Can Exposure to Certain Urban Green Spaces Trigger Frontal Alpha Asymmetry in the Brain?—Preliminary Findings from a Passive Task EEG Study
Abstract
1. Introduction
1.1. Frontal Alpha Asymmetry and Mental Health
1.2. Scope
2. Materials and Methods
2.1. Participants
2.2. Sites and Scenes Selection
2.3. Assessment of Participant’s Depression
2.4. Procedure
2.5. EEG Data Processing
3. Statistical Analysis
4. Results
4.1. Sample Characteristics
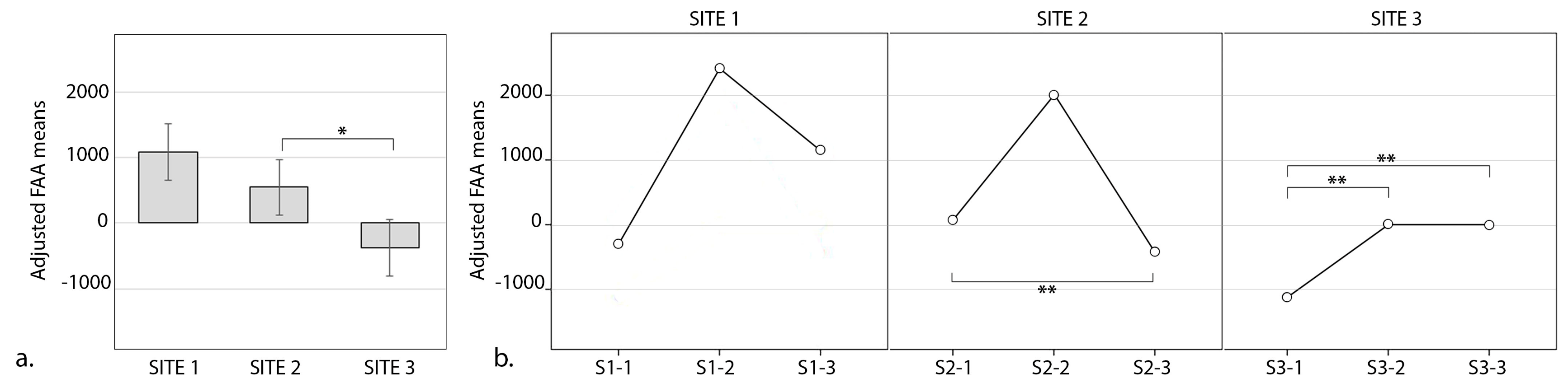
4.2. Differences between the Sites and Views
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helbich, M. Toward dynamic urban environmental exposure assessments in mental health research. Environ. Res. 2018, 161, 129–135. [Google Scholar] [CrossRef]
- Helbich, M. Dynamic Urban Environmental Exposures on Depression and Suicide (NEEDS) in the Netherlands: A protocol for a cross-sectional smartphone tracking study and a longitudinal population register study. BMJ Open 2019, 9, e030075. [Google Scholar] [CrossRef] [PubMed]
- Medina, J. Brain Rules. In Brain Rules; Pear Press: Seattle, WA, USA, 2018. [Google Scholar]
- United Nations. 2018 Revision of the World Urbanization Prospects; United Nations: Tokyo, Japan, 2018. [Google Scholar]
- Beveridge, C.E.; Rocheleau, P. Frederick Law Olmsted; Rizzoli International Publications: New York, NY, USA, 1995. [Google Scholar]
- Mueller, D.P. The current status of urban-rural differences in psychiatric disorder. An emerging trend for depression. J. Nerv. Ment. Dis. 1981, 169, 18–27. [Google Scholar] [CrossRef]
- Peen, J.; Schoevers, R.A.; Beekman, A.T.; Dekker, J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr. Scand. 2010, 121, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.G.; Jonides, J.; Kaplan, S. The cognitive benefits of interacting with nature. Psychol. Sci. 2008, 19, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; CUP: Cambridge, UK, 1989. [Google Scholar]
- Hartig, T.; Staats, H. Guest’s editors’ introduction: Restorative environments. J. Environ. Psychol. 2003, 23, 103–107. [Google Scholar] [CrossRef]
- Ulrich, R.S. Human responses to vegetation and landscapes. Landsc. Urban Plan. 1986, 13, 29–44. [Google Scholar] [CrossRef]
- Kaplan, S. Meditation, Restoration, and the Management of Mental Fatigue. Environ. Behav. 2001, 33, 480–506. [Google Scholar] [CrossRef]
- Hartig, T.; Staats, H. The need for psychological restoration as a determinant of environmental preferences. J. Environ. Psychol. 2006, 26, 215–226. [Google Scholar] [CrossRef]
- Van den Berg, A.E.; Hartig, T.; Staats, H. Preference for Nature in Urbanized Societies: Stress, Restoration, and the Pursuit of Sustainability. J. Soc. Issues 2007, 63, 79–96. [Google Scholar] [CrossRef]
- Hartig, T.; Mang, M.; Evans, G.W. Restorative effects of natural environment experiences. Environ. Behav. 1991, 23, 3–26. [Google Scholar] [CrossRef]
