Comparative Activation Process of Pb, Cd and Tl Using Chelating Agents from Contaminated Red Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Soil Preparation
2.1.2. Preparation of Contaminated Soil
2.1.3. Experimental Reagents
2.2. Experimental Design and Operation
2.2.1. Preliminary and Dynamic Activation Experiment
2.2.2. Influence of Chelating Agent Dosage and Solution pH on the Activation Effect of Heavy Metals
2.2.3. Determination of Heavy Metal Speciations before and after Activation
2.3. Statistical Analysis and Processing Methods
3. Results
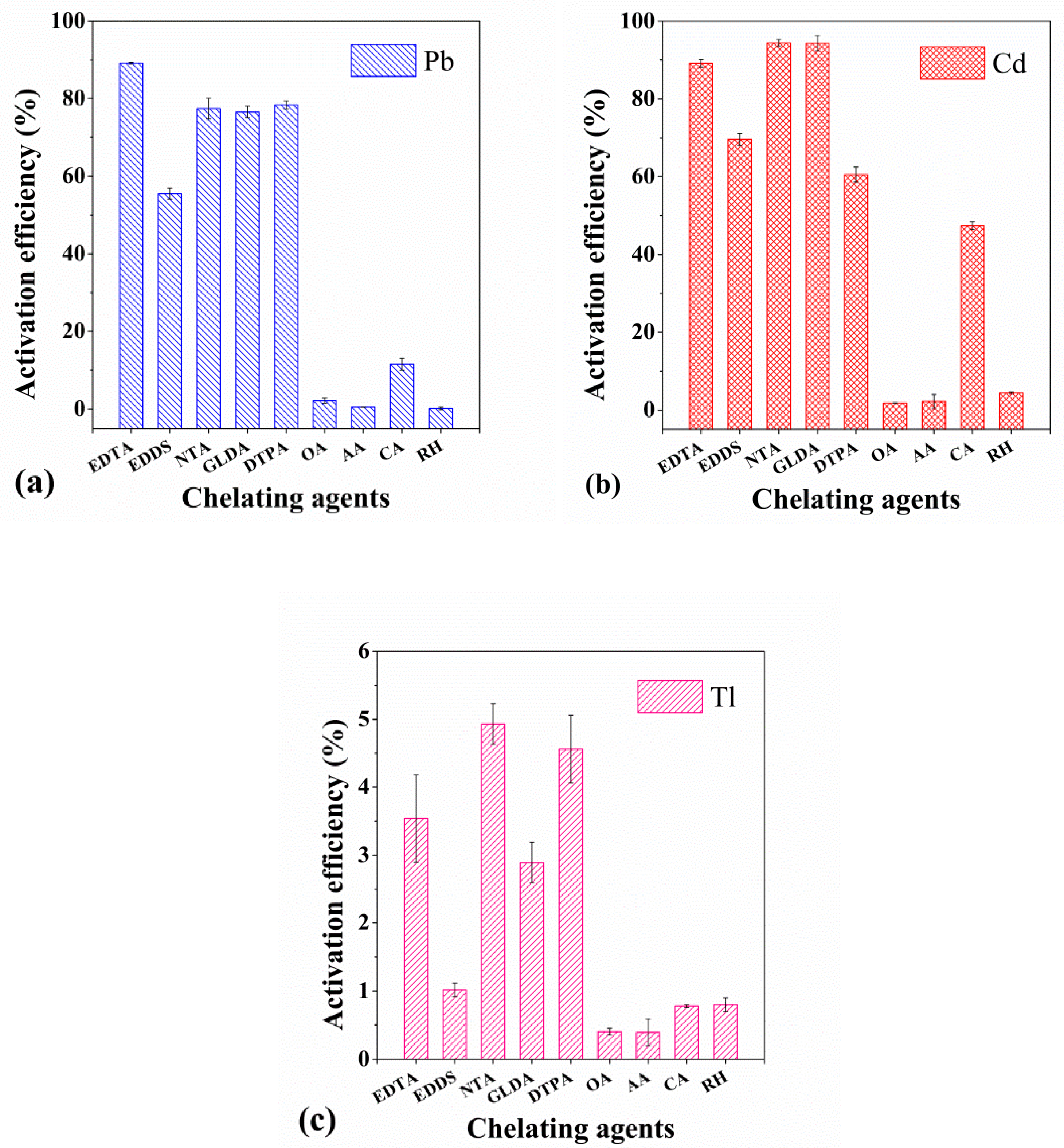
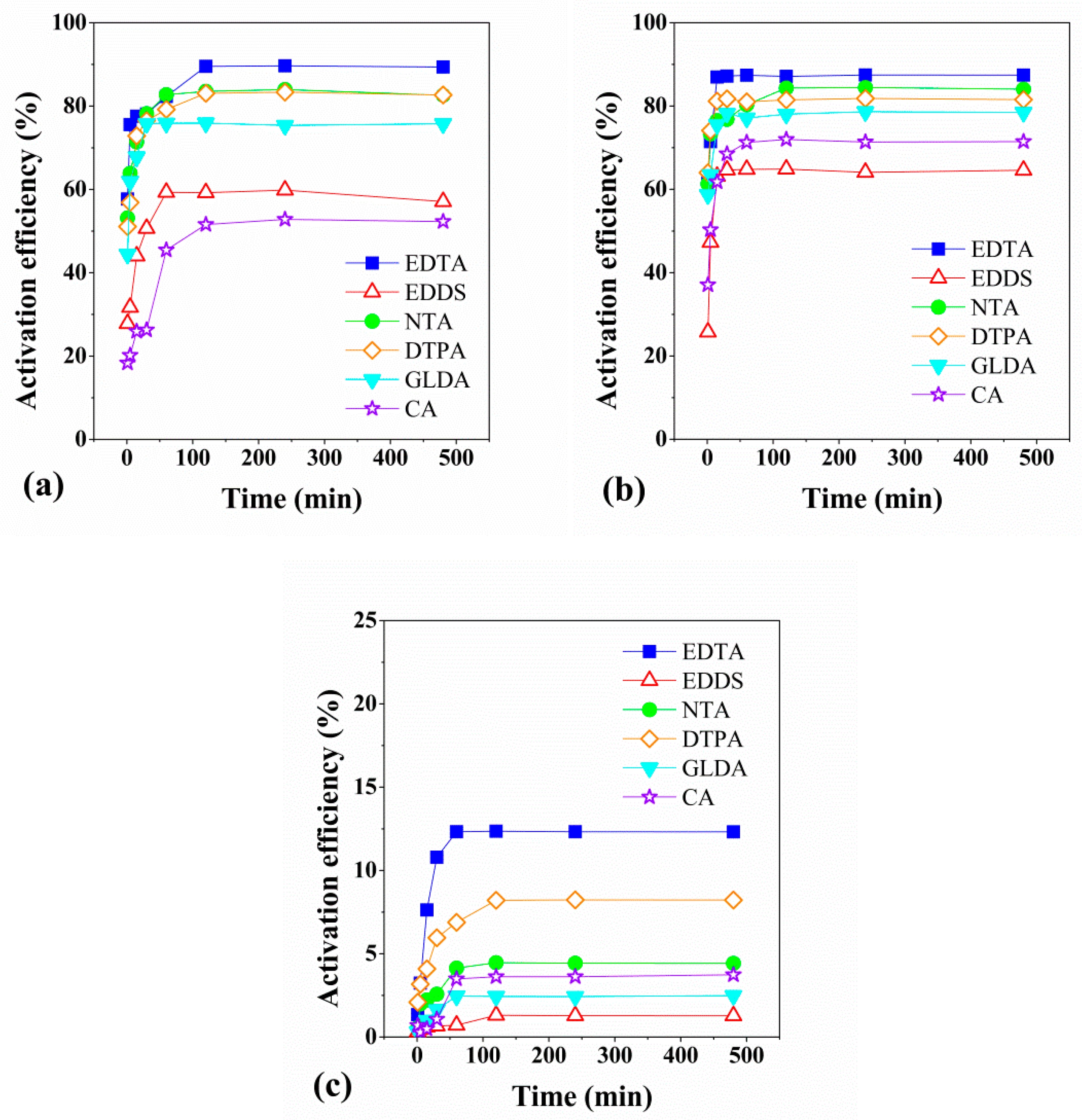
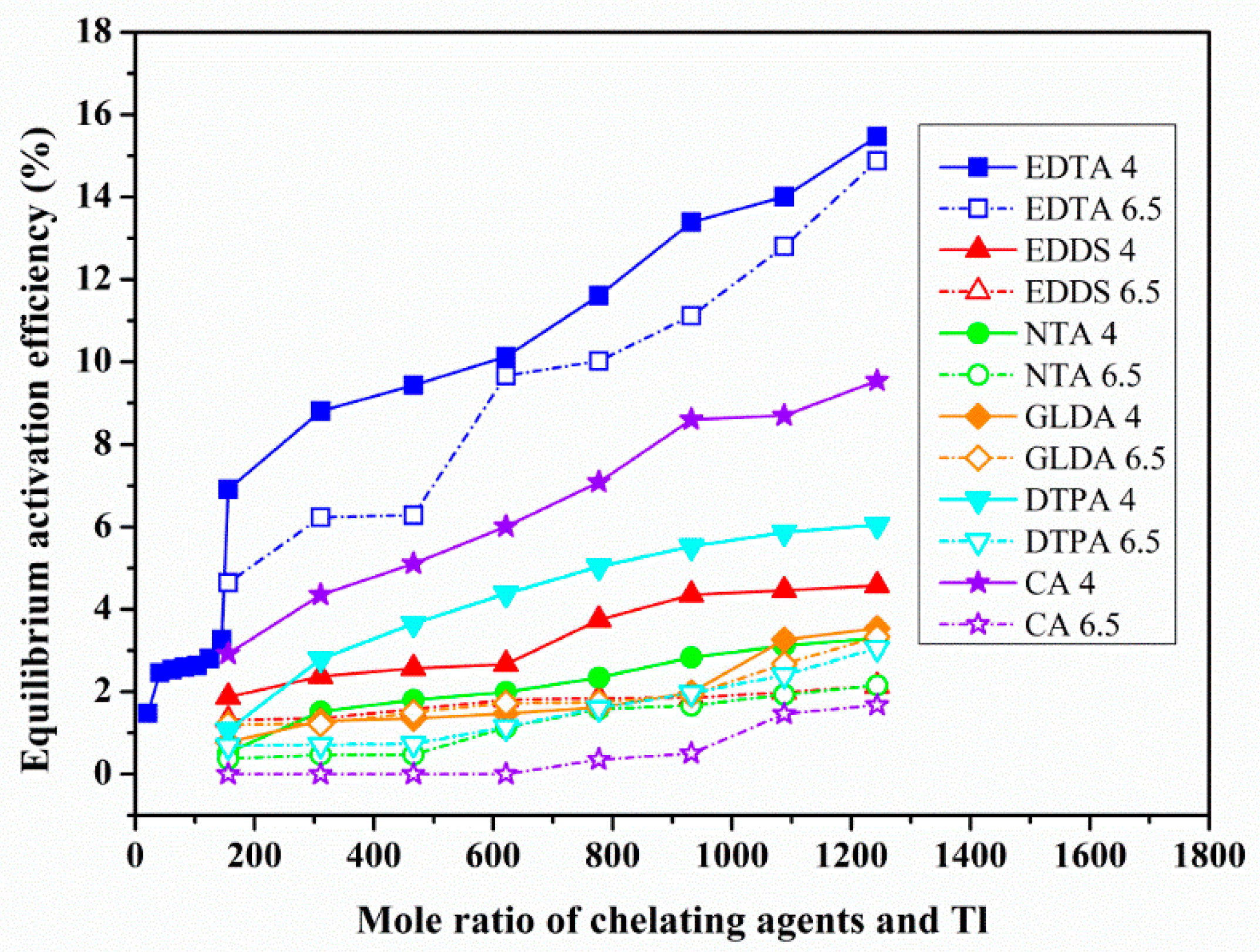
3.1. Screening of Chelating Agents and Dynamic Activation Experiment
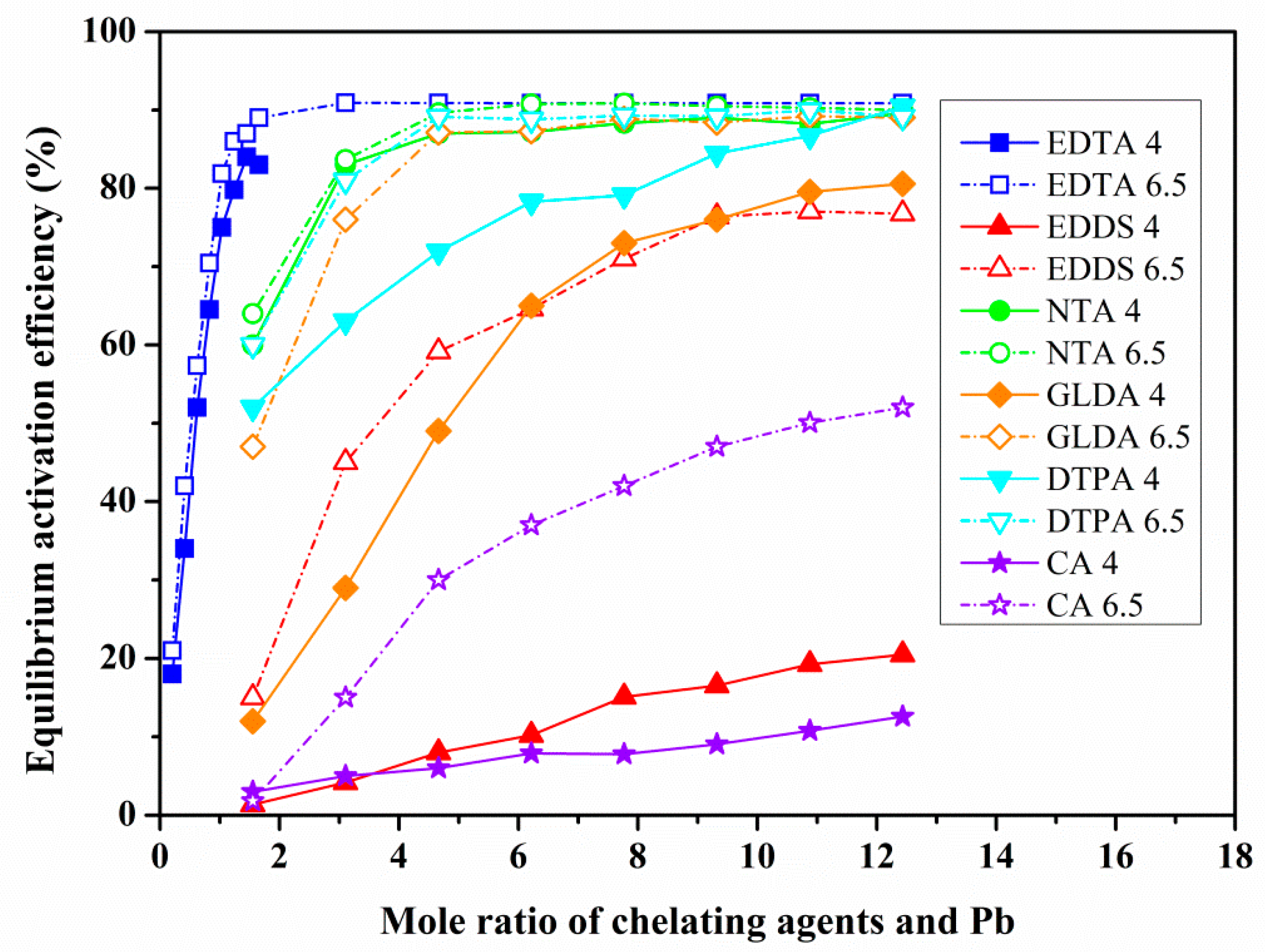
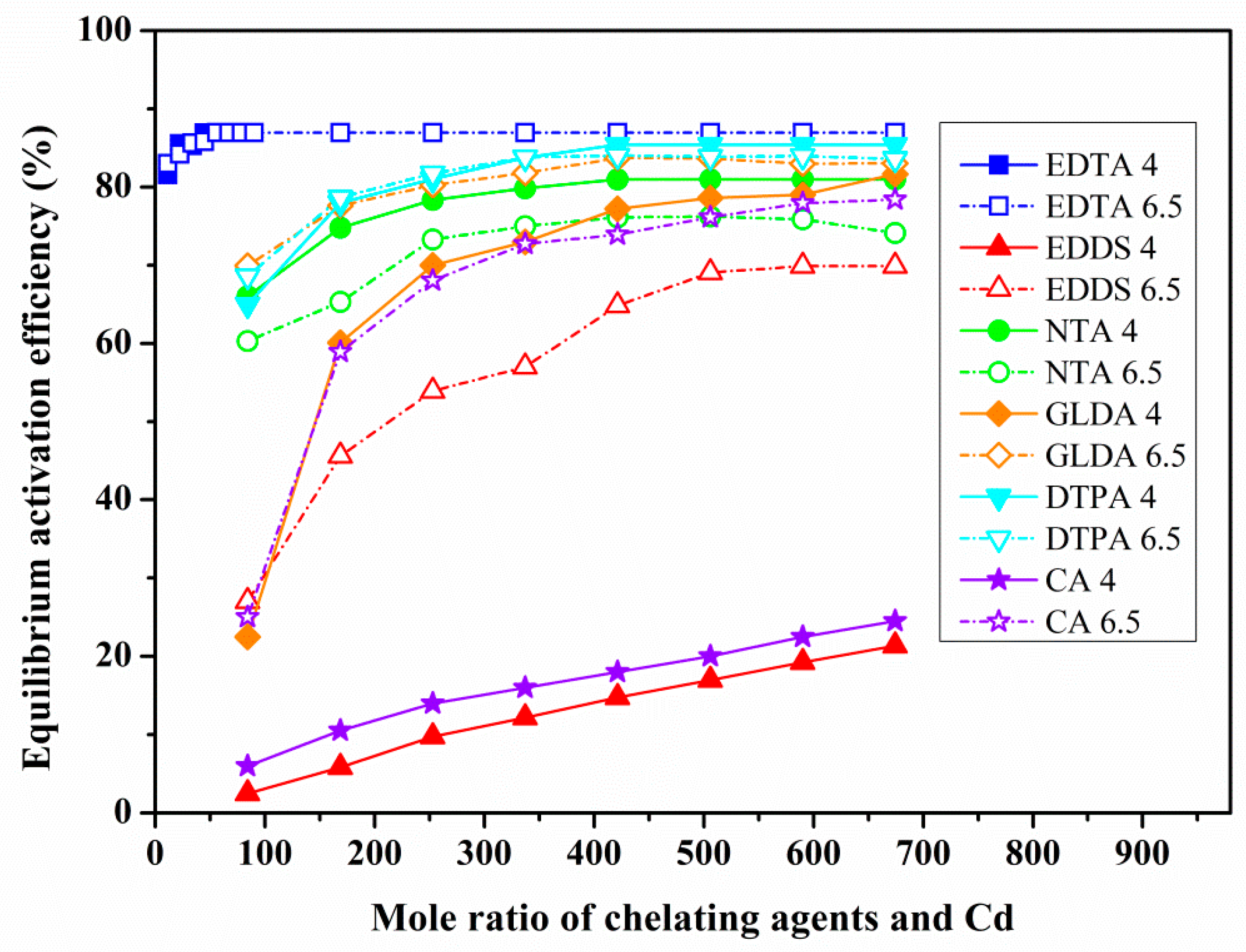
3.2. The Effect of Chelating Agent Dosage and Solution pH on Heavy Metals Activation
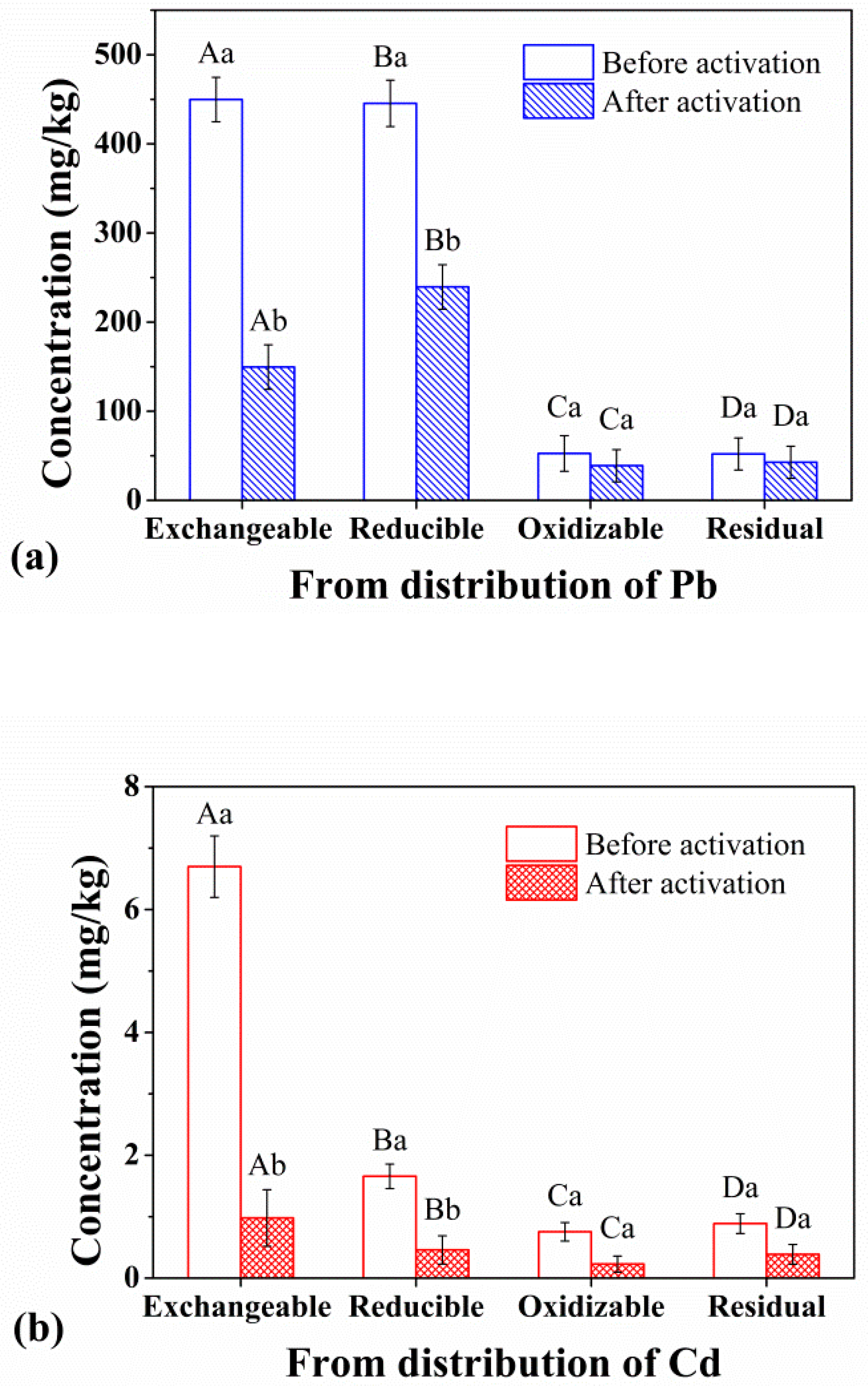
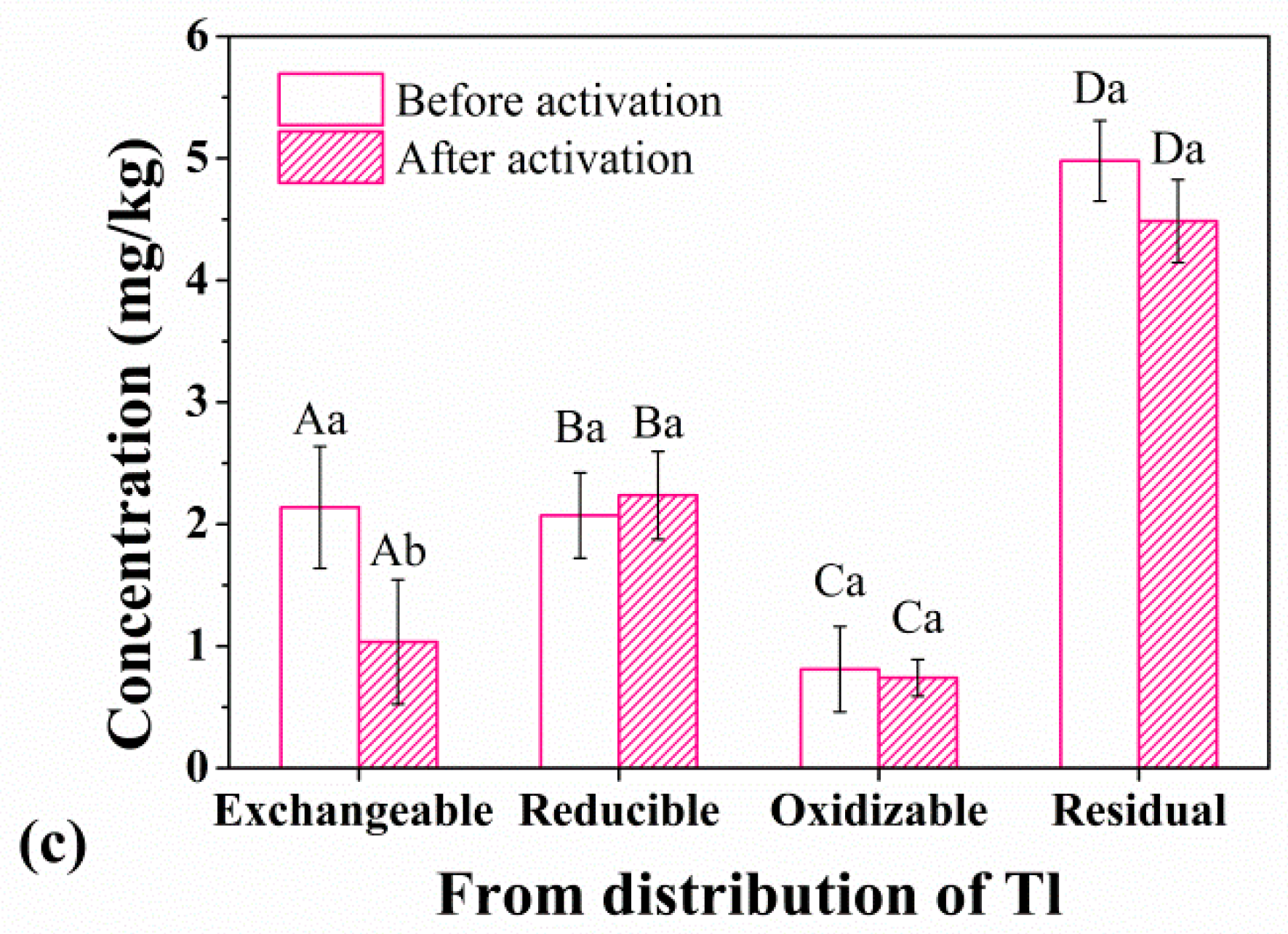
3.3. Speciation Characteristics of Heavy Metals before and after Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barrutia, O.; Garbisu, C.; Hernández-Allica, J.; García-Plazaola, J.I.; Becerril, J.M. Differences in EDTA-assisted metal phytoextraction between metallicolous and non-metallicolous accessions of Rumex acetosa L. Environ. Pollut. 2010, 158, 1710–1715. [Google Scholar] [CrossRef]
- Pietrzak, U.; Uren, N.C. Remedial options for copper-contaminated vineyard soils. Soil Res. 2011, 49, 44. [Google Scholar] [CrossRef]
- Cui, J.L.; Luo, C.-L.; Tang, C.W.-Y.; Chan, T.-S.; Li, X.-D. Speciation and leaching of trace metal contaminants from e-waste contaminated soils. J. Hazard. Mater. 