Factors Associated with In-Hospital Mortality after Continuous Renal Replacement Therapy for Critically Ill Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategies
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Data Analysis
2.5. Assessment of Methodological Quality
3. Results
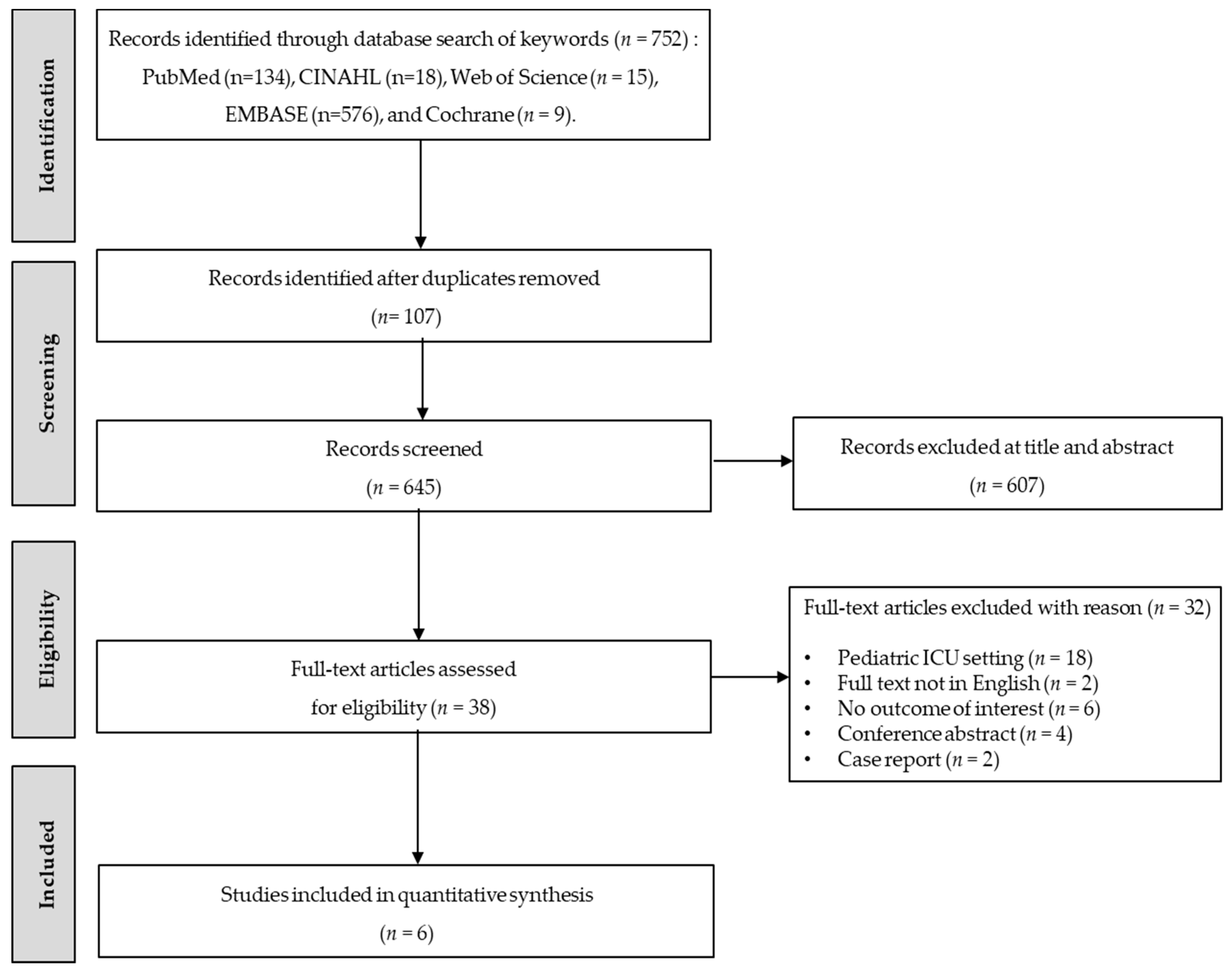
3.1. Literature Search
3.2. Characteristics of the Included Studies
3.3. Risk Factors for In-Hospital Mortality
3.3.1. Age
3.3.2. Body Mass Index
3.3.3. APACHE II
3.3.4. SOFA
3.3.5. Systolic Blood Pressure
3.3.6. Diastolic Blood Pressure
3.3.7. Serum Creatinine Level
3.3.8. Serum Sodium Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tandukar, S.; Palevsky, P.M. Continuous renal replacement therapy: Who, when, why, and how. Chest 2019, 155, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.R.; Leem, A.Y.; Park, M.S.; Kim, Y.S.; Chung, K.S. Optimal timing of initiating continuous renal replacement therapy in septic shock patients with acute kidney injury. Sci. Rep. 2019, 9, 11981. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Jang, G.S.; An, Y.J.; Han, M.; Park, I.; Kim, I.Y.; Seong, E.Y.; Lee, D.W.; Lee, S.B.; Kwak, I.S.; et al. Long-term outcomes in acute kidney injury patients who underwent continuous renal replacement therapy: A single-center experience. Clin. Exp. Nephrol. 2018, 22, 1411–1419. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, P.; Yang, Y.; Jiang, H.; He, Y.; Xu, C.; Yan, H.; Guo, Q.; Luo, Q.; Chen, J. Long-term renal and overall survival of critically ill patients with acute renal injury who received continuous renal replacement therapy. Ren. Fail. 2017, 39, 736–744. [Google Scholar] [CrossRef]
- Hansrivijit, P.; Yarlagadda, K.; Puthenpura, M.M.; Ghahramani, N.; Thongprayoon, C.; Vaitla, P.; Cheungpasitporn, W. A meta-analysis of clinical predictors for renal recovery and overall mortality in acute kidney injury requiring continuous renal replacement therapy. J. Crit. Care 2020, 16, 13–22. [Google Scholar] [CrossRef]
- Ahmed, A.R.; Obilana, A.; Lappin, D. Renal replacement therapy in the critical care setting. Crit. Care Res. Pract. 2019, 2019, 6948710. [Google Scholar] [CrossRef]
