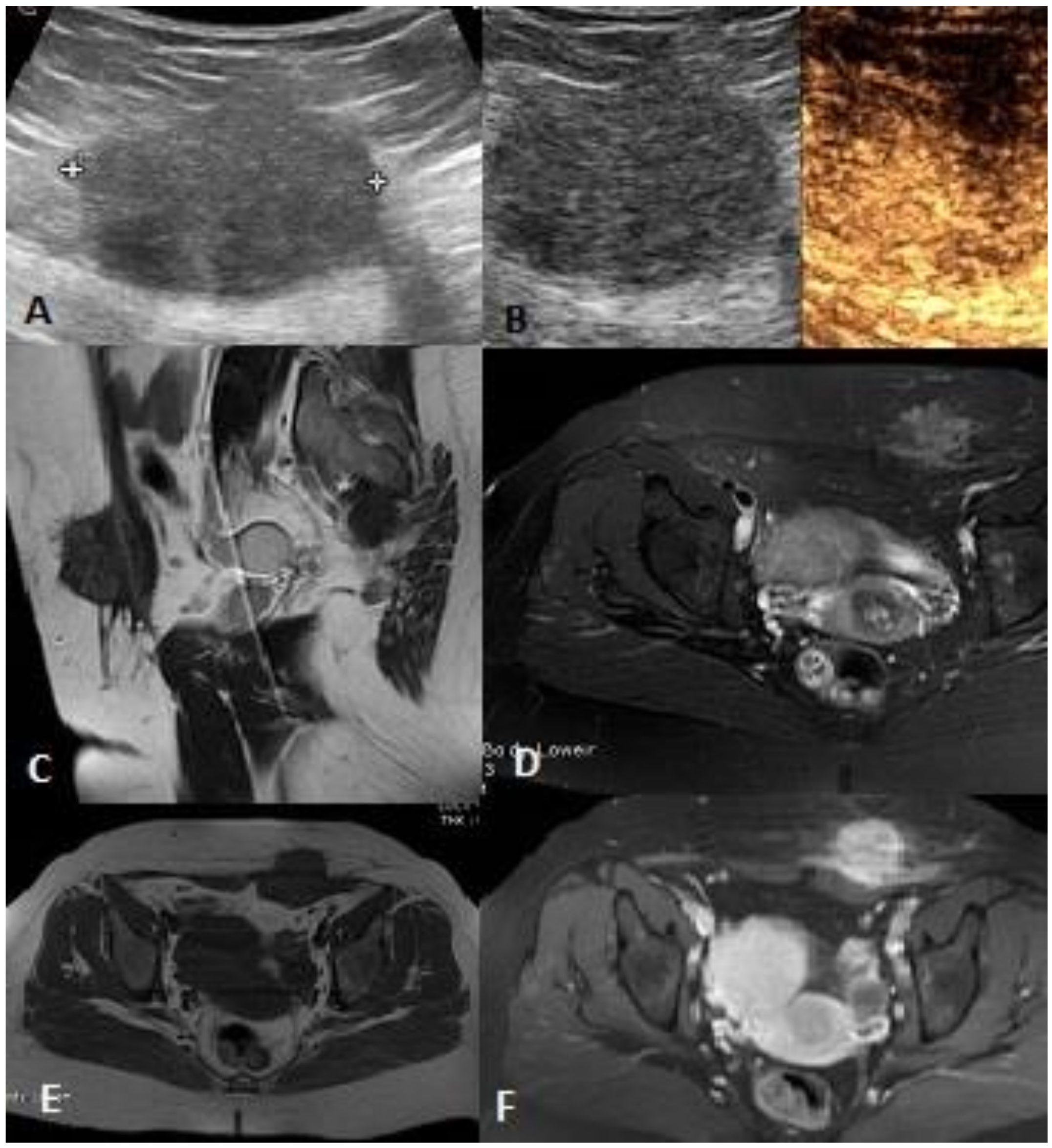
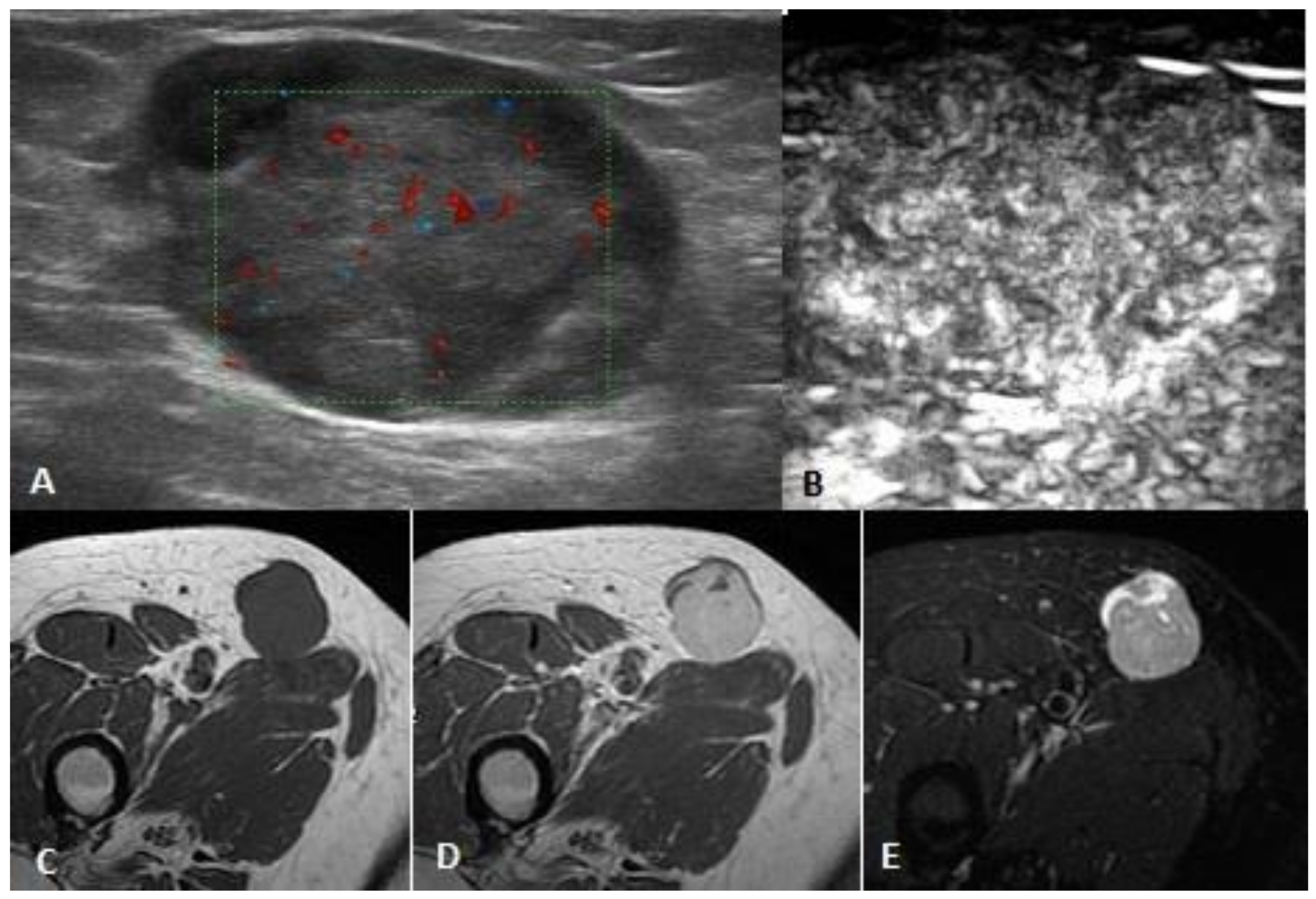
| Soft Tissue Sarcoma (STS) and Non STS Malignant Tumors | Total 36 |
|---|---|
| Liposarcoma | 6 |
| Primary Pleomorphic Sarcoma | 6 |
| Leiomyosarcoma | 4 |
| Dermatofibrous Sarcoma | 4 |
| Epitheliod Sarcoma | 2 |
| Myxofibrous Sarcoma | 2 |
| Carcinoma Metastasis | 1 |
| Lymphoma | 5 |
| Other malignant tumors | 6 |
| Benign and aggressive tumors and other Benign Non-Tumoral Lesions | Total 27 |
| Lipoma and Angiolipoma | 5 |
| Schwannoma | 2 |
| Fibroma | 1 |
| Leiomyoma | 1 |
| Hemangioma | 1 |
| Desmoid Tumor, also referred to as Extra-abdominal Aggressive Fibromatosis-Intermediate tumor (benign histology, aggressive in vivo behaviour) | 5 |
| Other benign tumors | 2 |
| Cysts | 1 |
| Flogistic lesions | 3 |
| Scar tissue, post surgery reaction | 3 |
| Haematoma | 1 |
| Endometriosis | 2 |
| Patient, US and PD Characteristics | All Patients (n = 63) | Benign (n = 27) | Malignant (n = 36) | p-Value (Ben vs Mal) |
|---|---|---|---|---|
| Gender (Female/Male) | 30/33 | 13/14 | 17/19 | 0.942 |
| Age (years) | 57.9 ± 15.9 | 51.7 ± 15.1 | 62.5 ± 15.1 | 0.006 |
| Location (upper vs lower part of the body) | 23/40 | 13/14 | 10/26 | 0.097 |
| Primary tumor/recurrent | 54/9 | 23/4 | 31/5 | 0.917 |
| US and PD variables | ||||
| Size (≤3.5/3.6–5/5.1–10/ > 10 cm) | 15/17/23/8 | 8/8/9/2 | 7/9/14/6 | 0.579 |
| Size (≤3.5 vs >3.5 cm) | 15/48 | 8/19 | 7/29 | 0.348 |