2017, 329, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gupta, K.; Debnath, S.; Ghosh, U.C.; Chattopadhyay, D.; Mukhopadhyay, A.J. Arsenic bioaccumulation in rice and edible plants and subsequent transmission through food chain in Bengal basin: A review of the perspectives for environmental health. Toxicol. Environ. Chem. 2012, 94, 429–441. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nima, J.; Divya, P.L. Adsorption of chromium(VI) from aqueous solutions by glycidylmethacrylate-grafted-densified cellulose with quaternary ammonium groups. Appl. Surf. Sci. 2013, 279, 441–449. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Tompkins, K.M.; Pavicevic, P.G. Phytoremediation: An Affordable Green Technology for the Clean-Up of Metal-Contaminated Sites in Sri Lanka. Ceylon J. Sci. (Biol. Sci.) 2006, 35, 25–39. [Google Scholar]
- Souza, E.C.; Vessoni-Penna, T.C.; Oliveira, R.P. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int. Biodeterior. Biodegrad. 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Robinson, B.H.; Leblanc, M.; Petit, D.; de Souza Oliveira, R.P.; Kirkman, J.H.; Gregg, P.E.H. The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant. Soil 1998, 203, 47–56. [Google Scholar] [CrossRef]
- Ali, H.; Naseer, M.; Sajad, M.A. Phytoremediation of heavy metals by Trifolium alexandrinum. Int. J. Environ. Sci. 2012, 2, 1459–1469. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Tahir, U.; Yasmin, A.; Khan, U.H. Phytoremediation: Potential flora for synthetic dyestuff metabolism. J. King Saud Univ. Sci. 2015, 28, 119–130. [Google Scholar] [CrossRef]
- Burken, J.G.; Schnoor, J.L. Phytoremediation: Plant uptake of atrazine and role of root exudates. J. Environ. Eng. 1996, 122, 958–963. [Google Scholar] [CrossRef]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.R.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. J. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Thayalakumaran, T.; Vogeler, I.; Scotter, D.R.; Percival, H.J.; Robinson, B.H.; Clothier, B.E. Leaching of copper from contaminated soil following the application of EDTA. II. Intact core experiments and model testing. Aust. J. Soil Res. 2003, 41, 335–350. [Google Scholar]