- Mottes, T.A.; Goldstein, S.L.; Basu, R.K. Process based quality improvement using a continuous renal replacement therapy dashboard. BMC Nephrol. 2019, 20, 17. [Google Scholar] [CrossRef]
- Gemmell, L.; Docking, R.; Black, E. Renal replacement therapy in critical care. BJA Educ. 2017, 17, 88–93. [Google Scholar] [CrossRef]
- Karkar, A.; Ronco, C. Prescription of CRRT: A pathway to optimize therapy. Ann. Intensive Care 2020, 10, 32. [Google Scholar] [CrossRef]
- Prasad, B.; Urbanski, M.; Ferguson, T.W.; Karreman, E.; Tangri, N. Early mortality on continuous renal replacement therapy (CRRT): The prairie CRRT study. Can. J. Kidney Health Dis. 2016, 3, 36. [Google Scholar] [CrossRef]
- Siddiqui, A.H.; Valecha, G.; Modi, J.; Saqib, A.; Weerasinghe, C.; Siddiqui, F.; El Sayegh, S. Predictors of 15-day survival for the intensive care unit patient on continuous renal replacement therapy: A retrospective analysis. Cureus 2020, 12, e8175. [Google Scholar] [CrossRef] [PubMed]
- Slessarev, M.; Salerno, F.; Ball, I.M.; McIntyre, C.W. Continuous renal replacement therapy is associated with acute cardiac stunning in critically ill patients. Hemodial. Int. 2019, 23, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Duyu, M.; Turkozkan, C. Clinical features and risk factors associated with mortality in critically ill children requiring continuous renal replacement therapy. Res. Sq. 2020, 1–20. [Google Scholar] [CrossRef]
- Kao, C.C.; Yang, J.Y.; Chen, L.; Chao, C.T.; Peng, Y.S.; Chiang, C.K.; Huang, J.W.; Hung, K.Y. Factors associated with poor outcomes of continuous renal replacement therapy. PLoS ONE 2017, 24, e0177759. [Google Scholar] [CrossRef]
- Prowle, J.R.; Bellomo, R. Continuous renal replacement therapy: Recent advances and future research. Nat. Rev. Nephrol. 2010, 6, 521–529. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses; the PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Haynes, R.B.; Sacket, D.L.; Guyatt, G.H.; Tugwell, P. Clinical Epidemiology: How Clinical Practice Research, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; ISBN 078-174-524-1. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 June 2019).
