Association between Body Composition, Physical Activity, Food Intake and Bone Status in German Children and Adolescents
Abstract
1. Introduction
2. Materials & Methods
2.1. Study Design and Participants
2.2. Anthropometric Data and Body Composition
2.3. Bone Status
2.4. Lifestyle Questionnaire
2.5. Food Intake
2.6. Statistics
3. Results
3.1. Participants
3.2. Food Intake
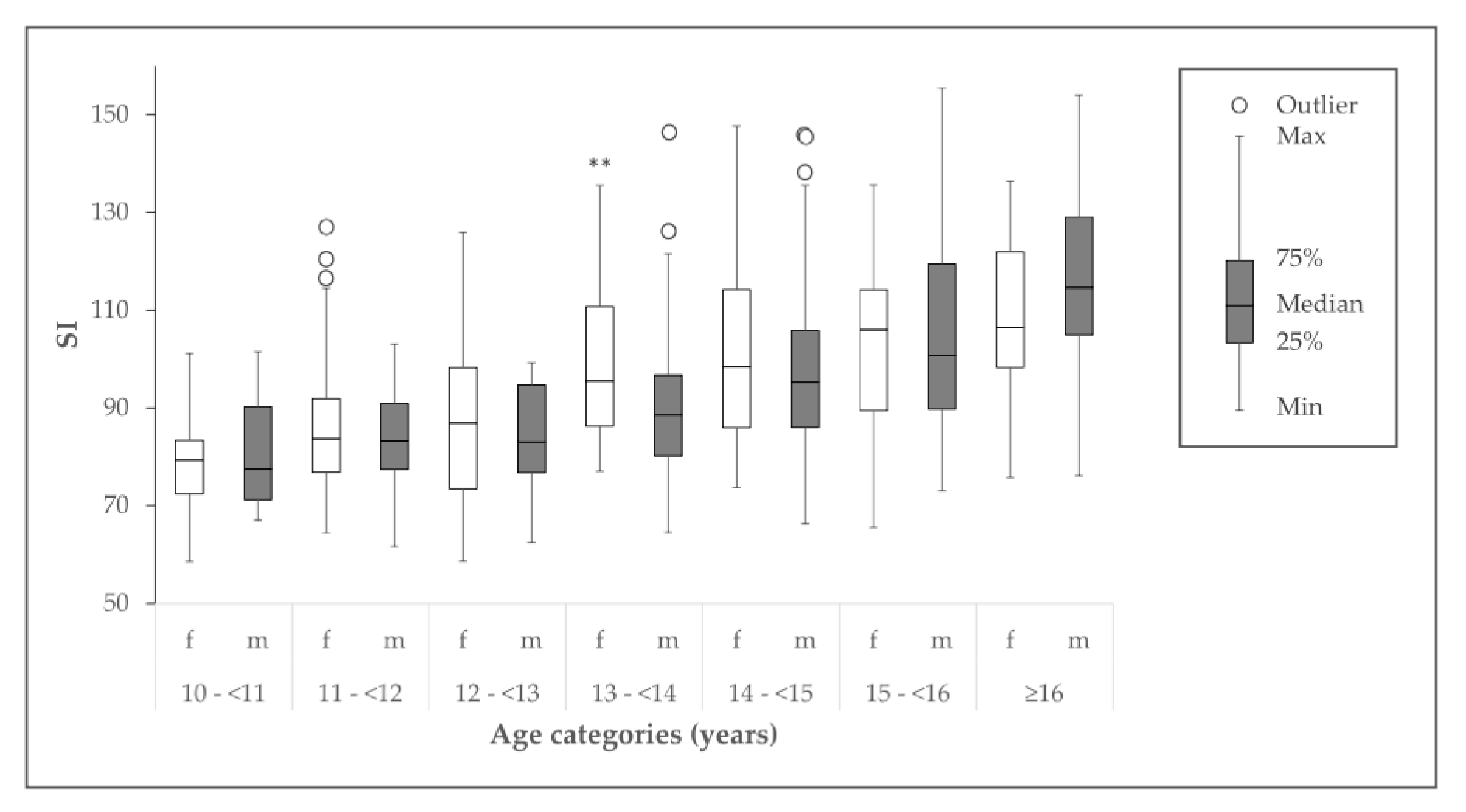
3.3. Bone Status and Influencing Factors
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Osteoporosis: Social and economic impact. Radiol. Clin. North. Am. 2010, 48, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Rizzoli, R.; Slosman, D.; Bonjour, J.P. Familial resemblance for bone mineral mass is expressed before puberty. J. Clin. Endocrinol. Metab. 1998, 83, 358–361. [Google Scholar] [CrossRef]
- Faulkner, R.A.; Bailey, D.A. Osteoporosis: A pediatric concern? Med. Sport Sci. 2007, 51, 1–12. [Google Scholar]
- Heaney, R.P.; Abrams, S.; Dawson-Hughes, B.; Looker, A.; Marcus, R.; Matkovic, V.; Weaver, C. Peak bone mass. Osteoporos. Int. 2001, 11, 985–1009. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.L.; Slemenda, C.W.; Johnston, C.C. The contribution of bone loss to postmenopausal osteoporosis. Osteoporos. Int. 1990, 1, 30–34. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Munhoz, L.; Aoki, E.M.; Cortes, A.R.G.; de Freitas, C.F.; Arita, E.S. Osteoporotic alterations in a group of different ethnicity Brazilian postmenopausal women: An observational study. Gerodontology 2018, 35, 101–109. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Nguyen, U.D.T.; Nguyen, T.V. Association between lean mass, fat mass, and bone mineral density: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 30–38. [Google Scholar] [CrossRef]
- Gabel, L.; Macdonald, H.M.; Nettlefold, L.; McKay, H.A. Physical activity, sedentary time, and bone strength from childhood to early adulthood: A mixed longitudinal HR-pQCT study. J. Bone Min. Res. 2017, 32, 1525–1536. [Google Scholar] [CrossRef]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone formation by applied dynamic loads. J. Bone Joint Surg. Am. 1984, 66, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Witzke, K.A.; Snow, C.M. Effects of plyometric jump training on bone mass in adolescent girls. Med. Sci. Sports Exerc. 2000, 32, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.H.; Gabr, S.A.; Rizk, A.A. Physical fitness, adiposity, and diets as surrogate measures of bone health in schoolchildren: A biochemical and cross-sectional survey analysis. J. Clin. Densitom. 2018, 21, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D.; Mughal, M.Z.; Adams, J.E.; Wilkinson, J.; Berry, J.L.; Edwards, L.; Kift, R.; Marjanovic, E.; Vail, A.; Webb, A.R.; et al. Sun exposure behavior, seasonal vitamin D deficiency, and relationship to bone health in adolescents. J. Clin. Endocrinol. Metab. 