- Yang, B.; Zhang, J.; Zhang, Y.; Deng, S.; Yu, G.; Wu, J.; Zhang, H.; Liu, J. Promoting effect of EDTA on catalytic activity of highly stable Al–Ni bimetal alloy for dechlorination of 2-chlorophenol. Chem. Eng. J. 2014, 250, 222–229. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Begum, Z.A.; Rahman, I.M.M.; Tate, Y.; Sawai, H.; Maki, T.; Hasegawa, H. Remediation of toxic metal contaminated soil by washing with biodegradable aminopolycarboxylate chelants. Chemosphere 2012, 87, 1161–1170. [Google Scholar] [CrossRef]
- Salido, A.L.; Hasty, K.L.; Lim, J.M.; Butcher, D.J. Phytoremediation of arsenic and lead in contaminated soil using Chinese Brake Ferns (Pteris vittata) and Indian mustard (Brassica juncea). Int. J. Phytoremed. 2003, 5, 89–103. [Google Scholar] [CrossRef]
- Wu, L.; Luo, Y.; Xing, X.; Christie, P. EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agric. Ecosyst. Environ. 2004, 102, 307–318. [Google Scholar] [CrossRef]
- Yang, G.; Miao, C.; Xia, J.; Luo, C.; Mao, L.; Zhou, P.; Shi, W. Effect of citric acid on phytoextraction and antioxidative defense in Solanum nigrum L. as a hyperaccumulator under Cd and Pb combined pollution. Environ. Earth Sci. 2012, 65, 1923–1932. [Google Scholar]
- Deshpande, S.; Shiau, B.J.; Wade, D.; Sabatini, D.A.; Harwell, J.H. Surfactant selection for enhancing ex situ soil washing. Water Res. 1999, 33, 351–360. [Google Scholar] [CrossRef]
- Gabos, M.B.; Abreu, C.A.D.; Coscione, A.R. EDTA assisted phytorremediation of a Pb contamined soil: Metal leaching and uptake by jack beans. Sci. Agric. 2009, 66, 506–514. [Google Scholar] [CrossRef]