- Lin, Y.F.; Ko, W.J.; Chu, T.S.; Chen, Y.S.; Wu, V.C.; Chen, Y.M.; Wu, M.S.; Chen, Y.M.; Tsai, C.W.; Shiao, C.C.; et al. The 90-day mortality and the subsequent renal recovery in critically ill surgical patients requiring acute renal replacement therapy. Am. J. Surg. 2009, 198, 325–332. [Google Scholar] [CrossRef]
- Kritmetapak, K.; Peerapornratana, S.; Srisawat, N.; Somlaw, N.; Lakananurak, N.; Dissayabutra, T.; Phonork, C.; Leelahavanichkul, A.; Tiranathanagul, K.; Susantithapong, P.; et al. The impact of macro-and micronutrients on predicting outcomes of critically ill patients requiring continuous renal replacement therapy. PLoS ONE 2016, 11, e0156634. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Chen, Q.; Chen, M.; Cheng, L.; Jiang, H.; Sun, S. D-dimer is a predictor of 28-day mortality in critically ill patients receiving continuous renal replacement therapy. Arch. Med. Res. 2016, 47, 356–364. [Google Scholar] [CrossRef]
- Cho, A.Y.; Yoon, H.J.; Lee, K.Y.; Sun, I.O. Clinical characteristics of sepsis-induced acute kidney injury in patients undergoing continuous renal replacement therapy. Ren. Fail. 2018, 40, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kee, Y.K.; Kim, D.; Kim, S.J.; Kang, D.K.; Choi, K.B.; Oh, H.J.; Ryu, D.R. Factors associated with early mortality in critically ill patients following the initiation of continuous renal replacement therapy. J. Clin. Med. 2018, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Keleshian, V.; Kashani, K.B.; Kompotiatis, P.; Barsness, G.W.; Jentzer, J.C. Short, and long-term mortality among cardiac intensive care unit patients started on continuous renal replacement therapy. J. Crit. Care 2020, 55, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Eldadah, B.; Halter, J.B.; Hazzard, W.R.; Himmelfarb, J.; Horne, F.M.; Kimmel, P.L.; Molitoris, B.A.; Murthy, M.; O’Hare, A.M.; et al. Acute kidney injury in older adults. J. Am. Soc. Nephrol. 2011, 21, 28–38. [Google Scholar] [CrossRef]
- Carlson, N.; Hommel, K.; Olesen, J.B.; Soja, A.M.; Vilsbøll, T.; Kamper, A.-L.; Torp-Pedersen, C.; Gislason, G. Dialysis- requiring acute kidney injury in Denmark 2000–2012: Time trends of incidence and prevalence of risk factors—A nationwide Study. PLoS ONE 2016, 11, e0148809. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.; Kim, Y. The effect of nutritional supply on clinical outcomes and nutritional status in critically ill patients receiving continuous renal replacement therapy. J. Nutr. Health 2015, 48, 211–220. [Google Scholar] [CrossRef]
- Compher, C.; Higashiguch, T.; Yu, J.; Jensen, G.L. Does low body mass index predict the hospital mortality of adult Western or Asian patients? JPEN J. Parenter. Enter. Nutr. 2018, 42, 467–472. [Google Scholar] [CrossRef]
