Effects of Heavy Metals in Lake Water and Sediments on Bottom Invertebrates Inhabiting the Brackish Coastal Lake Łebsko on the Southern Baltic Coast
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Sample Collection
2.3. Laboratory Procedures
- (1)
- radio frequency (RF) power: 1.2 kW;
- (2)
- gas flow through a nebulizer: 0.7 L·min−1;
- (3)
- auxiliary gas flow: 1.0 L·min−1;
- (4)
- plasma gas flow: 12.0 L·min−1;
- (5)
- Charge Coupled Device (CCD) temperature: −40 °C;
- (6)
- viewing height for radial plasma observation: 8 mm for 5 s.
2.4. Statistical Analysis
3. Results
3.1. Physicochemical Variables of Water
3.2. Heavy Metal Analysis
3.2.1. Water
3.2.2. Sediments
3.3. Structure of Benthic Invertebrate Communities
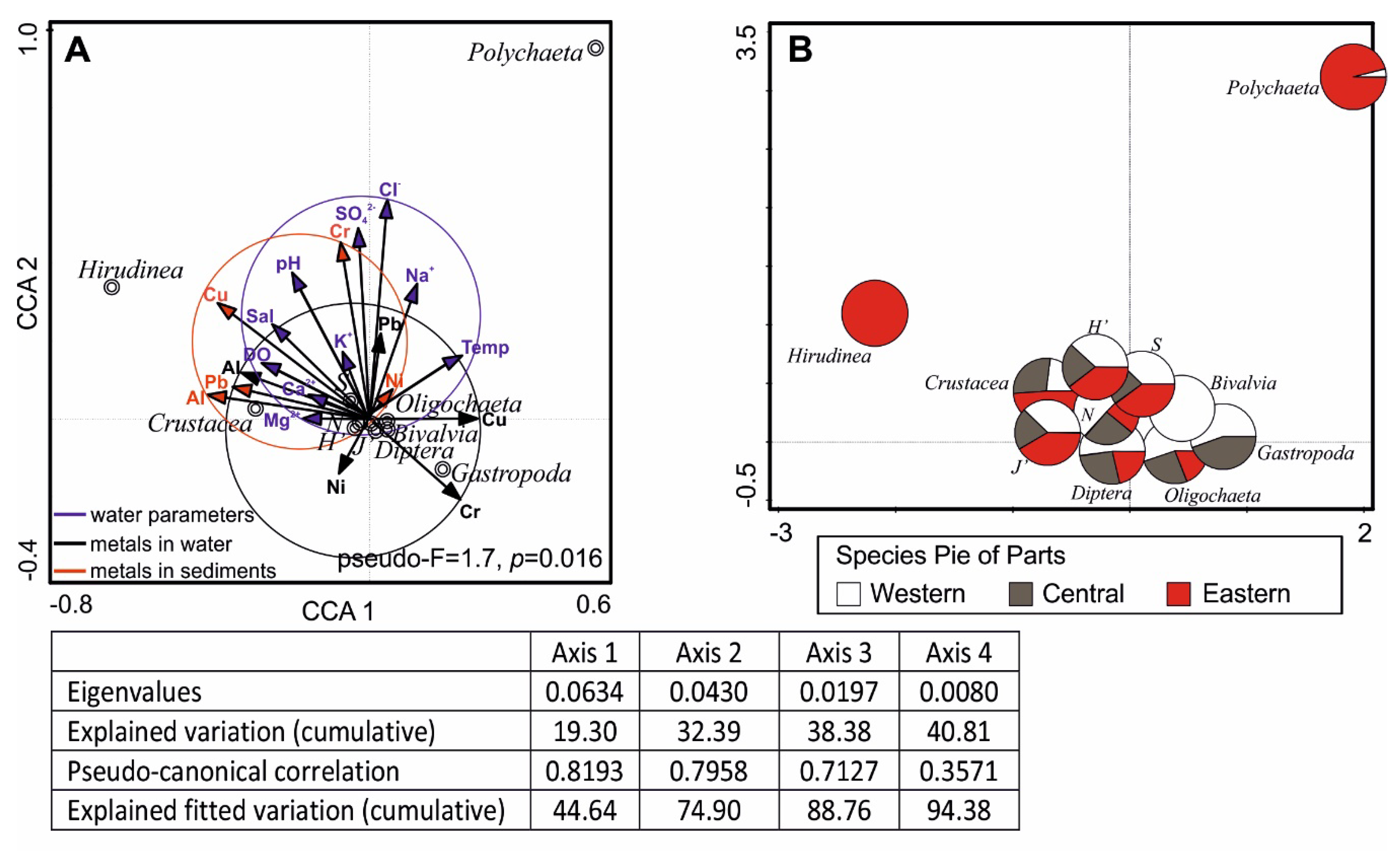
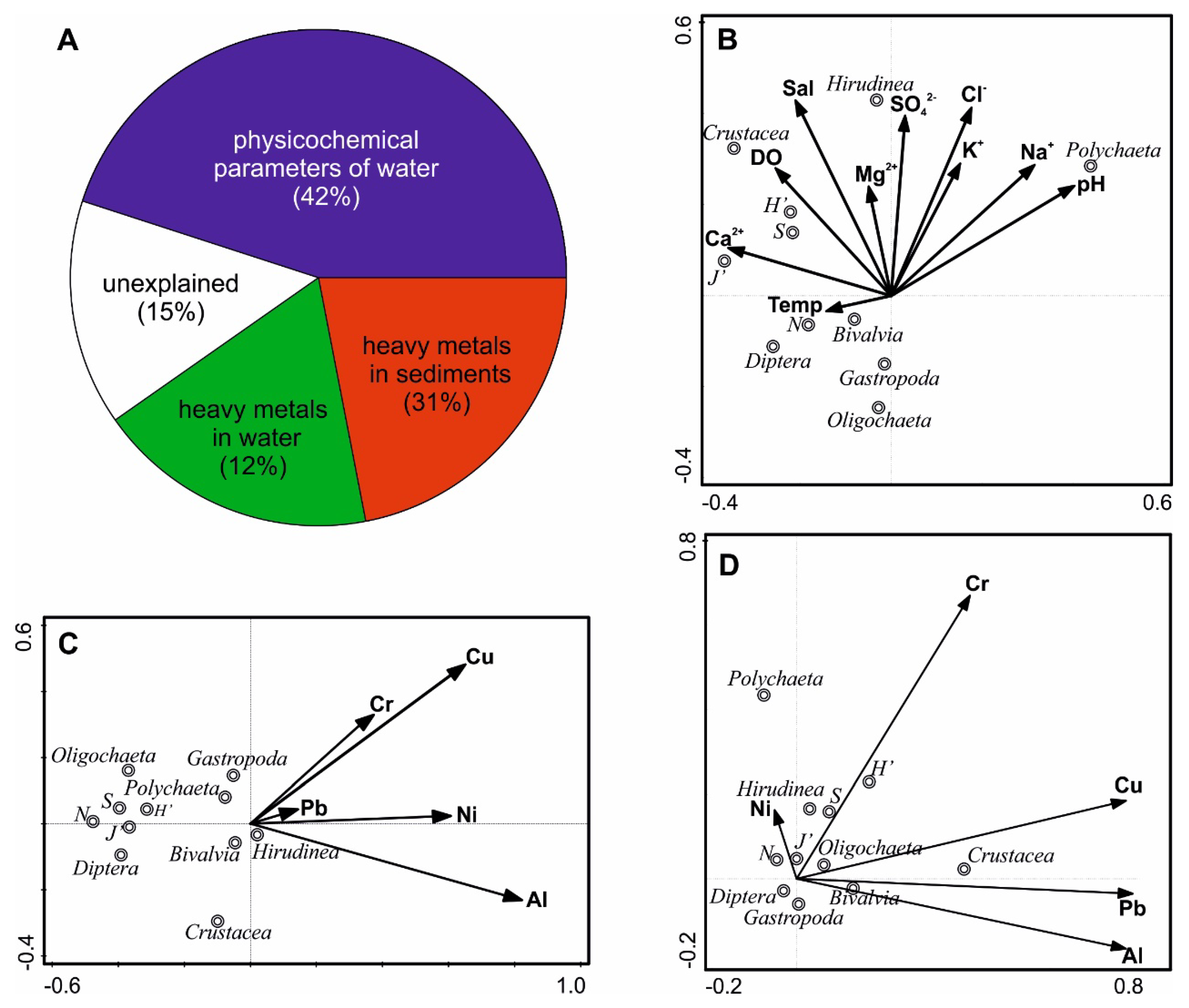
3.4. Interactions Between Macrofauna and Environmental Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baustian, M.M.; Meselhe, E.; Jung, H.; Sadid, K.; Duke-Sylvester, S.M.; Visser, J.M.; Allison, A.A.; Moss, L.C.; Ramatchandirane, C.; van Maren, D.S.; et al. Development of an Integrated Biophysical Model to represent morphological and ecological processes in a changing deltaic and coastal ecosystem. Environ. Model. Softw. 2018, 109, 402–419. [Google Scholar] [CrossRef]
- Nemr, A.E. Heavy Metals, Algae and Microbial Activities in marine Systems; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2015. [Google Scholar]
- Deljanin, I.; Antanasijević, D.; Bjelajac, A.; Urošević, M.A.; Nikolić, M.; Perić-Grujić, A.; Ristić, M. Chemometrics in biomonitoring: Distribution and correlation of trace elements in tree leaves. Sci. Total Environ. 2016, 361, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals and metalloids as micronutrients for plants and animals. Heavy Metals in soils. Environ. Pollut. 2012, 22, 195–209. [Google Scholar] [CrossRef]
