Photochemical Generation of Methyl Chloride from Humic Aicd: Impacts of Precursor Concentration, Solution pH, Solution Salinity and Ferric Ion
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Irradiation Experiments
2.3. Analysis Methods
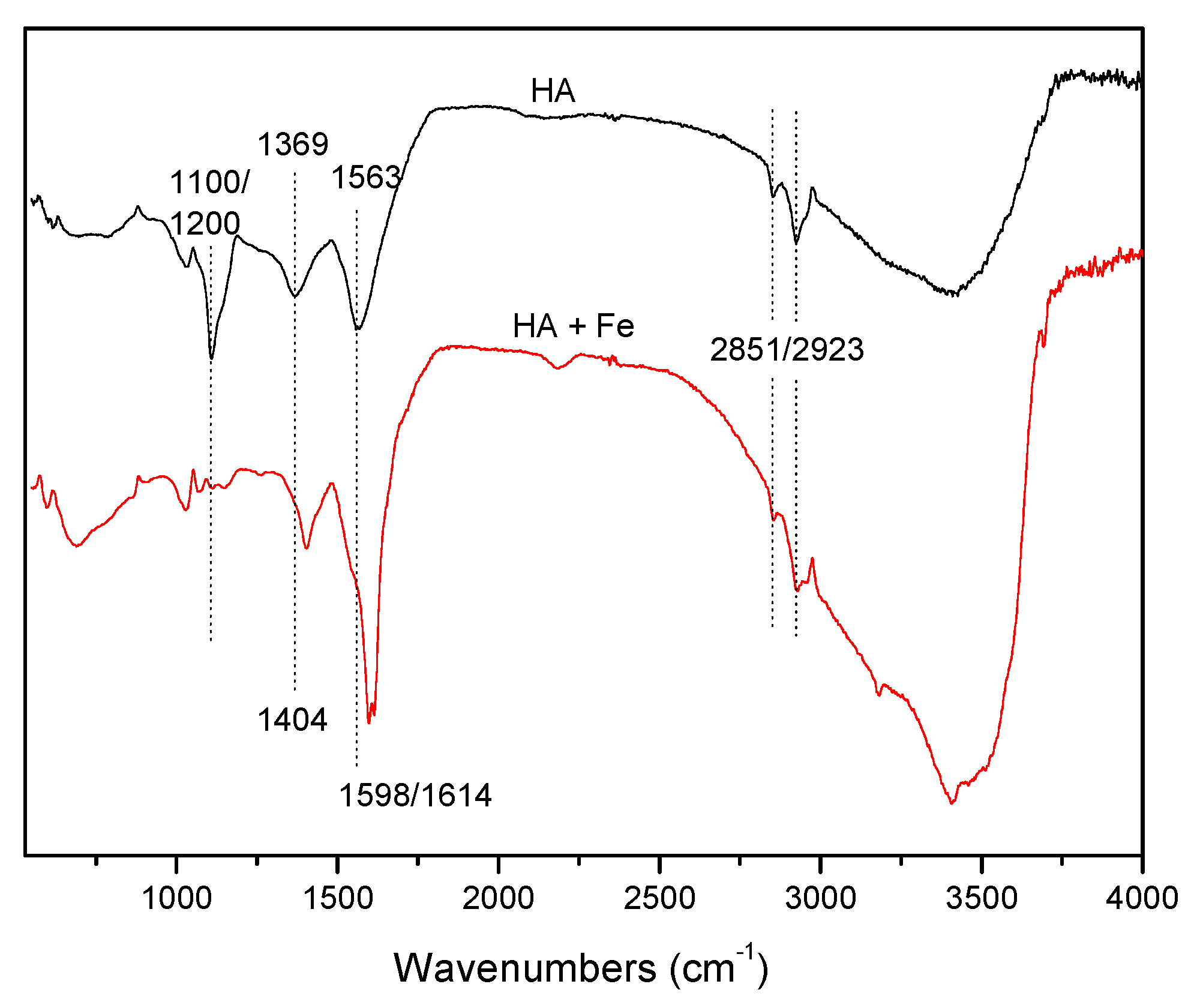
2.4. Characteristics of HA and HA-Fe (III)
3. Results and Discussion
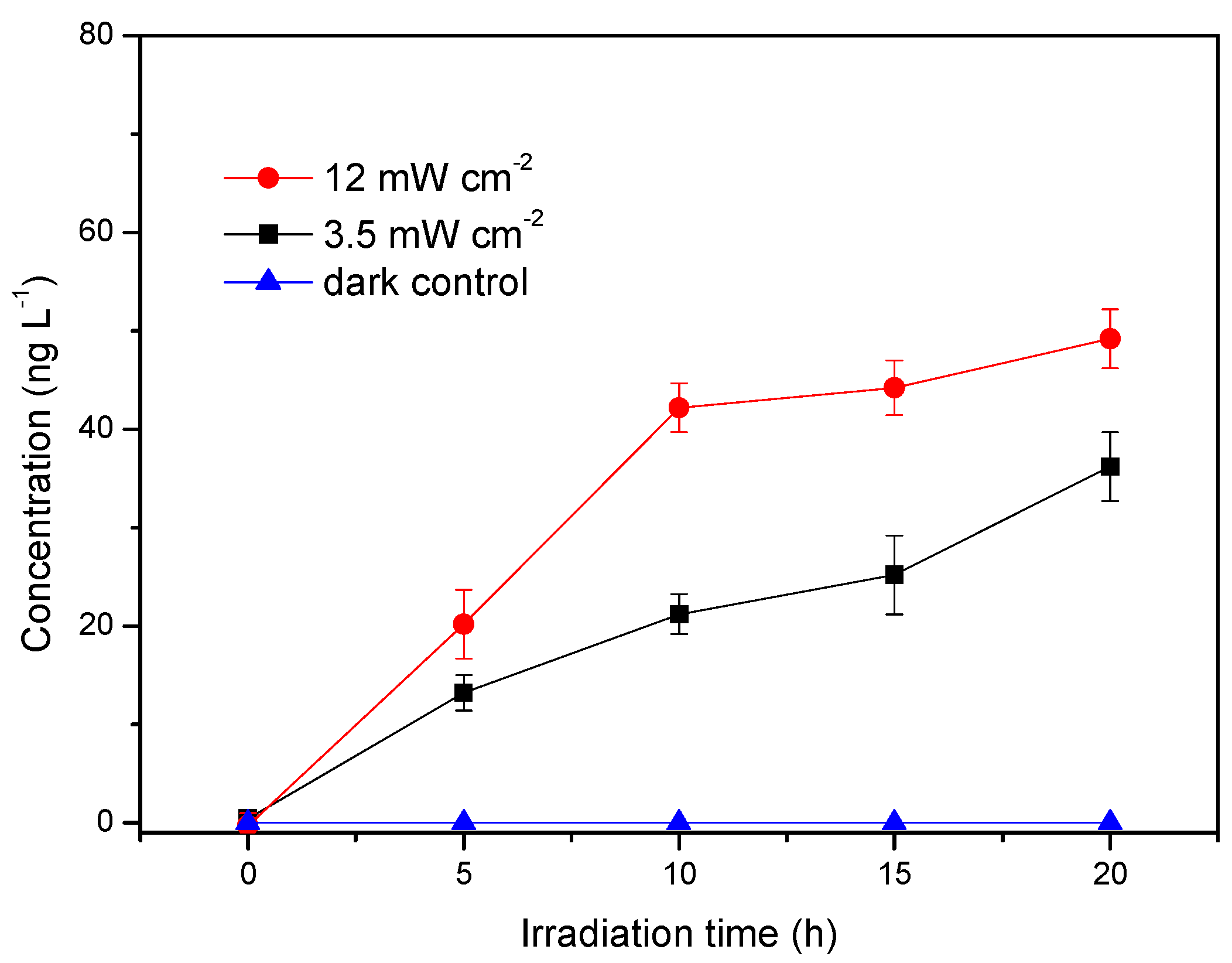
3.1. Influence of Light Intensity
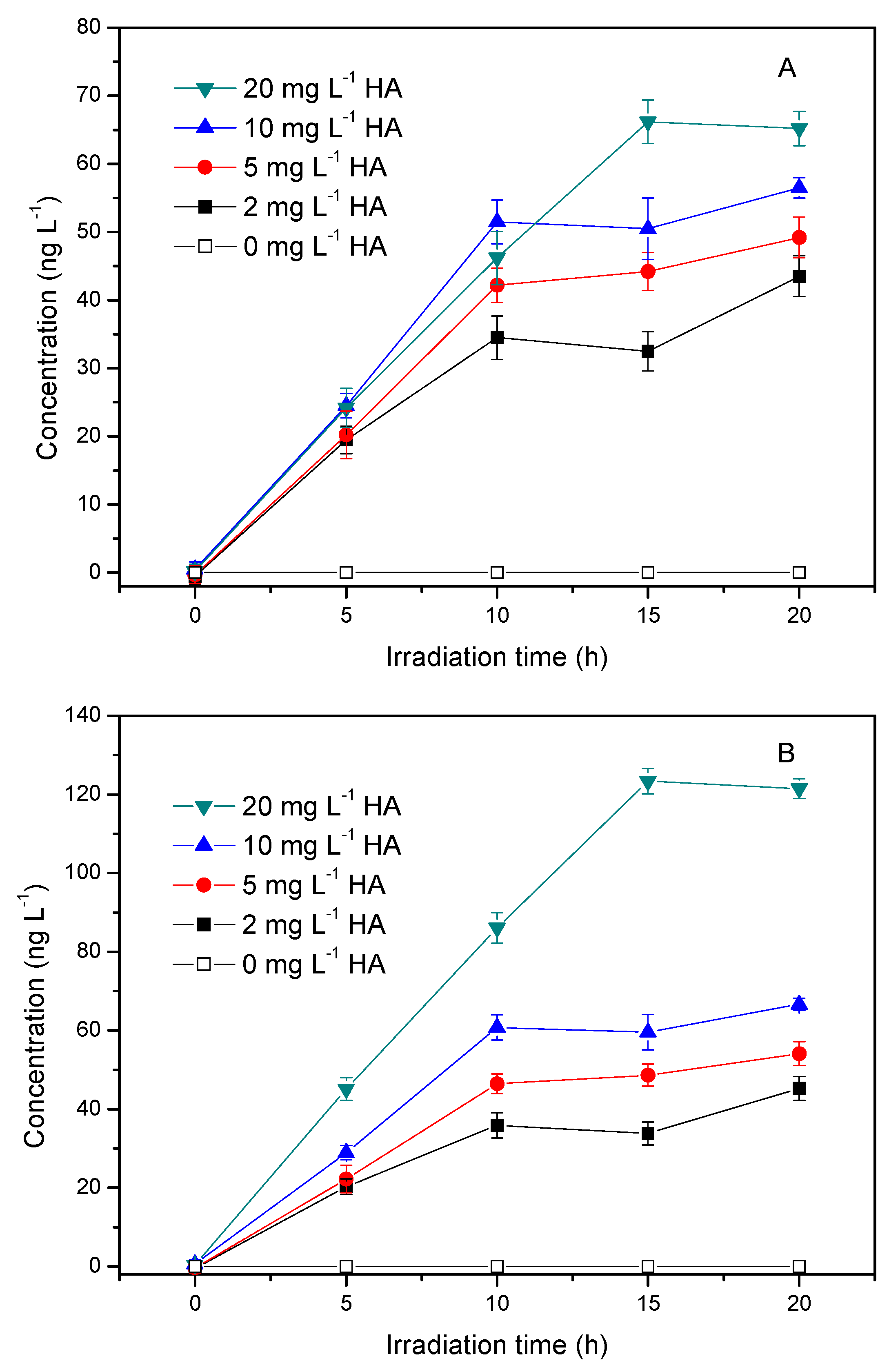
3.2. Influence of HA Concentration
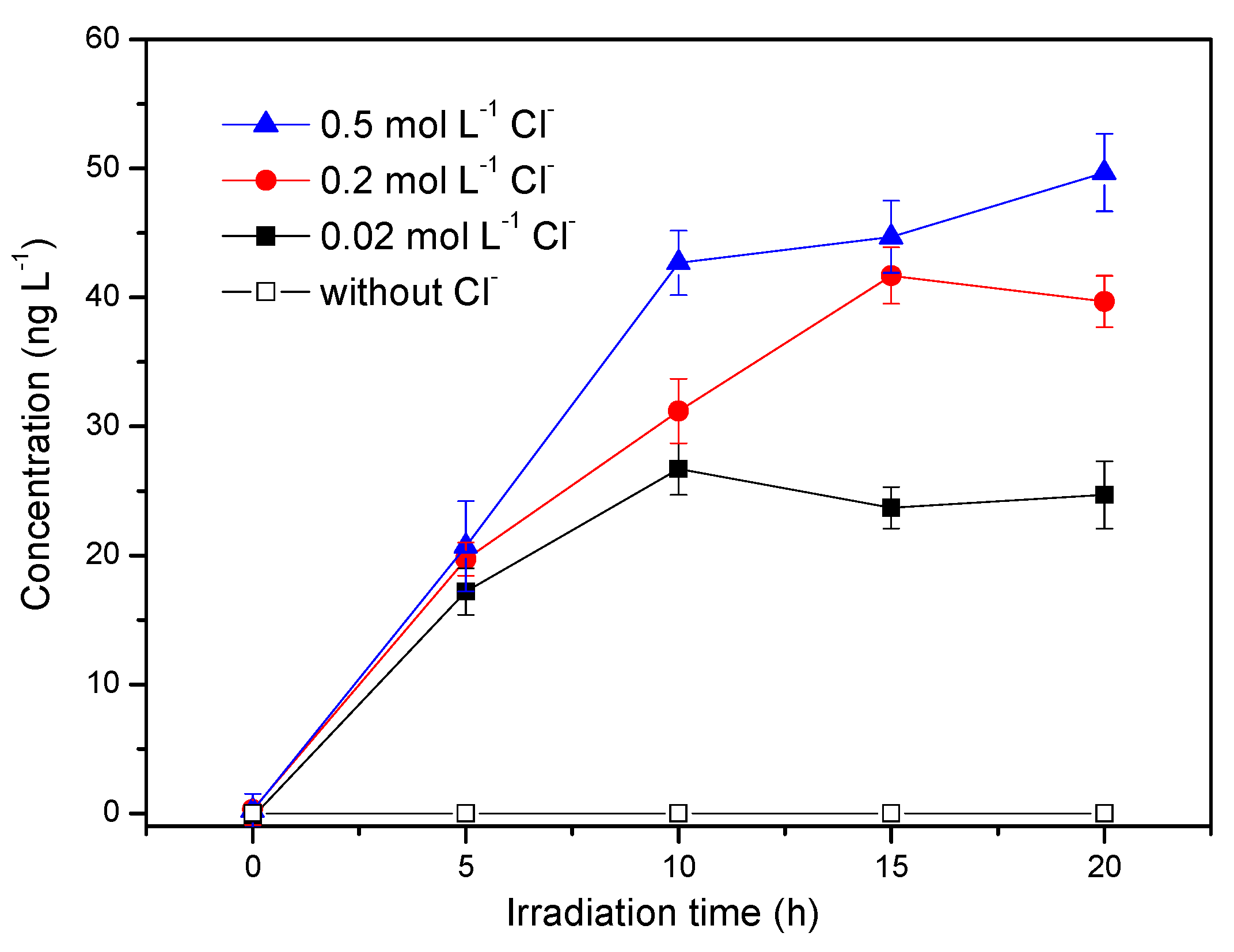
3.3. Influence of Chloride
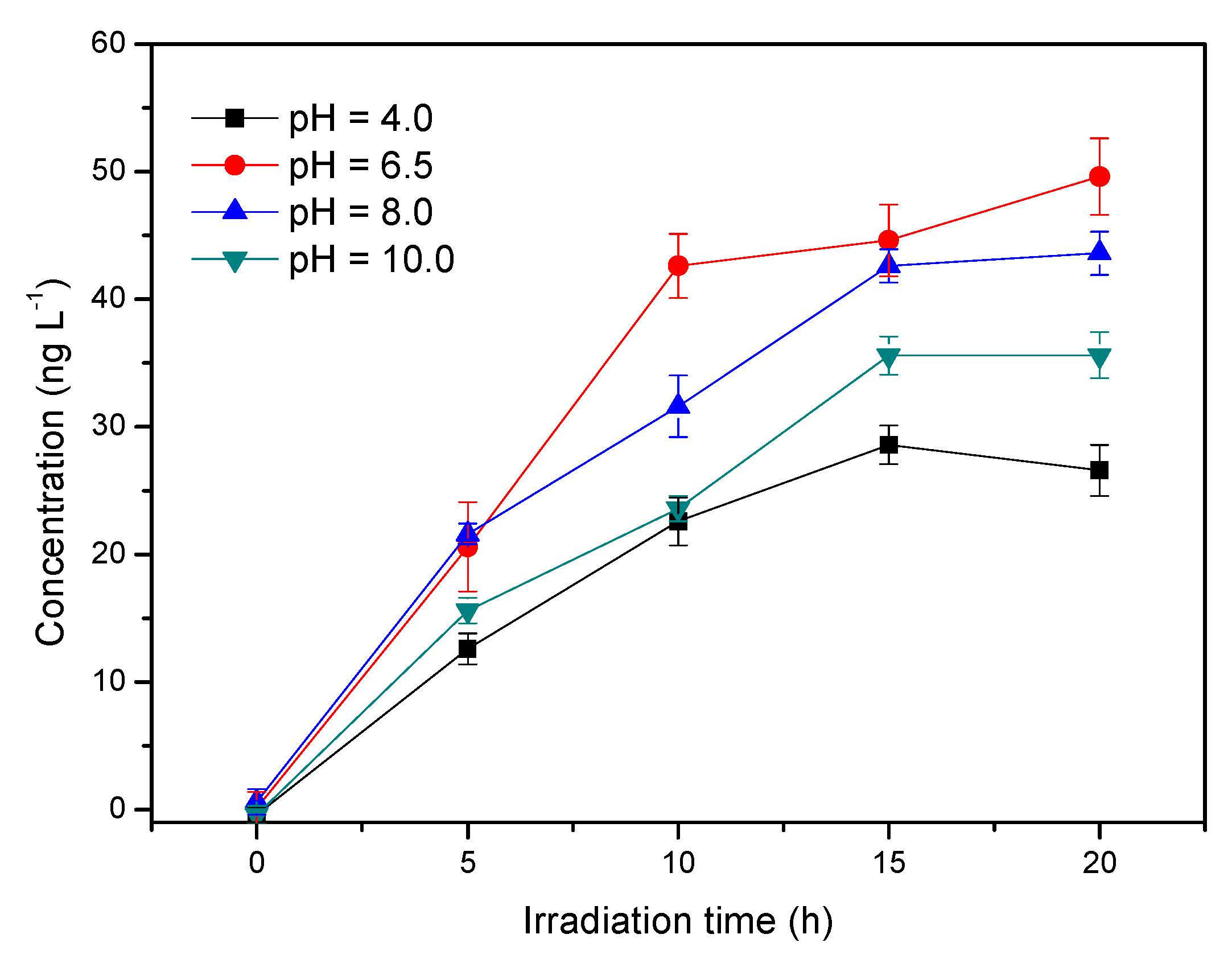
3.4. Influence of pH
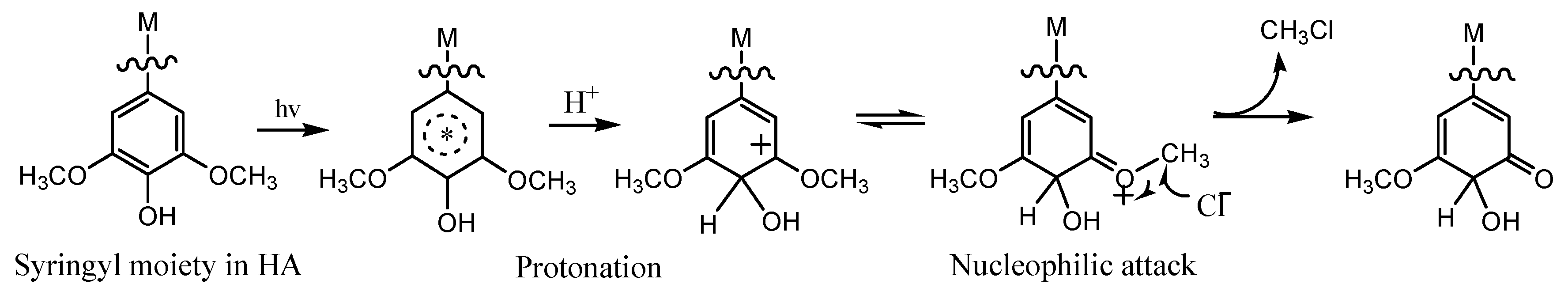
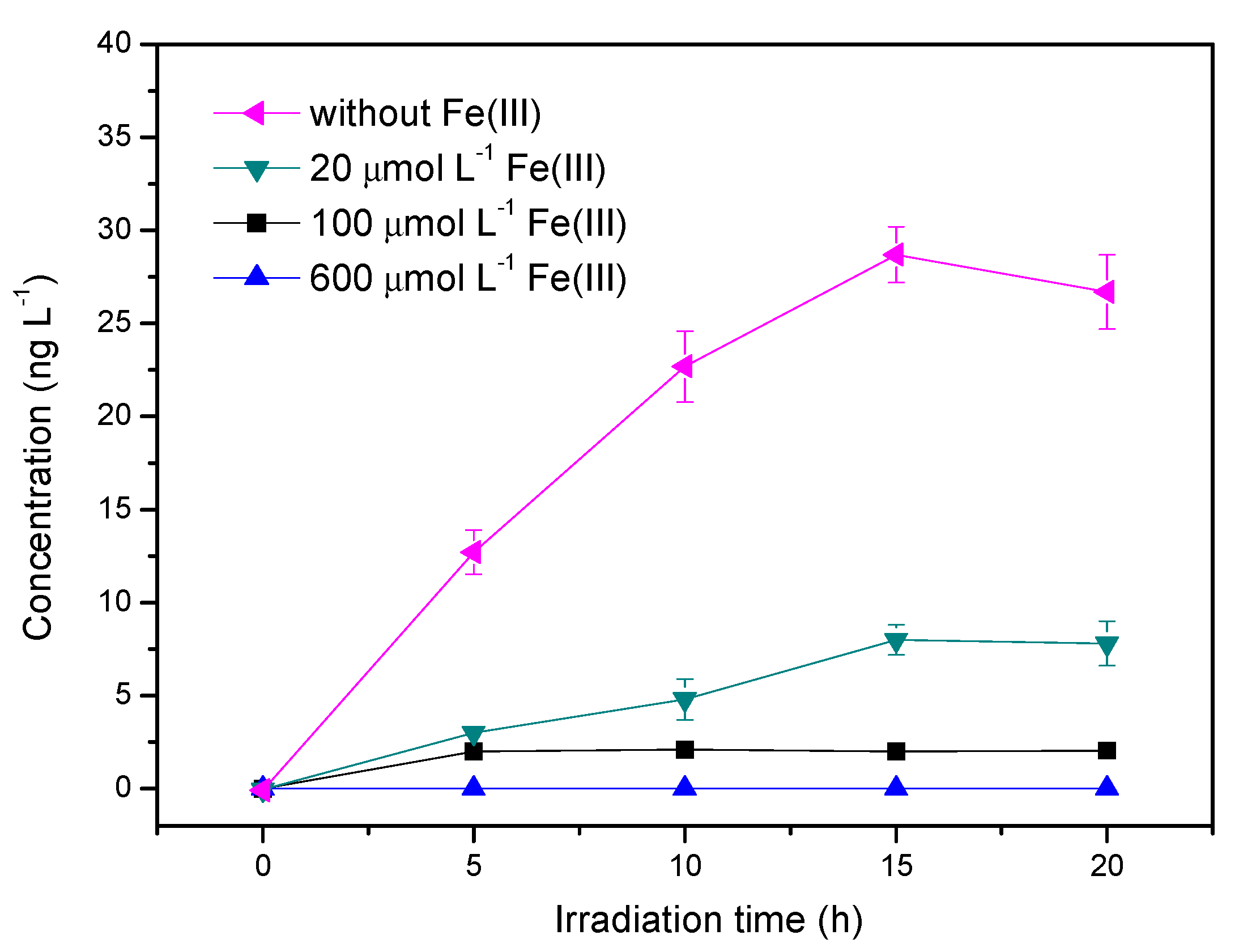
3.5. Influence of Ferric Ions
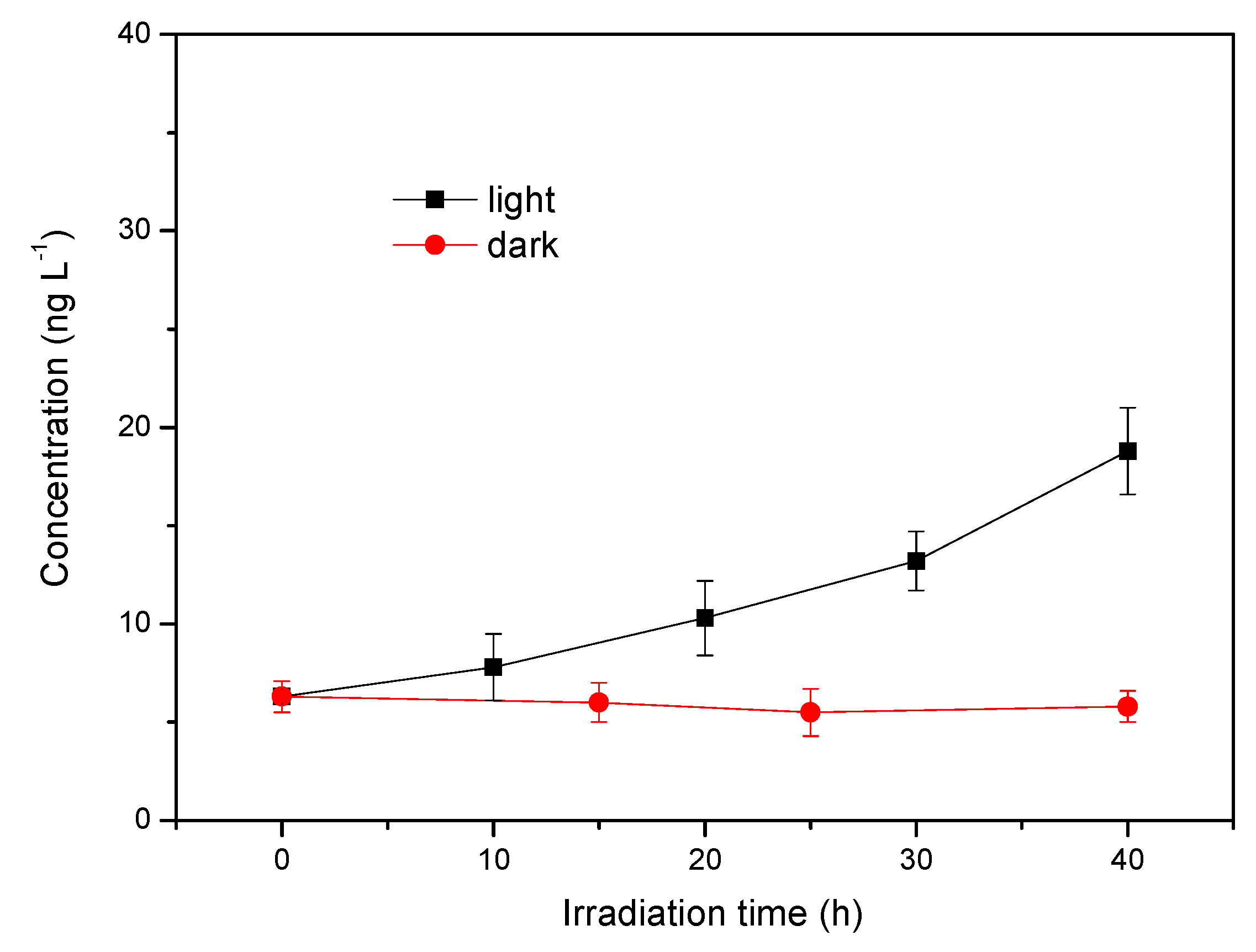
3.6. Generation of Methyl Chloride in Natural Seawater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khalil, M.A.K.; Rasmussen, R. Atmospheric methyl chloride. Atmos. Environ. 1999, 33, 1305–1321. [Google Scholar] [CrossRef]
- Wallington, T.J.; Pivesso, B.P.; Lira, A.M.; Anderson, J.E.; Nielsen, C.J.; Andersen, N.J.; Hodnebrog, O. CH3Cl, CH2Cl2, CHCl3 and CCl4: Infrared spectra, radiative efficiencies, and global warming potentials. J. Quant. Spectrosc. Radiat. 2016, 174, 56–64. [Google Scholar] [CrossRef]