2016, 101, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Hynes, K.L.; Dwyer, T. The association between breastfeeding, maternal smoking in utero, and birth weight with bone mass and fractures in adolescents: A 16-year longitudinal study. Osteoporos. Int. 2013, 24, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Hong, K.; Kang, S.W.; Joung, H. A milk and cereal dietary pattern is associated with a reduced likelihood of having a low bone mineral density of the lumbar spine in Korean adolescents. Nutr. Res. 2013, 33, 59–66. [Google Scholar] [CrossRef] [PubMed]
- van den Hooven, E.H.; Gharsalli, M.; Heppe, D.H.M.; Raat, H.; Hofman, A.; Franco, O.H.; Rivadeneira, F.; Jaddoe, V.W. Associations of breast-feeding patterns and introduction of solid foods with childhood bone mass: The Generation R Study. Br. J. Nutr. 2016, 115, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-Mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Plachta-Danielzik, S.; Gehrke, M.I.; Kehden, B.; Kromeyer-Hauschild, K.; Grillenberger, M.; Willhöft, C.; Bosy-Westphal, A.; Müller, M.J. Body fat percentiles for German children and adolescents. Obes. Facts. 2012, 5, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Chula de Castro, J.A.; Rodrigues de Lima, T.; Santos Silva, D.A. Body composition estimation in children and adolescents by bioelectrical impedance analysis: A systematic review. J. Bodyw. Mov. Ther. 2018, 22, 134–146. [Google Scholar] [CrossRef]
- Wünsche, K.; Wünsche, B.; Fähnrich, H.; Mentzel, H.-J.; Vogt, S.; Abendroth, K.; Kaiser, W.A. Ultrasound bone densitometry of the os calcis in children and adolescents. Calcif. Tissue Int. 2000, 67, 349–355. [Google Scholar]
- Lewiecki, E.M.; Gordon, C.M.; Baim, S.; Leonard, M.B.; Bishop, N.J.; Bianchi, M.L.; Kalkwarf, H.J.; Langman, C.B.; Plotkin, H.; Rauch, F.; et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 2008, 43, 1115–1121. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, B.; Gong, J.; Xu, H.; Bai, Z. The correlation between calcaneus stiffness index calculated by QUS and total body BMD assessed by DXA in Chinese children and adolescents. J. Bone Miner. Metab. 2014, 32, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, M.; Lebiedowski, M.; Lorenc, R.S.; Trempe, J. Ultrasound bone measurement in pediatric subjects. Calcif. Tissue Int. 1995, 56, 368–371. [Google Scholar] [CrossRef]
- Krems, C.; Lührmann, P.M.; Neuhäuser-Berthold, M. Physical activity in young and elderly subjects. J. Sports Med. Phys. Fitness. 2004, 44, 71–76. [Google Scholar] [PubMed]
- Müller, M.J.; Bosy-Westphal, A.; Klaus, S.; Kreymann, G.; Lührmann, P.M.; Neuhäuser-Berthold, M.; Noack, R.; Pirke, K.M.; Platte, P.; Selberg, O.; et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am. J. Clin. Nutr. 2004, 80, 1379–1390. [Google Scholar]