- Elmorsi, R.R.; Abou-El-Sherbini, K.S.; Mostafa, G.A.H.; Hamed, M.A. Distribution of essential heavy metals in the aquatic ecosystem of Lake Manzala, Egypt. Heliyon 2019, 8, e02276. [Google Scholar] [CrossRef] [PubMed]
- Obolewski, K.; Glińska-Lewczuk, K.; Szymańska, M.; Mrozińska, N.; Bąkowska, M.; Astel, A.; Lew, S.; Paturej, E. Patterns of salinity regime in coastal lakes based on structure of benthic invertebrates. PLoS ONE 2018, 13, e0207825. [Google Scholar] [CrossRef]
- Wang, J.; Du, H.; Xu, Y.; Chen, K.; Liang, J.; Ke, H.; Cheng, S.-Y.; Liu, M.; Deng, H.; He, T. Environmental and ecological risk assessment of trace metal contamination in mangrove ecosystems: A case from Zhangjiangkou Mangrove National Nature Reserve, China. BioMed Res. Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Astel, A.; Bigus, K.; Obolewski, K.; Niedzielski, P.; Stec, M.; Astel, K.; Tsakovski, S. Heavy metals risk assessment in water and bottom sediments of ICOLLs in northern Poland. Global Nest. J. 2019, 21, 438–448. [Google Scholar] [CrossRef]
- Astel, A.M.; Bigus, K.; Obolewski, K.; Glińska-Lewczuk, K. Spatiotemporal assessment of water chemistry in intermittently open/closed coastal lakes of Southern Baltic Estuarine. Estuar. Coast. Shelf Sci. 2016, 182, 47–59. [Google Scholar] [CrossRef]
- Snelgrove, P.V.R.; Buttman, C.A. Animal-sediment relationship revisited: Cause versus effect. Oceanogr. Mar. Biol. 1994, 32, 111–177. [Google Scholar]