- Zeremski-Skoric, T.; Sekulic, P.; Maksimovic, I.; Seremesic, S.; Ninkov, J.; Milic, S.; Vasin, J. Chelate-assisted phytoextraction: Effect of EDTA and EDDS on copper uptake by Brassica napus L. J. Serb. Chem. Soc. 2010, 75, 1279–1289. [Google Scholar] [CrossRef]
- Sun, Y.B.; Zhou, Q.-X.; An, J.; Liu, W.-T.; Liu, R. Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma 2009, 150, 106–112. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, F.B.; Liu, C.S.; Huang, W.; Yu, W.M. Iron Redox Cycling Coupled to Transformation and Immobilization of Heavy Metals: Implications for Paddy Rice Safety in the Red Soil of South China. Adv. Agron. 2016, 137, 279–317. [Google Scholar]
- Huang, X.; Luo, D.; Chen, X.; Wei, L.; Liu, Y.; Wu, Q.; Xiao, T.; Mai, X.; Liu, G.; Liu, L. Insights into Heavy Metals Leakage in Chelator-Induced Phytoextraction of Pb- and Tl-Contaminated Soil. Int. J. Environ. Res. Public Health 2019, 16, 1328. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.A.; Wissocq, A.; Benedetti, M.F.; Latrille, C. Thallium (Tl) sorption onto illite and smectite: Implications for Tl mobility in the environment. Geochim. Cosmochim. Acta 2018, 230, 1–16. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Luo, D.G.; Liu, L.R.; Tan, Z.C.; Lai, A.; Liu, G.W.; Li, J.H.; Long, J.Y.; Huang, X.X.; Chen, Y.H. Leaching variations of heavy metals in chelator-assisted phytoextraction by Zea mays L. exposed to acid rainfall. Environ. Sci. Pollut. Res. 2017, 24, 24409–24418. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Usman, A.R.A.; Ahmad, M.; Kim, K.-R.; Vithanage, M.; Ok, Y.S. Role of chelating agents on release kinetics of metals and their uptake by maize from chromated copper arsenate-contaminated soil. Environ. Technol. 2013, 34, 747–755. [Google Scholar] [CrossRef]