- Grossman, A.S.; Grant, K.E.; Blass, W.E.; Wuebbles, D.J. Radiative forcing calculations for CH3Cl and CH3Br. J. Geophys. Res. Atoms. 1997, 102, 13651–13656. [Google Scholar] [CrossRef]
- Keppler, F.; Eiden, R.; Niedan, V.; Pracht, J.; Schöler, H.F. Halocarbons produced by natural oxidation processes during degradation of organic matter. Nature 2000, 403, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Yokouchi, Y.; Noijiri, Y.; Barrie, L.A.; Toom-Sauntry, D.; Machida, T.; Inuzuka, Y.; Akimoto, H.; Li, H.J.; Fujinuma, Y.; Aoki, S. A strong source of methyl chloride to the atmosphere from tropical coastal land. Nature 2000, 403, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rhew, R.C.; Miller, B.R.; Weiss, R.F. Natural methyl bro-mide and methyl chloride emissions from coastal saltmarshes. Nature 2000, 403, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Díaz, J.D.; Shimabuku, K.K.; Enumah, J.M.Z.O.; Pignatello, J.J.; Mitch, W.A.; Dodd, M.C. Sunlight-driven photochemical halogenation of dissolved organic matter in seawater: A natural abiotic source of organobromine and organoiodine. Environ. Sci. Technol. 2014, 48, 7418–7427. [Google Scholar] [CrossRef]
- Moore, R.M. Methyl halide production and loss rates from field incubation experiments. Mar. Chem. 2006, 101, 213–219. [Google Scholar] [CrossRef]
- Moore, R.M. A photochemical source of methyl chloride in saline waters. Environ. Sci. Technol. 2008, 42, 1933–1937. [Google Scholar] [CrossRef]