- Obolewski, K.; Glińska-Lewczuk, K. Connectivity and complexity of coastal lakes as determinants for their restoration–A case study of the southern Baltic Sea. Ecol. Eng. 2020, 155, 105948. [Google Scholar] [CrossRef]
- Jarosiewicz, A.; Obolewski, K.; Ożgo, M. Long-term trends in nutrient concentrations in polish coastal rivers. Ocean. Coast. Manag. 2015, 118, 37–46. [Google Scholar] [CrossRef]
- Netto, S.A.; Fonseca, G. Regime shifts in coastal lagoons: Evidence from freeliving marine nematodes. PLoS ONE 2017, 12, e0172366. [Google Scholar] [CrossRef] [PubMed]
- Netto, S.A.; Domingos, A.M.; Kurtz, M.N. Effects of artificial breaching of a temporarily open/closed estuary on benthic macroinvertebrates (Camacho Lagoon, Southern Brazil). Estuar. Coast. 2012, 35, 1069–1081. [Google Scholar] [CrossRef]
- Cieśliński, R. Geographic Determinants of Hydrochemical Variability of the Southern Baltic Coast; University of Gdańsk: Gdańsk, Poland, 2011. (In Polish) [Google Scholar]
- Hakanson, L. The quantitative impact of pH, bioproduction and Hg-contamination on the Hg-content of fish (pike). Environ. Pollut. B 1980, 1, 285–304. [Google Scholar] [CrossRef]
- Obolewski, K.; Bąkowska, M. Epiphytic invertebrate patterns in coastal lakes along a gradient of salinity and water exchange with the sea. Estuar. Coast. Shelf Sci. 2017, 197, 150–158. [Google Scholar] [CrossRef]
- Choiński, A. Changes in the morphometry of the coastal lakes. In Current State of the Lakes on the Southern Coast of the Baltic Sea; Obolewski, K., Ed.; WN PWN: Warsaw, Poland, 2017; pp. 20–39. [Google Scholar]