- Rhee, H.; Jang, K.S.; Park, J.M.; Kang, J.S.; Hwang, N.K.; Kim, I.Y.; Song, S.H.; Seong, E.Y.; Lee, D.W.; Lee, S.B.; et al. Short- and long-term mortality rates of elderly acute kidney injury patients who underwent continuous renal replacement therapy. PLoS ONE 2016, 11, e0167067. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- Anzueto, A.; Frutos-Vivar, F.; Esteban, A.; Bensalami, N.; Marks, D.; Raymondos, K.; Apezteguía, C.; Arabi, Y.; Hurtado, J.; González, M.; et al. Influence of body mass index on outcome of the mechanically ventilated patient. Thorax 2011, 66, 66–73. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Bai, Z.H.; Lv, J.H.; Sun, J.L.; Pei, H.H.; Zhang, Z.L. Higher body mass index is not a protective risk factor for 28-days mortality in critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren. Fail. 2019, 41, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, H.I.; Mahmood, K.; Ziaullaha, S.; Kashif, S.M.; Sharif, A. Better prognostic marker in ICU—APACHE II, SOFA or SAP II! Pak. J. Med. Sci. 2016, 32, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Bahtouee, M.; Eghbali, S.S.; Maleki, N.; Rastgou, V.; Motamed, N. Acute physiology and chronic health evaluation II score for the assessment of mortality prediction in the intensive care unit: A single-centre study from Iran. Nurs. Crit. Care 2019, 24, 375–380. [Google Scholar] [CrossRef]
- Singh, S.; Patra, A.K.; Patel, B.; Ramesh, G.S.; Sharma, V.K.; Ravishankar, V.; Bassannar, D. Acute renal failure in the ICU setting: A prospective observational study. Med. J. Armed Forces India 2016, 72, 236–241. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Kang, X.; Shi, Y.; Bai, Z.H.; Lv, J.H.; Sun, J.L.; Pei, H.H. SOFA score is superior to APACHE-II score in predicting the prognosis of critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren. Fail. 2020, 42, 638–645. [Google Scholar] [CrossRef]
- Reilly, R.F. Attending rounds: A patient with intradialytic hypotension. Clin. J. Am. Soc. Nephrol. 2014, 9, 798–803. [Google Scholar] [CrossRef]
- Stefánsson, B.V.; Brunelli, S.M.; Cabrera, C.; Rosenbaum, D.; Anum, E.; Ramakrishnan, K.; Jensen, D.E.; Stålhammar, N. Intradialytic hypotension and risk of cardiovascular disease. Clin. J. Am. Soc. Nephrol. 2014, 9, 2124–2132. [Google Scholar] [CrossRef]
- Shawwa, K.; Kompotiatis, P.; Jentzer, J.C.; Wiley, B.M.; Williams, A.W.; Dillon, J.J.; Albright, R.C.; Kashani, K.B. Hypotension within one-hour from starting CRRT is associated with in-hospital mortality. J. Crit. Care 2019, 54, 7–13. [Google Scholar] [CrossRef]
- Wang, X.T.; Wang, C.; Zhang, H.M.; Liu, D.W. Clarifications on continuous renal replacement therapy and hemodynamics. Chin. Med. J. 2017, 130, 1244–1248. [Google Scholar] [CrossRef]
- Lindner, G.; Funk, G.C.; Schwarz, C.; Kneidinger, N.; Kaider, A.; Schneeweiss, B.; Kramer, L.; Druml, W. Hypernatremia in the critically ill is an independent risk factor for mortality. Am. J. Kidney Dis. 2007, 50, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Bae, E.; Kim, D.K.; Kim, Y.S.; Han, J.S.; Joo, K.W. Dysnatremia, its correction, and mortality in patients undergoing continuous renal replacement therapy: A prospective observational study. BMC Nephrol. 2016, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Sileanu, F.E.; Murugan, R.; Lucko, N.; Shaw, A.W.; Clermont, G. Classifying AKI by urine output versus serum creatinine level. J. Am. Soc. Nephrol. 2015, 26, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.; Kellum, J.A. Acute kidney injury: Epidemiology and diagnostic criteria. Curr. Opin. Crit. Care 2006, 12, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.R.; Letteri, J.J.; Uchino, S.; Bellomo, R.; Ronco, C. Recent clinical advances in the management of critically ill patients with acute renal failure. Blood Purif. 2006, 24, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Y. Effect of renal replacement therapy modalities on renal recovery and mortality for acute kidney injury: A PRISMA-compliant systematic review and meta-analysis. Semin. Dial. 2020, 33, 127–132. [Google Scholar] [CrossRef] [PubMed]