- World Health Organization (WHO). Energy and protein requirement. Report of a Joint FAO/WHO/UNU Expert Consultation. Technical Report Series 724. WHO: Geneva, Switzerland, 1985. [Google Scholar]
- Carskadon, M.A.; Acebo, C. A self-administered rating scale for pubertal development. J. Adolesc. Health 1993, 14, 190–195. [Google Scholar] [CrossRef]
- Petersen, A.C.; Crockett, L.; Richards, M.; Boxer, A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J. Youth Adolesc. 1988, 17, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Crockett, L.J. Pubertal Development Scale: Pubertal categories. Pennsylvania State University, Department of Human Development and Family Studies, University Park. 1988; Unpublished work. [Google Scholar]
- Schweter, A. Einfluss verschiedener Lebensstilfaktoren auf die Knochenmasse von Kindern und Jugendlichen: Möglichkeiten der Gesundheitsförderung im Setting Schule. PhD Thesis; University of Education Schwäbisch Gmünd: Schwäbisch Gmünd, Germany, 2014. Available online: https://phsg.bsz-bw.de/frontdoor/index/index/docId/27 (accessed on 15 May 2020).
- Mensink, G.B.M.; Burger, M. Was isst du? Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. 2004, 47, 219–226. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Heseker, H.; Richter, A.; Stahl, A.; Vohmann, C. Ernährungsstudie als KiGGS-Modul (EsKiMo). Forschungsbericht. Robert-Koch-Institut, Universität Paderborn: Berlin, Paderborn, Germany, 2007; pp. 1–137. [Google Scholar]
- Waijers, P.M.C.M.; Feskens, E.J.M.; Ocké, M.C. A critical review of predefined diet quality scores. Br. J. Nutr. 2007, 97, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Kleiser, C.; Mensink, G.B.M.; Scheidt-Nave, C.; Kurth, B.-M. HuSKY: A healthy nutrition score based on food intake of children and adolescents in Germany. Br. J Nutr. 2009, 102, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Forschungsinstitut für Kinderernährung (FKE) Dortmund. Empfehlungen für die Ernährung von Kindern und Jugendlichen. Die Optimierte Mischkost optimiX ®, 2nd ed.; FKE Dortmund: Dortmund, Germany, 2008. [Google Scholar]
- Alexy, U.; Clausen, K.; Kersting, M. Die Ernährung gesunder Kinder und Jugendlicher nach dem Konzept der Optimierten Mischkost. Ernährungs Umschau 2008, 55, 168–177. [Google Scholar]
- Cohen, J. Statistical Power Analysis for Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Max Rubner Institut (MRI); Bundesforschungsinstitut für Ernährung und Lebensmittel (BFEL). Nationale Verzehrsstudie Ergebnisbericht, Teil 2. Die bundesweite Befragung zur Ernährung von Jugendlichen und Erwachsenen; MRI, BFEL: Karlsruhe, Germany, 2008. Available online: https://www.bmel.de/SharedDocs/Downloads/Ernaehrung/NVS_ErgebnisberichtTeil2.pdf?__blob=publicationFile (accessed on 8 April 2020).
