Vancomycin for Dialytic Therapy in Critically Ill Patients: Analysis of Its Reduction and the Factors Associated with Subtherapeutic Concentrations
Abstract
1. Introduction
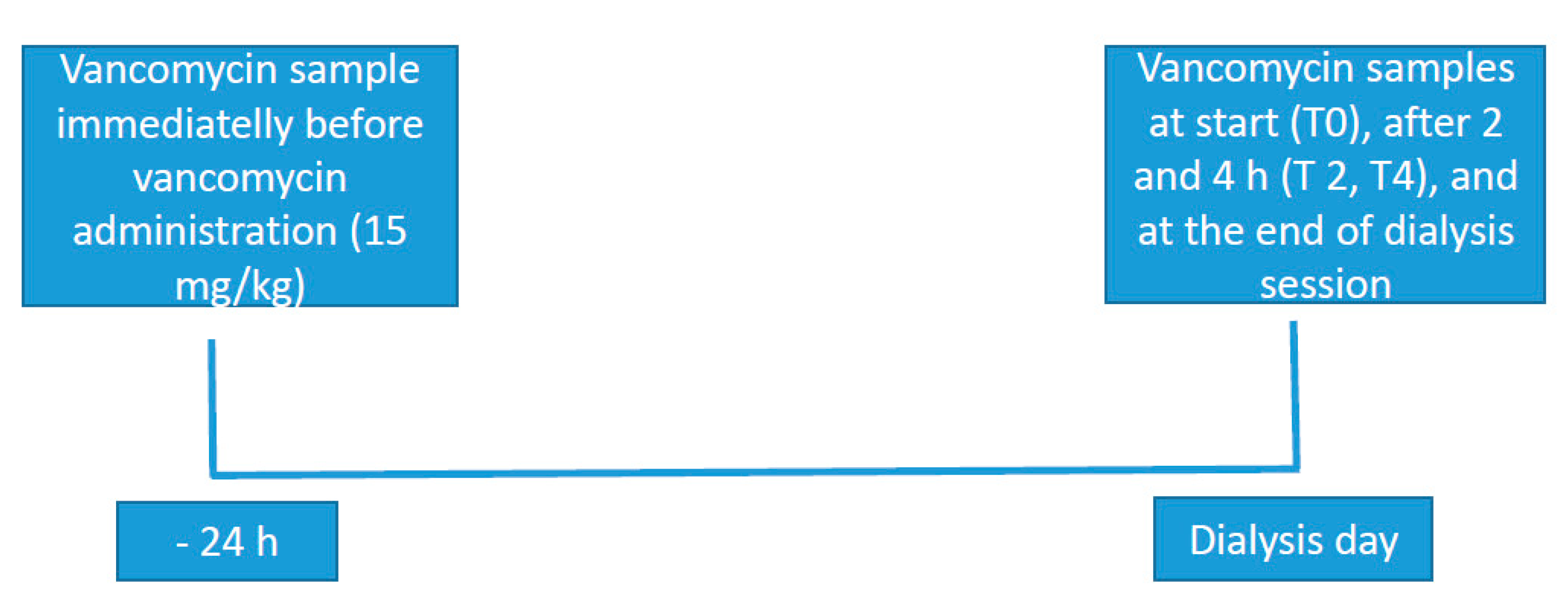
2. Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alberti, C.; Brun-Buisson, C.; Burchardi, H.; Martin, C.; Goodman, S.; Artigas, A.; Sicignano, A.; Palazzo, M.; Moreno, R.; Boulmè, R.; et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002, 28, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Metha, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A. Dialytic treatment for septic patients with acute kidney injury. Kidney Blood Press Res. 2011, 34, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Aegerter, P.; Jars-Guincestre, M.C.; Guidetfor, B. Current epidemiology of septic shock: The CUB-Réa Network. Am. J. Respir. Crit. Med. 2003, 168, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef]
- Lewis, S.J.; Mueller, B.A. Antibiotic Dosing in Patients With Acute Kidney Injury: Enough But Not Too Much. Intensive Care Med. 2016, 31, 164–176. [Google Scholar] [CrossRef]
- Eyler, R.F.; Mueller, B.A. Antibiotic dosing in critically ill patients with acute kidney injury. Nat. Rev. Nephorol. 2011, 7, 226–235. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef]
- Schier, R.W.; Wang, W. Acute Renal Failure and Sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.A.; Kuhl, D.A.; Hickerson, W.L. Pharmacokinetics of systemically administered antibiotics in patients with thermal injury. Clin. Infect. Dis. 1992, 14, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Keynan, Y. Vancomycin revisited—60 years later. Front. Public Health 2014, 2, 217. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T.; Blake, P.G.; Tood, S.I. Handbook of Dialysis, 5th ed.; Wolters Kluwer: New York, NY, USA, 2015. [Google Scholar]
- Pai, A.B.; Pai, M.P. Vancomycin dosing in high flux hemodialysis: A limited sampling algorithm. Am. J. Health Syst. Pharm. 2004, 61, 1812–1816. [Google Scholar] [CrossRef]
- Klansuwan, N.; Ratanajamit, C.; Kasiwong, S.; Wangsiripaisan, A. Clearance of vancomycin during high-efficiency hemodialysis. J. Med. Assoc. Thai. 2006, 89, 986–991. [Google Scholar]