- Amir, M.; Mohsen, S.; Afsaneh, M. Simultaneous Desorption and Desorption Kinetics of Phenanthrene, Anthracene, and Heavy Metals from Kaolinite with Different Organic Matter Content. Soil Sediment Contam. 2018, 27, 200–220. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Zhu, Y.; Li, Z.; Kumar, R. Recycled chitosan nanofibril as an effective Cu(II), Pb(II) and Cd(II) ionic chelating agent: Adsorption and desorption performance. Carbohydr. Polym. 2014, 111, 469–476. [Google Scholar] [CrossRef]
- Papassiopi, N.; Tambouris, S.; Kontopoulos, A. Removal of Heavy Metals from Calcareous Contaminated Soils by EDTA Leaching. Water Air Soil Pollut. 1999, 109, 1–15. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, F.J.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Nemcsok, D.S.; Kocsis, T. Bonding interactions in EDTA complexes. J. Mol. Struct. Theochem. 2010, 950, 93–97. [Google Scholar] [CrossRef]
- Doong, R.A.; Wu, Y.-W.; Lei, W.-G. Surfactant enhanced remediation of cadmium contaminated soils. Water Sci. Technol. 1998, 37, 65–71. [Google Scholar] [CrossRef]
- Chauhan, G.; Pant, K.K.; Nigam, K.D.P. Chelation technology: A promising green approach for resource management and waste minimization. Environ. Sci. Process. Impacts 2015, 17, 12–40. [Google Scholar] [CrossRef]
- Pochodylo, A.L.; Aristilde, L. Molecular dynamics of stability and structures in phytochelatin complexes with Zn, Cu, Fe, Mg, and Ca: Implications for metal detoxification. Environ. Chem. Lett. 2017, 15, 495–500. [Google Scholar] [CrossRef]
- Saglam, A.; Bektas, S.; Patir, S.; Genc, O.; Denizli, A. Novel metal complexing ligand: Thiazolidine carrying poly(hydroxyethylmethacrylate) microbeads for removal of cadmium(II) and lead(II) ions from aqueous solutions. React. Funct. Polym. 2001, 47, 185–192. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Hedayati, A.; Ansari, K. Experimental investigation and optimization of supercritical carbon dioxide extraction of toxic heavy metals from solid waste using different modifiers and chelating agents. J. Supercrit. Fluids 2016, 117, 131–137. [Google Scholar] [CrossRef]