- Ulrich, R. View through a window may influence recovery. Science 1984, 224, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.B.; Davidson, R.J. Left frontal hypoactivation in depression. J. Abnorm. Psychol. 1991, 100, 535. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Schwartz, G.E.; Hardin, E.E.; Baldwin, C.M.; Kline, J.P. Differential resting quantitative electroencephalographic alpha patterns in women with environmental chemical intolerance, depressives, and normals. Biol. Psychiatry 1998, 43, 376–388. [Google Scholar] [CrossRef]
- Gotlib, I.H. EEG alpha asymmetry, depression, and cognitive functioning. Cogn. Emot. 1998, 12, 449–478. [Google Scholar] [CrossRef]
- Debener, S.; Beauducel, A.; Nessler, D.; Brocke, B.; Heilemann, H.; Kayser, J. Is resting anterior EEG alpha asymmetry a trait marker for depression? Neuropsychobiology 2000, 41, 31–37. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Nitschke, J.B.; Oakes, T.R.; Hendrick, A.M.; Horras, K.A.; Larson, C.L.; Abercrombie, H.C.; Schaefer, S.M.; Koger, J.V.; Benca, R.M. Brain electrical tomography in depression: The importance of symptom severity, anxiety, and melancholic features. Biol. Psychiatry 2002, 52, 73–85. [Google Scholar] [CrossRef]
- Sackeim, H.A.; Greenberg, M.S.; Weiman, A.L.; Gur, R.C.; Hungerbuhler, J.P.; Geschwind, N. Hemispheric asymmetry in the expression of positive and negative emotions: Neurologic evidence. Arch. Neurol. 1982, 39, 210–218. [Google Scholar] [CrossRef]
- Banerjee, S.; Argaez, C. CADTH Rapid Response Reports. In Neurofeedback and Biofeedback for Mood and Anxiety Disorders: A Review of Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2017. [Google Scholar]
- Harmon-Jones, E.; Allen, J.J. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. J. Abnorm. Psychol. 1997, 106, 159. [Google Scholar] [CrossRef]
- Walker, J.; Lawson, R.; Kozlowski, G. Current status of QEEG and neurofeedback in the treatment of clinical depression. Neurother. Cent. Dallas 2006, 343, 1–21. [Google Scholar]
- Rosenfeld, J.P. An EEG biofeedback protocol for affective disorders. Clin. Electroencephalogr. 2000, 31, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Baehr, E.; Rosenfeld, J.P.; Baehr, R. Clinical use of an alpha asymmetry neurofeedback protocol in the treatment of mood disorders: Follow-up study one to five years post therapy. J. Neurother. 2001, 4, 11–18. [Google Scholar] [CrossRef]
- Duvinage, M.; Castermans, T.; Petieau, M.; Hoellinger, T.; Cheron, G.; Dutoit, T. Performance of the Emotiv Epoc headset for P300-based applications. Biomed. Eng. Online 2013, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Gascon, M.; Triguero-Mas, M.; Martínez, D.; Dadvand, P.; Forns, J.; Plasència, A.; Nieuwenhuijsen, M.J. Mental health benefits of long-term exposure to residential green and blue spaces: A systematic review. Int. J. Environ. Res. Public Health 2015, 12, 4354–4379. [Google Scholar] [CrossRef]
- Shanahan, D.F.; Bush, R.; Gaston, K.J.; Lin, B.B.; Dean, J.; Barber, E.; Fuller, R.A. Health Benefits from Nature Experiences Depend on Dose. Sci. Rep. 2016, 6, 28551. [Google Scholar] [CrossRef]
- Van den Bosch, M. Live long in nature and long live nature! Lancet Planet. Health 2017, 1, e265–e266. [Google Scholar] [CrossRef]
- Meteorological Service Singapore. Climate of Singapore. Available online: http://www.weather.gov.sg/climate-climate-of-singapore/ (accessed on 25 November 2019).