- Ariano, R.E.; Fine, A.; Sitar, D.S.; Rexrode, S.; Zelenitsky, S.A. Adequacy of a vancomycin dosing regimen in patients receiving high-flux hemodialysis. Am. J. Kidney Dis. 2005, 46, 681–687. [Google Scholar] [CrossRef]
- López, K.J.V.; Bertoluci, D.F.; Vicente, K.M.; Dell’Aquilla, A.M.; Santos, S.R.C.J. Simultaneous determination of cefepime, vancomycin and imipenem in human plasma of burn patients by high-performance liquid chromatography. J. Chromatogr. B 2007, 860, 241–245. [Google Scholar] [CrossRef]
- Santos, S.R.C.J.; Sanches-Giraud, C.; Silva, J.R.C.V.; Souza, F.F.; Gomez, D.S.; Campos, E.V.; Azevedo, R.P.; Ferreira, M.C.; Nascimento, J.W.L. Pharmacokinetic-Pharmacodynamic Correlation for Meropenem in one Burn Child by Drug Plasma Monitoring using a Bioanalytical Liquid Cromatographic Method. Rev Port. Farmacoter. 2012, 3, 224–232. [Google Scholar]
- Petejovaa, N.; Martineka, A.; Zahalkovab, J.; Duricovad, J.; Brozmanovad, H.; Urbaneke, K.; Grundmannd, M.; Kacirovad, I. Vancomycin removal during low-flux and high-flux extended daily hemodialysis in critically ill septic patients. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2012, 156, 342–347. [Google Scholar] [CrossRef]
- Frimodt-Moller, N. How predictive is PK/PD for antibacterial agents? Int. J. Antimicrob. Agents 2002, 19, 333–339. [Google Scholar] [CrossRef]
- Rybak, M.J. Pharmacodynamics: Relation to antimicrobial resistance. Am. J. Med. 2006, 119, S37–S44. [Google Scholar] [CrossRef] [PubMed]
- Bussab, W.O. Estatística Básica, 4th ed.; Editora Saraiva: São Paulo, Brazil, 1987. [Google Scholar]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Schetz, M. Drug dosing in continuous renal replacement therapy: General rules. Curr. Opin. Crit. Care 2007, 13, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Gomersall, C.D.; Tian, Q.; Joynt, G.M.; Freebairn, R.; Lipman, J. Principles of antibacterial dosing in continuous renal replacement therapy. Crit. Care Med. 2009, 37, 2268–2282. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Mueller, B.A. Antibiotic dosing in critically ill patients receiving CRRT: Underdosing is overprevalent. Semin. Dial. 2014, 27, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Crew, P.; Heintz, S.J.; Heintz, B.H. Vancomycin dosing and monitoring for patients with end-stage renal disease receiving intermittent hemodialysis. Am. J. Health Syst. Pharm. 2015, 72, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.; Mushatt, D.M.; Mihm, L.B.; Dreisbach, A.W.; Simon, E.E. Antibiotic dosing in slow extended daily dialysis. Clin. Infect. Dis. 2009, 49, 433–437. [Google Scholar]