- Ho, Y.S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.-Q.; Zhang, Q.-P.; Liu, Q.-C.; Li, Y.-D.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Repo, E.; Warchol, J.K.; Kurniawan, T.A.; Sillanp, M.E.T. Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: Kinetic and equilibrium modeling. Chem. Eng. J. 2010, 161, 73–82. [Google Scholar] [CrossRef]
- Tohdee, K.; Kaewsichan, L. Enhancement of adsorption efficiency of heavy metal Cu(II) and Zn(II) onto cationic surfactant modified bentonite. J. Environ. Chem. Eng. 2018, 6, 2821–2828. [Google Scholar] [CrossRef]
- Mehmood, F.; Rashid, A.; Mahmood, T.; Dawson, L. Effect of DTPA on Cd solubility in soil—Accumulation and subsequent toxicity to lettuce. Chemosphere 2013, 90, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tian, T.; Wang, M.K.; Wang, G. Release of Pb in soils washed with various extractants. Geoderma 2016, 275, 74–81. [Google Scholar] [CrossRef]
- Naghipour, D.; Gharibi, H.; Taghavi, K.; Jaafari, J. Influence of EDTA and NTA on heavy metal extraction from sandy-loam contaminated soils. J. Environ. Chem. Eng. 2016, 4, 3512–3518. [Google Scholar] [CrossRef]
- Blixt, J.; Glaser, J.; Solymosi, P.; Toth, I. Equilibria and dynamics of thallium-EDTA (Tl(edta)X2-) complexes (X = halide, pseudohalide) studied by multinuclear NMR. Inorg. Chem. 1992, 31, 5288–5297. [Google Scholar] [CrossRef]
- Kim, C.S.; Ong, S.K. Recycling of lead-contaminated EDTA wastewater. J. Hazard. Mater. 1999, 69, 273–286. [Google Scholar] [CrossRef]
- Manouchehri, N.; Besancon, S.; Bermond, A. Major and trace metal extraction from soil by EDTA: Equilibrium and kinetic studies. Anal. Chim. Acta 2006, 559, 105–112. [Google Scholar] [CrossRef]