- McNeill, K.; Canonica, S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties. Environ. Sci. Processes Impacts 2016, 18, 1381–1399. [Google Scholar] [CrossRef]
- Sandron, S.; Rojas, A.; Wilson, R.; Davies, N.W.; Haddad, P.R.; Shellie, R.A.; Nesterenko, P.N.; Kelleher, B.P.; Paull, B. Chromatographic methods for the isolation, separation and characterisation of dissolved organic matter. Environ. Sci. Processes Impacts 2015, 17, 1531–1567. [Google Scholar] [CrossRef] [PubMed]
- Glover, C.M.; Rosario-Ortiz, F.L. Impact of halides on the photoproduction of reactive intermediates from organic matter. Environ. Sci. Technol. 2013, 47, 13949–13956. [Google Scholar] [CrossRef] [PubMed]
- Brigante, M.; Minella, M.; Mailhot, G.; Maurino, V.; Minero, C.; Vione, D. Formation and reactivity of the dichloride radical (Cl2−) in surface waters: A modelling approach. Chemosphere 2014, 95, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Jammoul, A.; Dumas, S.; D’anna, B.; George, C. Photoinduced oxidation of sea salt halides by aromatic ketones: A source of halogenated radicals. Atmos. Chem. Phys. 2009, 9, 4229–4237. [Google Scholar] [CrossRef]
- Hao, Z.; Yin, Y.; Cao, D.; Liu, J. Probing and comparing the photobromination and photoiodination of dissolved organic matter by using ultra-high-resolution mass spectrometry. Environ. Sci. Technol. 