- Sethi, S.K.; Krishnappa, V.; Nangethu, N.; Nemer, P.; Frazee, L.A.; Raina, R. Antibiotic Dosing in Sustained Low-Efficiency Dialysis in Critically Ill Patients. Can. J. Kidney Health Dis. 2018, 5. [Google Scholar] [CrossRef]
- Keough, L.A.; Krauss, A.; Hudson, J.Q. Inadequate antibiotic dosing in patients receiving sustained low efficiency dialysis. Int. J. Clin. Pharm. 2018, 40, 1–7. [Google Scholar] [CrossRef]
- Harris, L.E.; Reaves, A.B.; Krauss, A.G.; Hudson, J.G.Q.Q. Evaluation of antibiotic prescribing patterns in patients receiving sustained low-efficiency dialysis: Opportunities for pharmacists. Int. J. Pharm. Pract. 2013, 21, 55–61. [Google Scholar] [CrossRef]
- Lewis, S.J.; Mueller, B.A. Development of a vancomycin dosing approach for critically ill patients receiving hybrid hemodialysis using Monte Carlo simulation. SAGE Open Med. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Ahern, J.W.; Lai, C.; Rebuck, J.A.; Possidente, C.J.; Weidner, M. Experience with vancomycin in patients receiving slow low-efficiency dialysis. Hosp. Pharm. 2004, 39, 138–143. [Google Scholar] [CrossRef]
- Ezdon, D.; Brown, M.; Meshay, M.; Brophy, A.; Hickey, R.; Aggarwal, S.; Polisetty, R.; Cuhaci, B.; Mitchell, J.; Schlecht, H.P. Weight-based maintenance dosing of vancomycin in hemodialysis. In Proceedings of the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 9–12 September 2012; American Society for Microbiology: Washington, DC, USA, 2012. [Google Scholar]
- Golestaneh, L.; Gofran, A.; Mokrzycki, M.H.; Chen, J.L. Removal of vancomycin in sustained low-efficiency dialysis (SLED): A need for better surveillance and dosing. Clin. Nephrol. 2009, 72, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Czock, D.; Schöpke, T.; Hafer, C.; Bode-Böger, S.M.; Kuse, E.; Fliser, D. Pharmacokinetics and total elimination of meropenem and vancomycin in intensive care unit patients undergoing extended daily dialysis. Crit. Care Med. 2006, 34, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ponce, D.; Zamoner, W.; Freitas, F.M.; Balbi, A.; Awdishu, L. Vancomycin Removal During High-Volume Peritoneal Dialysis in Acute Kidney Injury Patients: A Prospective Cohort Clinical Study. Kidney Int. Rep. 2018, 4, 112–118. [Google Scholar] [CrossRef]