- Olszewska, A.A.; Marques, P.F.; Ryan, R.L.; Barbosa, F. What makes a landscape contemplative? Environ. Plan. B Urban Anal. City Sci. 2016, 45, 7–25. [Google Scholar] [CrossRef]
- Olszewska, A.A. Contemplative Values of Urban Parks and Gardens: Applying Neuroscience to Landscape Architecture. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2016. [Google Scholar]
- Treib, M. Attending. In Contemporary Landscapes of Contemplation; Routledge: Abingdon, UK, 2005; pp. 27–49. [Google Scholar]
- Krinke, R. Contemporary Landscapes of Contemplation; Routledge: Abingdon, UK, 2005. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory-II. San Antonio 1996, 78, 490–498. [Google Scholar]
- Edwards, A.L. Balanced latin-square designs in psychological research. Am. J. Psychol. 1951, 64, 598–603. [Google Scholar] [CrossRef]
- Shacham, S. A shortened version of the Profile of Mood States. J. Personal. Assess. 1983, 47, 305–306. [Google Scholar] [CrossRef]
- Epstein, Y.; Moran, D.S. Thermal comfort and the heat stress indices. Ind. Health 2006, 44, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Hyvonen, K.; Tornroos, K.; Salonen, K.; Korpela, K.; Feldt, T.; Kinnunen, U. Profiles of Nature Exposure and Outdoor Activities Associated With Occupational Well-Being Among Employees. Front. Psychol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Fong, K.C.; Hart, J.E.; James, P. A Review of Epidemiologic Studies on Greenness and Health: Updated Literature Through 2017. Curr. Environ. Health Rep. 2018, 5, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J.; Schwartz, G.E.; Saron, C.; Bennett, J.; Goleman, D.J. Frontal versus parietal EEG asymmetry during positive and negative affect. Psychophysiology 1979, 16, 202–203. [Google Scholar]
- Olszewska-Guizzo, A.A.; Paiva, T.O.; Barbosa, F. Effects of 3D Contemplative Landscape Videos on Brain Activity in a Passive Exposure EEG Experiment. Front. Psychiatry 2018, 9, 317. [Google Scholar] [CrossRef]
- Bornioli, A.; Parkhurst, G.; Morgan, P.L. The psychological wellbeing benefits of place engagement during walking in urban environments: A qualitative photo-elicitation study. Health Place 2018, 53, 228–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Herrup, K.; Shi, B.E. Physiological Responses of the Youth Viewing a Japanese Garden. In Proceedings of 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 1550–1553. [Google Scholar]
- Triguero-Mas, M.; Gidlow, C.J.; Martínez, D.; De Bont, J.; Carrasco-Turigas, G.; Martínez-Íñiguez, T.; Hurst, G.; Masterson, D.; Donaire-Gonzalez, D.; Seto, E. The effect of randomised exposure to different types of natural outdoor environments compared to exposure to an urban environment on people with indications of psychological distress in Catalonia. PLoS ONE 2017, 12, e0172200. [Google Scholar] [CrossRef]


| Variable | Participants in Preliminary Phase (n = 22) | ||
|---|---|---|---|
| Age | M = 32.9 (SD = 12.7) | ||
| Gender | |||
| Male | 9 (41%) | ||
| Ethnicity | |||
| Chinese | 16 (73%) | ||
| Indian | 1 (5%) | ||
| Others | 5 (22%) | ||
| Education | |||
| Tertiary | 20 (91%) | ||
| Secondary | 2 (9%) | ||
| Profile of Nature Exposure [41] | |||
| High (92%–100%) | 0 | ||
| Versatile (70%–91%) | 3 (14%) | ||
| Unilateral (32%–69%) | 19 (86%) | ||
| Average (19%–31%) | 0 | ||
| Low (0%–19%) | 0 | ||
| Beck Depression Inventory-II | |||
| Low (1–16 pt.) | 19 (86%) | ||
| Moderate (17–30 pt.) | 3 (14%) | ||
| Significant (31–>40 pt.) | 0 | ||
| Inter-session break | M = 11 days (SD = 13 days) | ||
| Site 1 | Site 2 | Site 3 | |
| Temperature (°C) | M = 29.38 (SD = 0.63) | M = 27.73 (SD = 0.50) | M = 28.09 (SD = 0.62) |
| Humidity (%Rh) | M = 69.42 (SD = 0.62) | M = 72.13 (SD = 1.37) | M = 68.01 (SD = 0.30) |
| TDI | M = 26.76 (SD = 0.49) | M = 25.79 (SD = 0.41) | M = 26.52 (SD = 0.54) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska-Guizzo, A.; Sia, A.; Fogel, A.; Ho, R. Can Exposure to Certain Urban Green Spaces Trigger Frontal Alpha Asymmetry in the Brain?—Preliminary Findings from a Passive Task EEG Study. Int. J. Environ. Res. Public Health 2020, 17, 394. https://doi.org/10.3390/ijerph17020394
Olszewska-Guizzo A, Sia A, Fogel A, Ho R. Can Exposure to Certain Urban Green Spaces Trigger Frontal Alpha Asymmetry in the Brain?—Preliminary Findings from a Passive Task EEG Study. International Journal of Environmental Research and Public Health. 2020; 17(2):394. https://doi.org/10.3390/ijerph17020394
Chicago/Turabian StyleOlszewska-Guizzo, Agnieszka, Angelia Sia, Anna Fogel, and Roger Ho. 2020. "Can Exposure to Certain Urban Green Spaces Trigger Frontal Alpha Asymmetry in the Brain?—Preliminary Findings from a Passive Task EEG Study" International Journal of Environmental Research and Public Health 17, no. 2: 394. https://doi.org/10.3390/ijerph17020394
APA StyleOlszewska-Guizzo, A., Sia, A., Fogel, A., & Ho, R. (2020). Can Exposure to Certain Urban Green Spaces Trigger Frontal Alpha Asymmetry in the Brain?—Preliminary Findings from a Passive Task EEG Study. International Journal of Environmental Research and Public Health, 17(2), 394. https://doi.org/10.3390/ijerph17020394