2017, 51, 5464–5472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Thornton, D.C.O.; Bianchi, T.S.; Arnold, W.A.; Shields, M.R.; Chen, J.; Yvon-Lewis, S.A. Dissolved organic matter composition drives the marine production of brominated very short-lived substances. Environ. Sci. Technol. 2015, 49, 3366–3374. [Google Scholar] [CrossRef]
- Dallin, E.; Wan, P.; Krogh, E.; Gill, C.; Moore, R.M. New pH-dependent photosubstitution pathways of syringic acid in aqueous solution: Relevance in environmental photochemistry. J. Photochem. Photobiol. A Chem. 2009, 207, 297–305. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, Y.; Yue, E.; Zhang, S.; Blatchley, E.R., III; Li, J. Methyl chloride produced during UV irradiation of saline water. J. Hazard. Mater. 2019, 384, 121263. [Google Scholar] [CrossRef]
- Song, G.; Li, Y.; Hu, S.; Li, G.; Zhao, R.; Sun, X.; Xie, H. Photobleaching of chromophoric dissolved organic matter (CDOM) in the Yangtze River estuary: Kinetics and effects of temperature, pH and salinity. Environ. Sci. Processes Impacts 2017, 19, 861–873. [Google Scholar] [CrossRef]
- Mylon, S.E.; Chen, K.L.; Elimelech, M. Influence of natural organic matter and ionic composition on the kinetics and structure of hematite colloid aggregation: Implications to iron depletion in estuaries. Langmuir 2004, 20, 9000–9006. [Google Scholar] [CrossRef]