- Launay-Vachey, V.; Izzedine, H.; Mercadal, L.; Deray, G. Clinical review: Use of vancomycin in dialysis patients. Crit. Care 2002, 6, 313–316. [Google Scholar] [CrossRef]
| Pharmacokinetic | Formula |
|---|---|
| Distribution Volume (L) | VD = Vancomycin dose (mg)/concentration of vancomycin (mg/L) |
| Dialytic Clearence of vancomycin (L/h) | Cl vanco = (Initial—final concentration)/Initial concentration × QD/t |
| Half-life time | T½ life = 0.693 × VD/Cl |
| % reduction in vancomycin | % red = (C pre HD—C final HD)/C pre HD) × 100 |
| Variables | General (n = 55) | IHD (n = 27) | PHD 6 h (n = 17) | PHD 10 h (n = 11) | p |
|---|---|---|---|---|---|
| Age (Years) | 62.61 ± 13.12 | 63.30 ± 13.86 | 61.94 ± 14.15 | 62 ± 10.36 | 0.934 |
| Male sex (%) | 37 (67.3) | 17 (62.86) | 13 (76.47) | 6 (54.54) | 0.457 |
| Weight (kg) | 75.07 ± 24.20 | 71 (64–84.75) | 69 (57.32–77.75) | 62 (58.50–85.75) | 0.594 |
| Comorbidities | |||||
| Diabetes (%) | 28 (50.0) | 13 (48.15) | 10 (58.82) | 5 (45.45) | 0.726 |
| Hypertension (%) | 40 (72.72) | 24 (88.89) a | 10 (58.82) b | 6 (54.54) b | 0.030 |
| Cardiovascular disease (%) | 27 (49.09) | 18 (66.67) a | 5 (29.41) b | 4 (36.36) ab | 0.035 |
| Obesity (%) | 9 (16.36) | 3 (11.11) | 2 (11.76) | 4 (36.36) | 0.134 |
| Neoplasia (%) | 6 (10.90) | 2 (7.41) | 2 (11.76) | 2 (18.18) | 0.621 |
| Smoking (%) | 25 (45.45) | 11 (40.74) | 8 (47.06) | 6 (54.54) | 0.731 |
| Chronic Kidneyl Disease (%) | 4 (7.27) | 2 (7.40) | 1 (5.88) | 1 (9.09) | 0.950 |
| Other (%) | 30 (54.54) | 13 (48.15) | 10 (58.82) | 7 (63.64) | 0.626 |
| Hospitalization Diagnostic | |||||
| Sepsis (%) | 35 (63.64) | 22 (81.48) a | 7 (41.18) b | 6 (54.54) b | 0.02 |
| Acute abdomen (%) | 7 (12.73) | 3 (11.11) | 3 (17.65) | 1 (9.09) | 0.754 |
| Acute Coronarary Syndrome (%) | 6 (10.90) | 5 (18.6) | 1 (5.88) | 0 | 0.183 |
| Trauma (%) | 3 (5.45) | 1 (3.70) | 1 (5.88) | 1 (9.09) | 0.799 |
| Elective Surgery (%) | 7 (12.73) | 2 (7.41) | 3 (17.65) | 2 (18.18) | 0.508 |
| Other (%) | 3 (5.45) | 1 (3.70) | 0 | 2 (18.18) | 0.100 |
| Arterial Thrombosis (%) | 7 (12.73) | 5 (18.52) | 2 (11.76) | 0 | 0.172 |
| Infectious Focus | |||||