| Shape (fusiform/round/lobulated/soap_bubble/undefined) | 42/6/10/2/3 | 20/3/4/0/0 | 22/3/6/2/3 | 0.367 |
| Margins (regular/irregular infiltrating) | 44/19 | 18/9 | 26/10 | 0.634 |
| Echostructure (isoechoic/anechoic/hyperechoic/heterogeneous or hypoechoic) | 2/7/6/48 | 2/5/2/18 | 0/2/4/30 | 0.123 |
| Calcification (no/yes) | 55/8 | 23/4 | 32/4 | 0.662 |
| Vascularization (no/yes) | 25/38 | 12/15 | 13/23 | 0.503 |
| Septation (no/yes) | 51/12 | 23/4 | 28/8 | 0.459 |
| Tissue characteristcs (fat lesion/cyst/vascular lesions) ^ | 11/2/0 | 7/2/0 | 4/0/0 | 0.462 |
| Location in relation to the fascia (protective adipofascial system, PAFS/lubrificant adipofascial system, LAFS/both) | 15/14/34 | 6/8/13 | 9/6/21 | 0.469 |
| Relationship with the deep fascia (no contact, contact with acute angle, contact with larger acute angle, contact with obtuse angle) | 13/15/32/3 | 6/3/18/0 | 7/12/14/3 | 0.048 |
| CEUS and MRI Characteristics | All patients (n = 63) | Benign (n = 27) | Malignant (n = 36) | p-Value (Ben vs Mal) |
|---|---|---|---|---|
| CEUS variables | ||||
| Pattern (1/2/3/4/5/6/7) | 6/3/1/7/2/23/21 | 3/2/1/4/0/6/11 | 3/1/0/3/2/17/10 | 0.264 |
| Pattern dichotomy (1–5 probably benign vs. 6–7 probably malignant) | 19/44 | 10/17 | 9/27 | 0.303 |
| Vascularisation time (no or >20′ vs. <20′) | 20/43 | 9/18 | 11/25 | 0.815 |
| MRI variables | ||||
| Signal T1 (isointense/hypointense/hyperintense/inhomogeneous) | 3/49/5/4 | 2/20/3/0 | 1/29/2/4 | 0.227 |