- Tandy, S.; Bossart, K.; Mueller, R.; Ritschel, J.; Hauser, L.; Schulin, R.; Nowack, B. Extraction of heavy metals from soils using biodegradable chelating agents. Environ. Sci. Technol. 2004, 38, 937–944. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Zhang, W.; Lo, I.M.C. Copper extraction effectiveness and soil dissolution issues of EDTA-flushing of artificially contaminated soils. Chemosphere 2007, 68, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Hovsepyan, A.; Greipsson, S. EDTA-enhanced phytoremediation of lead-contaminated soil by corn. J. Plant Nutr. 2005, 28, 2037–2048. [Google Scholar] [CrossRef]
- Suthar, V.; Memon, K.S.; Mahmood-ul-Hassan, M. EDTA-enhanced phytoremediation of contaminated calcareous soils: Heavy metal bioavailability, extractability, and uptake by maize and sesbania. Environ. Monit. Assess. 2014, 186, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Luo, D.; Lai, A.; Liu, G.; Liu, L.; Long, J.; Zhang, H.; Chen, Y. Leaching characteristics of EDTA-enhanced phytoextraction of Cd and Pb by Zea mays L. in different particle-size fractions of soil aggregates exposed to artificial rain. Environ. Sci. Pollut. Res. 2017, 24, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Begum, Z.A.; Rahman, I.M.M.; Sawai, H.; Tate, Y.; Maki, T.; Hasegawa, H. Stability Constants of Fe(III) and Cr(III) Complexes with dl-2-(2-Carboxymethyl)nitrilotriacetic Acid (GLDA) and 3-Hydroxy-2,2′-iminodisuccinic acid (HIDS) in Aqueous Solution. J. Chem. Eng. Data 2012, 57, 2723–2732. [Google Scholar] [CrossRef]
- Begum, Z.A.; Rahman, I.M.M.; Tate, Y.; Egawa, Y.; Maki, T.; Hasegawa, H. Formation and Stability of Binary Complexes of Divalent Ecotoxic Ions (Ni, Cu, Zn, Cd, Pb) with Biodegradable Aminopolycarboxylate Chelants (DL-2-(2-Carboxymethyl)Nitrilotriacetic Acid, GLDA, and 3-Hydroxy-2,2′-Iminodisuccinic Acid, HIDS) in Aqueous Solutions. J. Solut. Chem. 2012, 41, 1713–1728. [Google Scholar]