| Lung (%) | 37 (67.27) | 21 (77.78) | 9 (52.94) | 7 (63.64) | 0.223 |
| Urinary (%) | 3 (5.45) | 2 (7.41) | 1 (5.88) | 0 | 0.657 |
| Abdominal (%) | 6 (10.90) | 1 (3.70) | 3 (17.65) | 2 (18.18) | 0.242 |
| Cutaneous (%) | 5 (9.09) | 1 (3.70) | 1 (5.88) | 3 (27.27) | 0.062 |
| Other (%) | 6 (10.90) | 3 (11.11) | 3 (17.65) | 0 | 0.343 |
| APACHE II | 29.64 ± 7.56 | 27.16 ± 7.930 a | 33.06 ± 7.07 b | 30.00 ± 5.59 ab | 0.042 |
| LIANO | 0.75 (0.6–0.87) | 0.72 (0.54–0.78) | 0.78 (0.71–0.84) | 0.89 (0.44–0.89) | 0.163 |
| Death (%) | 41 (74.54) | 19 (70.37) | 15 (88.23) | 7 (63.63) | 0.27 |
| Variables | General (n = 55) | IHD (n = 27) | PHD 6 h (n = 17) | PHD 10 h (n = 11) | p |
|---|---|---|---|---|---|
| Noradrenaline (mcg/kg/min) | 0.25 (0.05–0.5) | 0.05 (0.0–0.14) a | 0.5 (0.33–0.8) b | 0.45 (0.38–0.57) b | <0.001 |
| Urinary Volume (mL/24 h) | 200 (50–650) | 300 (133–950) | 150 (27.5–800) | 100 (0.0–260) | 0.072 |
| Hematocrit (%) | 27.85 ± 3.89 | 26.5 (23.8–28.9) | 27.9 (26.75–30.35) | 27.8 (26.8–29.9) | 0.062 |
| PCR (mg/L) | 27.1 (7.3–33.7) | 25.8 (5.50–31.82) | 23.2 (8.12–35.3) | 27.8 (23.8–35.1) | 0.518 |
| Albumin (g/dL) | 2.18 ± 0.49 | 2.21 ± 0.43 | 2.17 ± 0.51 | 2.11 ± 0.67 | 0.049 |
| Ultrafiltration (mL) | 1901.82 ± 1148.19 | 2018.52 ± 1138.84 | 1564.71 ± 1225.22 | 2136.36 ± 1026.91 | 0.339 |
| Kt/V | 0.89 ± 0.33 | 0.86 ± 0.28 | 0.82 ± 0.35 | 1.08 ± 0.36 | 0.093 |
| Intradialitic hypotension | 9 (16.36) | 4 (14.81) | 4 (23.52) | 1 (9.09) | 0.574 |
| Variables | General (n = 55) | IHD (n = 27) | PHD 6 h (n = 17) | PHD 10 h (n = 11) | p |
|---|---|---|---|---|---|
| Vancomycin days | 5 (2–10) | 7 (3–12) | 5 (2–6.5) | 4 (2–12) | 0.39 |
| Vancomycin dose (mg/kg/dia) | 15.5 ± 7.3 | 13.9 (8.6–15.4) | 14.7 (13.5–18.0) | 18.7 (12.0–24.6) | 0.05 |
| Vancomycin concentration before analysis (mg/L) | 19.6 (16–27.8) | 20.9 (17.8–31.5) | 20.2 (15.4–27.7) | 17.9 (14.1–23.0) | 0.61 |
| Distribution volume (L/Kg) | 0.6 (0.4–0.85) | 0.40 (0.29–0.62) a | 0.8 (0.61–1.0) b | 0.7 (0.59–1.29) b | <0.001 |
| Half-life time (h) | 17.4 (6.7–32.6) | 6.70 (4.2–11.9) a | 27.6 (19.5–74.4) b | 38.4 (30.62–74.78) b | <0.001 |
| Vancomycin dialytic Clearence (L/h) | 1.9 (1.13–3.23) | 3.2 (2.54–3.68) a | 1.3 (0.93–1.72) b | 1.0 (0.75–1.15) b | <0.001 |