| Signal T2 (isointense/hypointense/hyperintense/inhomogeneous) | 3/18/28/11 | 2/8/13/2 | 1/10/15/9 | 0.310 |
| STIR (homogeneous hypointense /inhomogeneous hypointense /homogeneous hyperintense/inhomogeneous hyperintense) | 7/6/27/18 | 2/3/10/10 | 5/3/17/8 | 0.520 |
| Gadolinium (no enhancement/homogeneous enhancement/inhomogeneous enhancement) * | 5/19/17 | 3/9/3 | 2/10/14 | 0.087 |
| Peritumoral edema (no/yes) | 42/20 | 19/8 | 23/12 | 0.697 |
| Septation (no/yes) | 46/16 | 23/4 | 23/12 | 0.082 |
| Fat rim (no/yes) | 18/44 | 5/22 | 13/22 | 0.109 |
| Margins (regular/irregular infiltrating) | 44/18 | 18/9 | 26/9 | 0.512 |
| MRI | Malignant | Benign | Total |
|---|---|---|---|
| Malignant | 19 | 7 | 26 |
| Benign | 17 | 20 | 37 |
| Total | 36 | 27 | 63 |
| Sensitivity | 52.8% | (37.0, 68.0) | |
| Specificity | 74.1% | (55.3, 86.8) | |
| Positive Predictive Value | 73.1% | (53.9, 86.3) | |
| Negative Predictive Value | 54.1% | (38.4, 69.0) | |
| CEUS | Malignant | Benign | Total |
|---|---|---|---|
| Malignant | 27 | 17 | 44 |
| Benign | 9 | 10 | 19 |
| Total | 36 | 27 | 63 |
| Sensitivity | 75.0% | (58.9, 86.3) | |
| Specificity | 37.0% | (21.5, 55.8) | |
| Positive Predictive Value | 61.4% | (46.6, 74.3) | |
| Negative Predictive Value | 52.6% | (31.7, 72.7) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marchi, A.; Pozza, S.; Charrier, L.; Cannone, F.; Cavallo, F.; Linari, A.; Piana, R.; Geniò, I.; Balocco, P.; Massè, A. Small Subcutaneous Soft Tissue Tumors (<5 cm) Can Be Sarcomas and Contrast-Enhanced Ultrasound (CEUS) Is Useful to Identify Potentially Malignant Masses. Int. J. Environ. Res. Public Health 2020, 17, 8868. https://doi.org/10.3390/ijerph17238868