- Hopwood, M.J.; Statham, P.J.; Milani, A. Dissolved Fe(II) in a river-estuary system rich in dissolved organic Matter. Estuar. Coast. Shelf Sci. 2014, 151, 1–9. [Google Scholar] [CrossRef]
- Moore, R.M.; Zafiriou, O.C. Photochemical production of methyl iodide in seawater. J. Geophys. Res. 1994, 99, 16415–16420. [Google Scholar] [CrossRef]
- Manley, S.L.; Barbero, P.E. Physiological constraints on bromoform (CHBr3) production by Ulva lactuca (Chlorophyta). Limnol. Oceanogr. 2001, 46, 1392–1399. [Google Scholar] [CrossRef]
- Lin, C.Y.; Manley, S.L. Bromoform production from seawater treated with bromoperoxidase. Limnol. Oceanogr. 2012, 57, 1857–1866. [Google Scholar] [CrossRef]
- Kohn, T.; Grandbois, M.; McNeill, K.; Nelson, K.L. Association with natural organic matter enhances the sunlight-mediated inactivation of MS2 coliphage by singlet oxygen. Environ. Sci. Technol. 2007, 41, 4626–4632. [Google Scholar] [CrossRef]
- Leifer, A. The Kinetics of Environmental Aquatic Photochemistry: Theory and Practice; American Chemical Society: Washington, DC, USA, 1988. [Google Scholar]
- Chiron, S.; Minero, C.; Vione, D. Photodegradation processes of the antiepileptic drug carbamazepine, relevant to estuarine waters. Environ. Sci. Technol. 2006, 40, 5977–5983. [Google Scholar] [CrossRef]
- Longstaffe, J.G.; Courtier-Murias, D.; Simpson, A.J. The pH-dependence of organofluorine binding domain preference in dissolved humic acid. Chemosphere 2013, 90, 270–275. [Google Scholar] [CrossRef]