| Vancomycin concentration (mg/L) at T0 | 26.0 (15.9–35.9) | 34.2 (22–44) a | 13.4 (10.7–18.4) b | 23.1 (13.6–31.0.6) ab | <0.001 |
| Vancomycin concentration at T 2 h (mg/L) | 17.7 (11.39–28.2) | 22.8 (17.45–33.04) a | 11.1 (8.36–15.26) b | 16.6 (9.45–19.03) b | <0.001 |
| Vancomycin concentration at T final (mg/L) | 14.2 (8.7–20.9) | 20.26 ± 9.91 a | 11.32 ± 5.71 b | 10.6 ± 5.96 b | <0.001 |
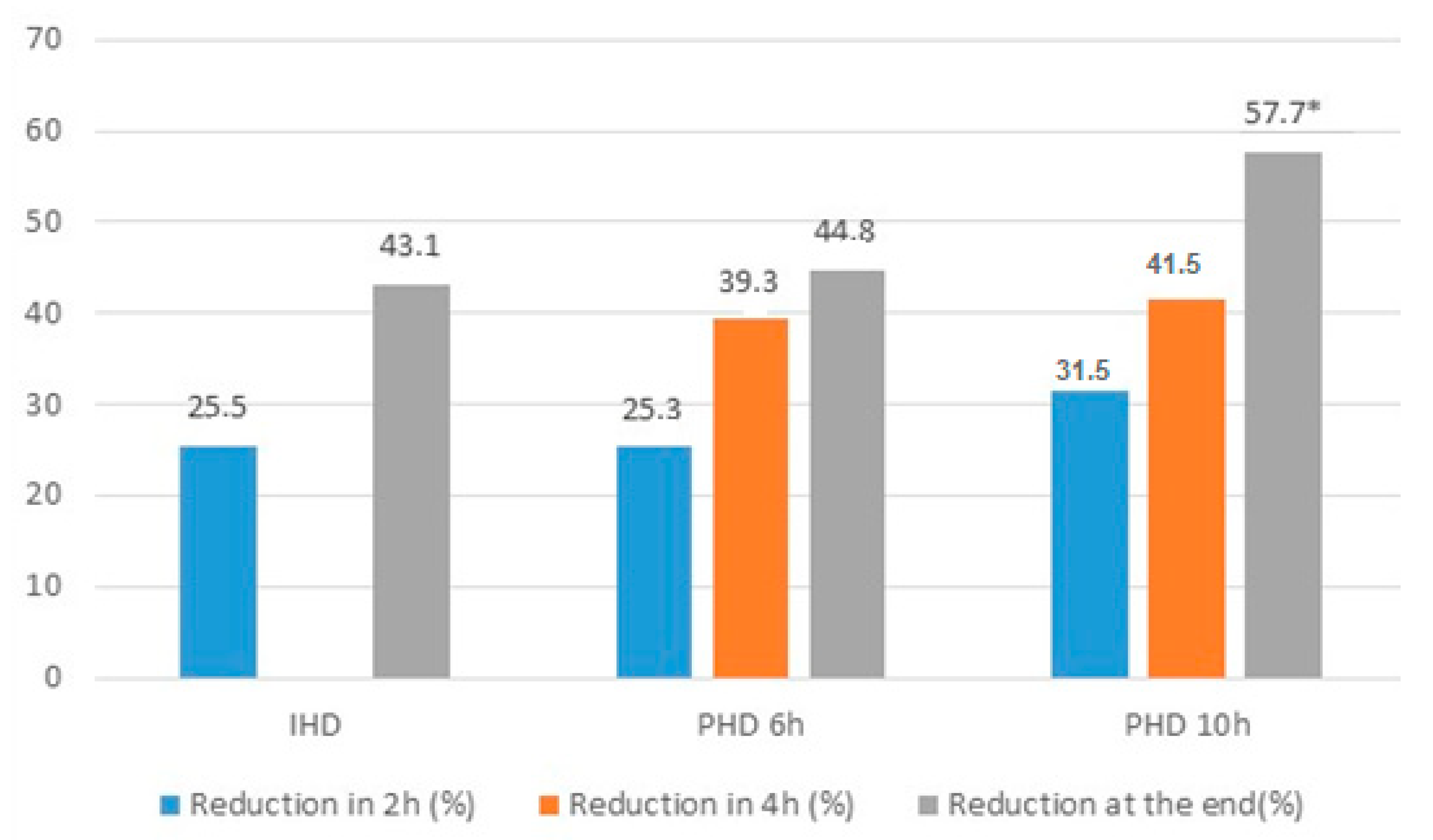
| % of reduction in 2 h | 26.6 ± 12.6 | 25.5 ± 12.5 | 25.3 ± 13.3 | 31.5 ± 11.8 | 0.36 |
| % Total reduction | 45.7 ± 12.79 | 43.1 (33.2–49.8) a | 44.7 (32.9–57.9) ab | 57.7 (40.5–64.3) b | 0.03 |
| AUC/MIC 24 h | 411.37 (326.64–580.17) | 504.2 (409.8–840.1) a | 410.65 (336.34–565.39) ab | 328.53 (302.36–372.39) b | 0.04 |
| AUC/MIC during analysis | 138.0 (99.6–215.2) | 169.8 (112.5–235.3) | 103.0 (94.8–178.1) | 124.7 (92.8–185.1) | 0.13 |
| Variables | General Population (n = 41) | AUC/MIC 24 h <400 (n = 19) | AUC/MIC 24 h >400 (n = 22) | p |
|---|---|---|---|---|
| Age (years) | 65.00 (56.50–72.50) | 65.00 (57.00–74.00) | 65.00 (53.75–72.25) | 0.724 |
| Male sex (%) | 15 (63.41) | 14 (73.68) | 12 (54.54) | 0.345 |
| Weight (kg) | 70.00 (60.50–82.50) | 71.00 (62.00–86.00) | 70.00 (54.25–74.25) | 0.36 |
| APACHE II | 32.00 (26.00–36.00) | 32.47 ± 6.04 | 28.91 ± 7.79 | 0.114 |
| LIANO | 0.77 (0.61–0.89) | 0.83 ± 0.72 | 0.75 ± 0.51 | 0.123 |
| Noradrenaline (mcg/kg/min) | 0.33 (0.06–0.53) | 0.38 (0.20–0.57) | 0.15 (0.00–0.52) | 0.11 |
| Urinary volume (ml/24 h) | 200.00 (40.00–425.00) | 100.00 (25.00–450.00) | 200.00 (45.00–500.00) | 0.591 |
| Hematocrite (%) | 28.10 ± 3.87 | 27.39 ± 3.75 | 28.71 ± 3.95 | 0.281 |
| PCR (mg/L) | 24.80 (6.60–33.65) | 25.78 ± 14.67 | 19.12 ± 14.30 | 0.15 |
| Albumin (g/dL) | 2.18 ± 0.49 | 2.14 ± 0.51 | 2.23 ± 0.48 | 0.587 |
| Conventional hemodialysis (%) | 16 (39.02) | 3 (15.79) | 13 (59.10) | 0.012 |
| Prolonged hemodialysis 6 and 10 h (%) | 25 (60.98) | 16 (84.21) | 9 (40.90) | 0.012 |
| Kt/V | 0.89 ± 0.33 | 0.90 ± 0.35 | 0.88 ± 0.31 | 0.814 |
| Dose of Vancomycin (mg/kg/day) | 14.71 (12.69–19.37) | 14.71 (10.71–20.00) | 14.60 (13.29–17.86) | 0.734 |
| Volume of distribution (L/Kg) | 0.67 (0.45–0.87) | 0.73 (0.61–1.34) | 0.56 (0.34–0.83) | 0.018 |
| Half-life time | 19.67 (9.36–38.14) | 28.38 (17.51–79.69) | 11.22 (5.99–26.28) | 0.004 |