| Authors (Year)/Country or Territories | Study Design | Follow-Up Period (Months) | Sample Characteristics | Indication for Initiation of CRRT | CRRT Modality | In-Hospital Mortality Rate (%) | NOS Quality | |
|---|---|---|---|---|---|---|---|---|
| Survivors | Non-Survivors | |||||||
| Lin et al. (2009)/Taiwan [20] | Prospective | 60 | n = 137 Mean age: 61.1 ± 14.8 (years) Male: 85 (62.0%) Female: 52 (38.0%) | n = 205 Mean age: 65.9 ± 15.5 (years) Male: 119 (58.0%) Female: 86 (42.0%) | Azotemia (BUN 80 mg/dL and serum creatinine 2 mg/dL, without evidence of dehydration), uremic symptoms, fluid overload refractory to diuretic use with a central venous pressure 14 mm Hg or pulmonary edema with a PaO2/FiO2 300 mmHg, hyperkalemia (serum K 5.5 mmol/L) refractory to medical treatment, oliguria(urine amount 200 mL/8 h) refractory to diuretics, metabolic acidosis (pH 7.2 in arterial blood gas) | CVVH | 59.9 (90 days) | 9 |
| Kritmetapak et al. (2016)/Thailand [21] | Prospective | 13 | N: 27 Mean age: 57.3 ±16.8 (years) Male: 20 (74.1%) Female: 7 (25.9%) | N: 43 Mean age: 62.8 ± 16.8 (years) Male: 27 (62.8%) Female: 16 (37.2%) | Hemodynamically unstable patients with refractory fluid overload, severe hyperkalemia, severe metabolic acidosis, severe azotemia, and uremic symptoms | CVVH | 38.6 (28 days) | 8 |
| Lu et al. (2016)/China [22] | Retrospective | 13 | N: unreported Mean age: 57 ± 14.4 (years) Male: unreported Female: unreported | N: unreported Mean age: 57 ± 14.4 (years) Male: unreported Female: unreported | Eliminating inflammatory mediators, cytokines, alleviating edema, protecting renal function | CVVH, CVVHDF | Unreported (28 days) | 8 |
| Cho et al. (2018)/Korea [23] | Retrospective | 60 | N = 128 Mean age: 64 ± 14 (years) Male: 79 (61.7%) Female: 49 (38.35) | N = 212 Mean age: 69 ± 12 (years) Male: 135 (63.7%) Female: 77 (36.3%) | Oliguria (urine output < 100 mL in a six-hour period and unresponsive to fluid resuscitation), serum potassium concentration > 6.5 mmol/L, severe acidemia (pH < 7.2), or presence of severe organ edema (e.g., pulmonary edema), severe sepsis associated with acute organ dysfunction and septic shock as sepsis with acute circulatory failure characterized by persistent arterial hypotension | CVVHDF | 62.4 (28 days) | 7 |
| Kee et al. (2018)/Korea [24] | Retrospective | 17 | N = 110 Mean age: 65.7 ± 15.3 (years) Male: 72 (65.5%) Female: 38 (34.5%) | N = 130 Mean age: 65.9 ± 14.2 (years) Male: 78 (60%) Female: 52 (40%) | Medically intractable or persistent electrolyte imbalance and/or metabolic acidosis, and decreased urine output with volume overload and/or progressive azotemia | CVVHDF | 54.2 (7 days) | 8 |