- Mentzel, H.-J.; Wünsche, K.; Malich, A.; Böttcher, J.; Vogt, S.; Kaiser, W. Einfluss sportlicher Aktivität von Kindern und Jugendlichen auf den Kalkaneus - Eine Untersuchung mit quantitativem Ultraschall. Fortschr Röntgenstr 2005, 177, 524–529. [Google Scholar] [CrossRef]
- Wang, K.C.; Wang, K.C.; Amirabadi, A.; Cheung, E.; Uleryk, E.; Moineddin, R.; Doria, A.S. Evidence-based outcomes on diagnostic accuracy of quantitative ultrasound for assessment of pediatric osteoporosis—A systematic review. Pediatr. Radiol. 2014, 44, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, X.-M.; Chen, T.-J.; Bai, M.-J. Rural-urban differences of dietary patterns, overweight, and bone mineral status in Chinese students. Nutrients 2016, 8, 537. [Google Scholar] [CrossRef]
- Zhao, L.-J.; Liu, Y.-J.; Liu, P.-Y.; Hamilton, J.; Recker, R.R.; Deng, H.-W. Relationship of obesity with osteoporosis. J. Clin. Endocrinol. Metab. 2007, 92, 1640–1646. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Méndez-Sánchez, L.; Muñoz-Aguirre, P.; Tucker, K.; Clark, P. Dietary patterns, bone mineral density, and risk of fractures: A systematic review and meta-analysis. Nutrients 2018, 10, 1922. [Google Scholar] [CrossRef]
- Torres-Costoso, A.; Vlachopoulos, D.; Ubago-Guisado, E.; Ferri-Morales, A.; Cavero-Redondo, I.; Martínez-Vizcaino, V.; Gracia-Marco, L. Agreement between dual-energy x-ray absorptiometry and quantitative ultrasound to evaluate bone health in adolescents: The PRO-BONE study. Pediatr. Exerc. Sci. 2018, 30, 466–473. [Google Scholar] [CrossRef]
- Baroncelli, G.I. Quantitative ultrasound methods to assess bone mineral status in children: Technical characteristics, performance, and clinical application. Pediatr. Res. 2008, 63, 220–228. [Google Scholar] [CrossRef]
- Dowd, K.P.; Szeklicki, R.; Minetto, M.A.; Murphy, M.H.; Polito, A.; Ghigo, E.; van der Ploeg, H.; Ekelund, U.; Maciaszek, J.; Stemplewski, R.; et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: ADEDIPAC study. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Heydenreich, J.; Schutz, Y.; Melzer, K.; Kayser, B. Comparison of conventional and individualized 1-MET values for expressing maximum aerobic metabolic rate and habitual activity related energy expenditure. Nutrients 2019, 11, 458. [Google Scholar] [CrossRef] [PubMed]
| Food Group | The Following Items Are Included in the BoneHEI: “How Often Do You Consume…” | Allocation of Points |
|---|---|---|
| Fruits and vegetables | Fresh/boiled/preserved/frozen fruits; cooked vegetables (prepared from fresh, frozen, preserved vegetables)/salad/raw vegetables | I/R ≤ 1: proportional points up to 100 I/R > 1: 100 points |
| Fish | Fish | |
| Bread | Bread; bread roll | I/R ≤ 1: proportional points up to 100 I/R > 1 and ≤ 2: 100 points I/R > 2: points proportionally subtracted from 100 |
| Milk and dairy products | Milk/cocoa/yoghurt/curd/buttermilk; cheese; cream cheese | I/R ≤ 1: proportional points up to 100 I/R > 1 and ≤ 2: points proportionally subtracted from 100 I/R > 2: 0 points |
| Meat and sausages | Meat; sausages/ham | I/R ≤ 1: 100 points I/R > 1 and ≤ 2: points proportionally subtracted from 100 I/R > 2: 0 points |