- Gan, L.; Yan, Z.; Ma, Y.; Zhu, Y.; Li, X.; Xu, J.; Zhang, W. pH dependence of the binding interactions between humic acids and bisphenol AA thermodynamic perspective. Environ. Pollut. 2019, 255, 113292. [Google Scholar] [CrossRef]
- Karpukhina, E.; Mikheev, I.; Perminova, I.; Volkov, D.; Proskurnin, M. Rapid quantification of humic components in concentrated humate fertilizer solutions by FTIR spectroscopy. J. Soils Sediments 2018, 19, 2729–2739. [Google Scholar] [CrossRef]
- Liu, Q.J.; Li, X.; Tang, J.P.; Zhou, Y.M.; Lin, Q.T.; Xiao, R.; Zhang, M. Characterization of goethite-fulvic acid composites and their impact on the immobility of Pb/Cd in soil. Chemosphere 2019, 222, 556–563. [Google Scholar] [CrossRef]
- Balmer, M.E.; Sulzberger, B. Atrazine degradation in irradiated iron/oxalate systems: Effects of pH and oxalate. Environ. Sci. Technol. 1999, 33, 2418–2424. [Google Scholar] [CrossRef]
- Ou, X.; Quan, X.; Chen, S.; Zhang, F.; Zhao, Y. Photocatalytic reaction by Fe(III)-citrate complex and its effect on the photodegradation of atrazine in aqueous solution. J. Photochem. Photobiol. A Chem. 2008, 197, 382–388. [Google Scholar] [CrossRef]
- Gu, B.H.; Schmitt, J.; Chen, Z.H.; Liang, L.Y.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef]