| Soil Properties | Value | |
|---|---|---|
| Particle size | <0.002 mm (%) | 16.00 |
| 0.002–0.02 mm (%) | 33.00 | |
| 0.02–2 mm (%) | 53.00 | |
| pH | 4.5 | |
| Available N (mg/kg) | 37.80 | |
| Available P (mg/kg) | 0.90 | |
| Available K (mg/kg) | 12.10 | |
| SOM (g/kg) | 15.40 | |
| CEC (cmol/kg) | 3.30 | |
| Total heavy metal concentration (mg/kg) | ||
| Pb | 52.30 | |
| Cd | 0.12 | |
| Tl | 0.63 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Luo, D.; Yao, G.; Huang, X.; Wei, L.; Liu, Y.; Wu, Q.; Mai, X.; Liu, G.; Xiao, T. Comparative Activation Process of Pb, Cd and Tl Using Chelating Agents from Contaminated Red Soils. Int. J. Environ. Res. Public Health 2020, 17, 497. https://doi.org/10.3390/ijerph17020497
Liu L, Luo D, Yao G, Huang X, Wei L, Liu Y, Wu Q, Mai X, Liu G, Xiao T. Comparative Activation Process of Pb, Cd and Tl Using Chelating Agents from Contaminated Red Soils. International Journal of Environmental Research and Public Health. 2020; 17(2):497. https://doi.org/10.3390/ijerph17020497
Chicago/Turabian StyleLiu, Lirong, Dinggui Luo, Guangchao Yao, Xuexia Huang, Lezhang Wei, Yu Liu, Qihang Wu, Xiaotao Mai, Guowei Liu, and Tangfu Xiao. 2020. "Comparative Activation Process of Pb, Cd and Tl Using Chelating Agents from Contaminated Red Soils" International Journal of Environmental Research and Public Health 17, no. 2: 497. https://doi.org/10.3390/ijerph17020497
APA StyleLiu, L., Luo, D., Yao, G., Huang, X., Wei, L., Liu, Y., Wu, Q., Mai, X., Liu, G., & Xiao, T. (2020). Comparative Activation Process of Pb, Cd and Tl Using Chelating Agents from Contaminated Red Soils. International Journal of Environmental Research and Public Health, 17(2), 497. https://doi.org/10.3390/ijerph17020497