| Tolerated food | Sweets/chocolate/chocolate bar/cake/pastry/cookies/drops/fruit gums; snacks/chips/salt sticks/cracker | |
| Soft drinks | Coke/lemonade/soft drinks/energy drinks/iced tea | |
| Caffeinated beverages | Coffee/black tea/green tea |
| Characteristics | Girls (n = 248) | Boys (n = 231) |
|---|---|---|
| Age (years) | 13.4 ± 1.9 | 13.6 ± 1.7 |
| Body mass (kg) | 51.3 ± 14.8 | 51.0 ± 13.7 |
| Height (cm) | 158 ± 10 | 161 ± 13 * |
| BMI a kg m−2 Strongly underweight (%) Underweight (%) Normal weight (%) Overweight (%) Obese (%) | 20.2 ± 4.8 2.4 6.0 73.4 9.7 8.5 | 19.3 ± 3.3 3.5 7.4 77.5 8.2 3.5 |
| Fat-free mass (kg) | 37.6 ± 7.2 | 41.6 ± 10.4 *** |
| Fat mass (kg) | 13.5 ± 7.9 | 9.6 ± 5.6 *** |
| Fat mass (%) | 24.9 ± 8.1 | 18.0 ± 7.3 *** |
| PAL b | 1.4 ± 0.1 | 1.5 ± 0.2 *** |
| RMR c (kJ d−1) (kcal kg−1 d−1) | 5952 ± 618 29.3 ± 5.1 | 6345 ± 746 *** 31.2 ± 5.3 *** |
| Puberty category score d Prepubescent (%) Early (%) Midpubescent (%) Advanced (%) Postpubescent (%) | 8.4 ± 2.9 3.5 10.5 18.6 57.0 10.5 | 7.0 ± 2.5 *** 10.2 20.9 36.2 31.1 1.7 |
| History of fractures (%) | 22.6 | 26.6 |
| Use of medication (%) | 6.5 | 7.9 |
| Staying outside (h d−1) | 3.6 ± 2.1 | 5.0 ± 5.6 ** |
| Breastfeeding e Prevalence (%) Duration (months) | 87.4 7.0 ± 4.2 | 83.8 7.8 ± 5.1 |
| Vitamin D supplementation f (%) | 72.7 | 73.5 |
| Food Group | Intake of Children | National Average Intake (14–18 years) | Recommended Intake (15–18 years) | BoneHEI Score |
|---|---|---|---|---|
| Fruits and vegetables (g d−1) | ||||
| Girls Boys | 471 ± 661 387 ± 516 ** | 323263 | 600 g d−1 700 g d−1 | 57 ± 33 45 ± 34 *** |
| Fish (g d−1) | ||||
| Girls Boys | 7 ± 14 17 ± 40 *** | 5 6 | 100 g wk−1 (14 g d−1) | 31 ± 39 47 ± 43 *** |
| Bread (g d−1) | ||||
| Girls Boys | 115 ± 136 170 ± 188 *** | 142 182 | 280 g d−1 350 g d−1 | 37 ± 31 43 ± 32 * |
| Milk and dairy products (g d−1) | ||||
| Girls Boys | 337 ± 377 381 ± 418 | 240 330 | 450 g d−1 500 g d-1 | 37 ± 29 42 ± 32 |
| Meat and sausages (g d−1) | ||||
| Girls Boys | 75 ± 103 166 ± 198 *** | 57 104 | 75 g d−1 85 g d−1 | 76 ± 39 52 ± 45 *** |
| Tolerated food (g d−1) | ||||
| Girls Boys | 77 ± 175 118 ± 271 | 69 81 | 1 serving d−1 (48 g) | 75 ± 39 70 ± 43 |
| Soft drinks (mL d−1) | ||||
| Girls Boys | 347 ± 694 563 ± 858 *** | 260 505 | 200 mL d−1 a | 76 ± 42 60 ± 47 *** |
| Caffeinated beverages (mL d−1) | ||||
| Girls Boys | 106 ± 322 103 ± 333 | 118 116 | 150 mL d−1 a | 90 ± 30 92 ± 27 |
| Total BoneHEI | ||||
| Girls Boys | 60 ± 13 56 ± 16 * | |||
| Bone Status Parameters | Girls (n = 248) | Boys (n = 231) |
|---|---|---|
| BUA (dB MHz−1) | 111 ± 18 | 110 ± 16 |
| BUA Z-score >−2.0 (%) ≤−2.0 (%) | 3.55 ± 1.22 100 0 | 3.87 ± 1.12 100 0 |
| SOS (m s−1) | 1570 ± 28 | 1571 ± 34 |
| SOS Z-score >−2.0 (%) ≤−2.0 (%) | −0.09 ± 0.98 98.0 2.0 | 0.37 ± 1.33 99.6 0.4 |
| SI | 94 ± 18 | 94 ± 19 |
| Sex | BUA (dB MHz−1) r (p-Value) | SOS (m s−1) r (p-Value) | SI r (p-Value) |
|---|---|---|---|
| Age (years) | |||
| Girls Boys | 0.543 (0.000) 0.566 (0.000) | 0.378 (0.000) 0.398 (0.000) | 0.523 (0.000) 0.536 (0.000) |
| BMI (kg m−2) | |||