- Cieśla, P.; Kocot, P.; Mytych, P.; Stasicka, Z. Homogeneous photocatalysis by transition metal complexes in the environment. J. Mol. Catal. A Chem. 2004, 224, 17–33. [Google Scholar] [CrossRef]
- Porasso, R.D.; Benegas, J.C.; Van den Hoop, M.G.T.; Paoletti, S. Analysis of trace metal humic acid interactions using counterion condensation theory. Environ. Sci. Technol. 2002, 36, 3815–3821. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Li, Y. Photoconversion of chlorinated saline wastewater DBPs in receiving seawater is overall a detoxification process. Environ. Sci. Technol. 2017, 51, 58–67. [Google Scholar] [CrossRef]
- Wu, F.; Deng, N. Photochemistry of hydrolytic iron (III) species and photoinduced degradation of organic compounds. A minireview. Chemosphere 2000, 41, 1137–1147. [Google Scholar]
- Zhang, K.; Parker, K.M. Halogen radical oxidants in natural and engineered aquatic systems. Environ. Sci. Technol. 2018, 52, 9579–9594. [Google Scholar] [CrossRef]
- Hirata, M.; Ikeda, M.; Fukuda, F.; Abe, M.; Sawada, H.; Hashimoto, S. Effect of temperature on the production rates of methyl halides in cultures of marine proteobacteria. Mar. Chem. 2017, 196, 126–134. [Google Scholar] [CrossRef]