| Vancomycin dialytic clearance(L/h) | 1.69 (1.03–3.05) | 1.19 (0.95–1.70) | 2.39 (1.15–3.55) | 0.017 |
| Vancomycin concentration (mg/L) at T0 | 23.59 (14.92–33.87) | 14.96 (13.19–24.20) | 32.83 (21.47–37.95) | 0.001 |
| % of reduction in 2 h | 26.52 ± 13.29 | 27.18 ± 12.23 | 25.94 ± 14.41 | 0.771 |
| % Total reduction | 46.98 ± 13.05 | 50.56 ± 14.62 | 43.90 ± 10.95 | 0.104 |
| AUC/MIC during dialysis | 120.44 (94.85–211.45) | 103.07 (87.35–169.25) | 172.43 (108.01–252.98) | 0.016 |
| Death (%) | 30 (73.17) | 16 (84.21) | 14 (63.64) | 0.259 |
| Variables | Odds Ratio | Confidence Interval | p |
|---|---|---|---|
| Prolonged hemodialysis | 11.59 | 1.219–110.171 | 0.033 |
| Distribution volume (L/Kg) | 0.197 | 0.0123–3.153 | 0.251 |
| Dialytic clearance of vancomycin (L/h) | 1.614 | 0.391–6.672 | 0.508 |
| Serum concentration of vancomycin (mg/L) at T0 | 0.791 | 0.664–0.942 | 0.009 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, F.M.d.; Zamoner, W.; Reis, P.F.d.; Balbi, A.L.; Ponce, D. Vancomycin for Dialytic Therapy in Critically Ill Patients: Analysis of Its Reduction and the Factors Associated with Subtherapeutic Concentrations. Int. J. Environ. Res. Public Health 2020, 17, 6861. https://doi.org/10.3390/ijerph17186861
Freitas FMd, Zamoner W, Reis PFd, Balbi AL, Ponce D. Vancomycin for Dialytic Therapy in Critically Ill Patients: Analysis of Its Reduction and the Factors Associated with Subtherapeutic Concentrations. International Journal of Environmental Research and Public Health. 2020; 17(18):6861. https://doi.org/10.3390/ijerph17186861
Chicago/Turabian StyleFreitas, Fernanda Moreira de, Welder Zamoner, Pamela Falbo dos Reis, André Luís Balbi, and Daniela Ponce. 2020. "Vancomycin for Dialytic Therapy in Critically Ill Patients: Analysis of Its Reduction and the Factors Associated with Subtherapeutic Concentrations" International Journal of Environmental Research and Public Health 17, no. 18: 6861. https://doi.org/10.3390/ijerph17186861
APA StyleFreitas, F. M. d., Zamoner, W., Reis, P. F. d., Balbi, A. L., & Ponce, D. (2020). Vancomycin for Dialytic Therapy in Critically Ill Patients: Analysis of Its Reduction and the Factors Associated with Subtherapeutic Concentrations. International Journal of Environmental Research and Public Health, 17(18), 6861. https://doi.org/10.3390/ijerph17186861