De Marchi A, Pozza S, Charrier L, Cannone F, Cavallo F, Linari A, Piana R, Geniò I, Balocco P, Massè A. Small Subcutaneous Soft Tissue Tumors (<5 cm) Can Be Sarcomas and Contrast-Enhanced Ultrasound (CEUS) Is Useful to Identify Potentially Malignant Masses. International Journal of Environmental Research and Public Health. 2020; 17(23):8868. https://doi.org/10.3390/ijerph17238868
Chicago/Turabian StyleDe Marchi, Armanda, Simona Pozza, Lorena Charrier, Filadelfo Cannone, Franco Cavallo, Alessandra Linari, Raimondo Piana, Irene Geniò, Paolo Balocco, and Alessandro Massè. 2020. "Small Subcutaneous Soft Tissue Tumors (<5 cm) Can Be Sarcomas and Contrast-Enhanced Ultrasound (CEUS) Is Useful to Identify Potentially Malignant Masses" International Journal of Environmental Research and Public Health 17, no. 23: 8868. https://doi.org/10.3390/ijerph17238868
APA StyleDe Marchi, A., Pozza, S., Charrier, L., Cannone, F., Cavallo, F., Linari, A., Piana, R., Geniò, I., Balocco, P., & Massè, A. (2020). Small Subcutaneous Soft Tissue Tumors (<5 cm) Can Be Sarcomas and Contrast-Enhanced Ultrasound (CEUS) Is Useful to Identify Potentially Malignant Masses. International Journal of Environmental Research and Public Health, 17(23), 8868. https://doi.org/10.3390/ijerph17238868