- Sato, N.; Hamamoto, K.; Kurihara, M.; Abe, M.; Hashimoto, S. Methyl halide production by cultures of marine thraustochytrids, Aurantiochytrium sp., Botryochytrium radiatum, and Schizochytrium sp. Mar. Chem. 2019, 208, 95–102. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Pu, Y.; Tong, T.; Zhu, X.; Sun, B.; Zhang, X. Photochemical Generation of Methyl Chloride from Humic Aicd: Impacts of Precursor Concentration, Solution pH, Solution Salinity and Ferric Ion. Int. J. Environ. Res. Public Health 2020, 17, 503. https://doi.org/10.3390/ijerph17020503
Liu H, Pu Y, Tong T, Zhu X, Sun B, Zhang X. Photochemical Generation of Methyl Chloride from Humic Aicd: Impacts of Precursor Concentration, Solution pH, Solution Salinity and Ferric Ion. International Journal of Environmental Research and Public Health. 2020; 17(2):503. https://doi.org/10.3390/ijerph17020503
Chicago/Turabian StyleLiu, Hui, Yingying Pu, Tong Tong, Xiaomei Zhu, Bing Sun, and Xiaoxing Zhang. 2020. "Photochemical Generation of Methyl Chloride from Humic Aicd: Impacts of Precursor Concentration, Solution pH, Solution Salinity and Ferric Ion" International Journal of Environmental Research and Public Health 17, no. 2: 503. https://doi.org/10.3390/ijerph17020503
APA StyleLiu, H., Pu, Y., Tong, T., Zhu, X., Sun, B., & Zhang, X. (2020). Photochemical Generation of Methyl Chloride from Humic Aicd: Impacts of Precursor Concentration, Solution pH, Solution Salinity and Ferric Ion. International Journal of Environmental Research and Public Health, 17(2), 503. https://doi.org/10.3390/ijerph17020503