| Keleshian et al. (2020)/USA [25] | Retrospective | 109 | N = 93 Mean age: 61.5 (years) Male: 63 (67.7%) Female: 30 (32.3%) | N = 105 Mean age: 64.8 (years) Male: 66 (62.9%) Female: 39 (37.1%) | Unreported | Unreported | 53.0 (in-hospital) | 8 |
| Risk Factors | No. of Studies | No. of Participants | OR/SMD | 95% CI | I2 (%) | p-Value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (years) | 4 | 992 | 0.26 * | 0.07 to 0.44 | 47.3 | 0.127 |
| BMI (kg/m2) | 3 | 652 | −0.17 * | −0.33 to −0.01 | 3.7 | 0.354 |
| Male | 5 | 1190 | 0.87 | 0.69 to 1.11 | 0 | 0.080 |
| Female | 5 | 1190 | 1.15 | 0.90 to 1.46 | 0 | 0.080 |
| Severity scoring | ||||||
| APACHE II | 3 | 752 | 1.05 * | 0.36 to 1.75 | 94.0 | <0.001 |
| SOFA | 2 | 752 | 1.06 * | 0.61 to 1.51 | 85.3 | <0.001 |
| Comorbidities | ||||||
| Diabetes mellitus, yes | 4 | 848 | 0.79 | 0.59 to 1.07 | 0 | 0.463 |
| Hypertension, yes | 3 | 650 | 1.11 | 0.67 to 1.86 | 53.0 | 0.119 |
| Heart failure, yes | 3 | 778 | 1.09 | 0.76 to 1.57 | 0 | 0.668 |
| Liver disease, yes | 2 | 410 | 1.94 | 0.77 to 4.90 | 50.1 | 0.157 |
| Sepsis, yes | 4 | 992 | 1.55 | 0.86 to 2.77 | 60.0 | 0.058 |
| CAD, yes | 2 | 268 | 0.61 | 0.33 to 1.10 | 0 | 0.879 |
| COPD, yes | 2 | 438 | 0.96 | 0.41 to 2.24 | 0 | 0.892 |
| Hemodynamic and clinical characteristics | ||||||
| Systolic BP (mmHg) | 2 | 580 | −0.38 * | −0.55 to −0.22 | 1.2 | 0.314 |
| Diastolic BP (mmHg) | 2 | 580 | −0.77 * | −0.43 to −0.10 | 0 | 0.555 |
| Hemoglobin (g/dL) | 2 | 580 | 0.04 * | −0.12 to 0.21 | 0 | 0.975 |
| White blood cell (103/mL) | 2 | 580 | 0.06 * | −0.16 to 0.27 | 40.2 | 0.196 |
| Platelet (103/mL) | 2 | 580 | −0.25 * | −0.72 to 0.23 | 87.6 | 0.005 |
| Serum creatinine (mg/dL) | 3 | 752 | −0.34 * | −0.48 to −0.19 | 0 | 0.541 |
| Serum sodium (mmol/L) | 2 | 580 | 0.21 * | 0.04 to 0.37 | 0 | 0.987 |
| Serum potassium (mmol/L) | 2 | 580 | −0.08 * | −0.24 to 0.09 | 0 | 0.427 |
| Serum calcium (mg/dL) | 2 | 580 | 0.08 * | −0.21 to 0.36 | 65.1 | 0.090 |
| Serum phosphate (mg/dL) | 2 | 580 | 0.14 * | −0.22 to 0.50 | 78.1 | 0.033 |
| Total bilirubin (mg/dL) | 2 | 580 | 0.21 * | −0.07 to 0.49 | 63.8 | 0.096 |
| Reasons for CRRT | ||||||
| Fluid overload | 3 | 610 | 1.22 | 0.86 to 1.74 | 0 | 0.488 |
| Severe acidosis | 3 | 752 | 1.20 | 0.58 to 2.48 | 27.4 | 0.252 |
| Oliguria | 3 | 752 | 0.83 | 0.47 to 1.47 | 47.4 | 0.149 |
| ICU mechanical assist device | ||||||
| ECMO or IABP, yes | 2 | 540 | 1.45 * | 0.77 to 2.88 | 61.5 | 0.107 |
| Age (Years) | ||||||
| Study | SMD | Lower Limit | Upper Limit | Z-Value | p-Value | SMD and 95% CI |
| Lin et al. (2009) [20] | 0.32 | 0.10 | 0.53 | 2.84 | 0.005 |  |
| Kritmetapak et al. (2016) [21] | 0.33 | −0.16 | 0.81 | 1.33 | 0.185 | |
| Cho et al. (2018) [23] | 0.39 | 0.17 | 0.61 | 3.46 | 0.001 | |
| Kee et al. (2018) [24] | 0.00 | −0.25 | 0.25 | 0.00 | 1.000 | |
| Total | 0.26 | 0.07 | 0.44 | 2.71 | 0.007 | |
| I2 = 47.3%, p = 0.127 | ||||||
| BMI (kg/m2) | ||||||
| Study | SMD | Lower Limit | Upper Limit | Z-Value | p-Value | SMD and 95% CI |
| Lin et al. (2009) [20] | −0.26 | −0.48 | −0.05 | −2.39 | 0.017 |  |
| Kritmetapak et al. (2016) [21] | −0.24 | −0.72 | 0.25 | −0.96 | 0.338 | |
| Kee et al. (2018) [24] | −0.02 | −0.28 | 0.23 | −0.18 | 0.857 | |
| Total | −0.17 | −0.33 | −0.01 | −2.07 | 0.038 | |
| I2 = 3.7%, p = 0.354 | ||||||
| APACHE II Score | ||||||