| Girls Boys | 0.477 (0.000) 0.417 (0.000) | 0.269 (0.000) 0.165 (0.012) | 0.435 (0.000) 0.319 (0.000) |
| Fat-free mass (kg) | |||
| Girls Boys | 0.576 (0.000) 0.618 (0.000) | 0.360 (0.000) 0.396 (0.000) | 0.540 (0.000) 0.563 (0.000) |
| Fat mass (kg) | |||
| Girls Boys | 0.451 (0.000) 0.226 (0.001) | 0.203 (0.001) −0.053 (0.424) | 0.392 (0.000) 0.094 (0.156) |
| Fat mass (%) | |||
| Girls Boys | 0.325 (0.000) −0.061 (0.359) | 0.108 (0.091) −0.266 (0.000) | 0.270 (0.000) −0.182 (0.006) |
| PAL a | |||
| Girls Boys | 0.171 (0.011) 0.180 (0.017) | 0.183 (0.006) 0.240 (0.001) | 0.196 (0.004) 0.223 (0.003) |
| Puberty category score b | |||
| Girls Boys | 0.548 (0.000) 0.555 (0.000) | 0.369 (0.000) 0.452 (0.000) | 0.532 (0.000) 0.543 (0.000) |
| BoneHEI | |||
| Girls Boys | −0.061 (0.373) −0.109 (0.113) | −0.093 (0.169) −0.056 (0.421) | −0.075 (0.271) −0.083 (0.231) |
| Staying outside (h d−1) | |||
| Girls Boys | 0.052 (0.426) 0.136 (0.042) | 0.034 (0.607) 0.047 (0.485) | 0.047 (0.468) 0.096 (0.150) |
| Duration of breastfeeding during infancy (months) | |||
| Girls Boys | −0.068 (0.323) 0.172 (0.018) | −0.090 (0.190) 0.098 (0.179) | −0.067 (0.327) 0.164 (0.023) |
| Regression Steps | B | SE B | β | R2 |
|---|---|---|---|---|
| Step 1 | 0.28 *** | |||
| Constant | 23.84 | 5.74 | ||
| Age (years) | 5.21 | 0.42 | 0.53 *** | |
| Step 2 | 0.32 *** | |||
| Constant | 28.43 | 5.68 | ||
| Age (years) | 3.15 | 0.60 | 0.32 *** | |
| Fat-free mass | 0.59 | 0.13 | 0.29 *** | |
| Step 3 | 0.33 *** | |||
| Constant | 25.70 | 5.72 | ||
| Age (years) | 2.88 | 0.60 | 0.29 *** | |
| Fat-free mass | 0.69 | 0.13 | 0.34 *** | |
| Sex (0 = male, 1 = female) | 4.31 | 1.57 | 0.12 ** | |
| Step 4 | 0.35 *** | |||
| Constant | −0.60 | 10.95 | ||
| Age (years) | 2.97 | 0.60 | 0.30 *** | |
| Fat-free mass | 0.65 | 0.13 | 0.32 *** | |
| Sex (0 = male, 1 = female) | 6.21 | 1.70 | 0.17 *** | |
| PAL | 17.55 | 6.25 | 0.13 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydenreich, J.; Schweter, A.; Lührmann, P. Association between Body Composition, Physical Activity, Food Intake and Bone Status in German Children and Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 7294. https://doi.org/10.3390/ijerph17197294
Heydenreich J, Schweter A, Lührmann P. Association between Body Composition, Physical Activity, Food Intake and Bone Status in German Children and Adolescents. International Journal of Environmental Research and Public Health. 2020; 17(19):7294. https://doi.org/10.3390/ijerph17197294
Chicago/Turabian StyleHeydenreich, Juliane, Antje Schweter, and Petra Lührmann. 2020. "Association between Body Composition, Physical Activity, Food Intake and Bone Status in German Children and Adolescents" International Journal of Environmental Research and Public Health 17, no. 19: 7294. https://doi.org/10.3390/ijerph17197294
APA StyleHeydenreich, J., Schweter, A., & Lührmann, P. (2020). Association between Body Composition, Physical Activity, Food Intake and Bone Status in German Children and Adolescents. International Journal of Environmental Research and Public Health, 17(19), 7294. https://doi.org/10.3390/ijerph17197294