| Study | SMD | Lower Limit | Upper Limit | Z-Value | p-Value | SMD and 95% CI |
| Lin et al. (2009) [20] | 0.64 | 0.42 | 0.86 | 5.64 | <0.001 |  |
| Kritmetapak et al. (2016) [21] | 2.36 | 1.74 | 2.98 | 7.47 | <0.001 | |
| Cho et al. (2018) [23] | 0.42 | 0.20 | 0.64 | 3.70 | <0.001 | |
| Total | 1.05 | 0.36 | 1.75 | 2.96 | 0.003 | |
| I2 = 94.0%, p < 0.001 | ||||||
| SOFA Score | ||||||
| Study | SMD | Lower Limit | Upper Limit | Z-Value | p-Value | SMD and 95% CI |
| Lin et al. (2009) [20] | 0.83 | 0.60 | 1.05 | 7.19 | <0.001 |  |
| Kritmetapak et al. (2016) [21] | 1.87 | 1.32 | 2.47 | 6.47 | <0.001 | |
| Cho et al. (2018) [23] | 0.74 | 0.51 | 0.97 | 6.40 | <0.001 | |
| Total | 1.06 | 0.61 | 1.51 | 4.60 | <0.001 | |
| I2 = 85.3%, p = 0.001 | ||||||
| Systolic BP (mmHg) | ||||||
| Study | SMD | Lower Limit | Upper Limit | Z-Value | p-Value | SMD and 95% CI |
| Cho et al. (2018) [23] | −0.31 | −0.53 | −0.09 | −2.75 | 0.006 |  |
| Kee et al. (2018) [24] | −0.48 | −0.74 | −0.23 | −3.68 | <0.001 | |
| Total | −0.38 | −0.55 | −0.22 | −4.46 | <0.001 | |
| I2 = 1.2%, p = 0.314 | ||||||
| Diastolic BP (mmHg) | ||||||
| Study | SMD | Lower Limit | Upper Limit | Z-Value | p-Value | SMD and 95% CI |
| Cho et al. (2018) [23] | −0.22 | −0.44 | −0.01 | −2.00 | 0.046 |  |
| Kee et al. (2018) [24] | −0.33 | −0.58 | −0.01 | −2.50 | 0.013 | |
| Total | −0.27 | −0.43 | −0.10 | −3.14 | 0.002 | |
| I2 = 0%, p = 0.555 | ||||||
| Serum Creatinine (mg/dL) | ||||||
| Study | SMD | Lower Limit | Upper Limit | Z-Value | p-Value | SMD and 95% CI |
| Lin et al. (2009) [20] | −0.29 | −0.51 | −0.07 | −2.61 | 0.009 |  |
| Kritmetapak et al. (2016) [21] | −0.59 | −1.08 | −0.10 | −2.37 | 0.018 | |
| Cho et al. (2018) [23] | −0.33 | −0.55 | −0.11 | −2.92 | 0.004 | |
| Total | −0.34 | −0.48 | −0.19 | −4.44 | <0.001 | |
| I2 = 0%, p = 0.541 | ||||||
| Serum Sodium (mmol/L) | ||||||
| Study | SMD | Lower Limit | Upper Limit | Z-Value | p-Value | SMD and 95% CI |
| Cho et al. (2018) [23] | 0.20 | −0.02 | 0.42 | 1.82 | 0.069 |  |
| Kee et al. (2018) [24] | 0.21 | −0.05 | 0.46 | 1.59 | 0.111 | |
| Total | 0.21 | 0.04 | 0.37 | 2.42 | 0.016 | |
| I2 = 0%, p = 0.987 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Son, Y.-J. Factors Associated with In-Hospital Mortality after Continuous Renal Replacement Therapy for Critically Ill Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8781. https://doi.org/10.3390/ijerph17238781
Lee H-J, Son Y-J. Factors Associated with In-Hospital Mortality after Continuous Renal Replacement Therapy for Critically Ill Patients: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(23):8781. https://doi.org/10.3390/ijerph17238781
Chicago/Turabian StyleLee, Hyeon-Ju, and Youn-Jung Son. 2020. "Factors Associated with In-Hospital Mortality after Continuous Renal Replacement Therapy for Critically Ill Patients: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 23: 8781. https://doi.org/10.3390/ijerph17238781
APA StyleLee, H.-J., & Son, Y.-J. (2020). Factors Associated with In-Hospital Mortality after Continuous Renal Replacement Therapy for Critically Ill Patients: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 17(23), 8781. https://doi.org/10.3390/ijerph17238781