- Savvides, C.; Papadopoulos, A.; Haralambous, K.J.; Loizidou, M. Sea sediments contaminated with heavy metals: Metal speciation and removal. Water Sci. Technol. 1995, 32, 65–73. [Google Scholar] [CrossRef]
- Pekey, H.; Karakaş, D.; Ayberk, S.; Tolun, L.; Bakoglu, M. Ecological risk assessment using trace elements from Surface sediments of İzmit Bay (Northeastern Marmara Sea) Turkey. Mar. Pollut. Bull. 2004, 48, 946–953. [Google Scholar] [CrossRef]
- Angulo, E. The Tomlinson Pollution Load Index applied to heavy metal, ‘Mussel-Watch’ data: A useful index to assess coastal pollution. Sci. Total Environ. 1996, 187, 19–56. [Google Scholar] [CrossRef]
- Ganugapenta, S.; Nadimikeri, J.; Chinnapolla, S.R.R.B.; Ballari, L.; Madiga, R.; Lakshami Prasad Tella, N.K. Assessment of heavy metal pollution from the sediment of Tupilipalem Coast, southeastcoast of India. Int. J. Sediment. Res. 2018, 33, 294–302. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Wiss. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Sarkar, S.K. Trace Metals in a Tropical Mangrowe Wetland; Springer: Berlin, Germany, 2018. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and Canodraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Biometris: Wageningen, The Netherlands, 2002. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA for PRIMER: Guide to Software and Statistical Methods; PRIMER–E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Stubbington, R.; Boulton, A.J.; Little, S.; Wood, P.J. Changes in invertebrate assemblage composition in benthic and hyporeheic zones during a severe suprasesonal drought. Freshw. Sci. 2015, 34, 344–354. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Gray, R.; Bebbington, J. Environmental accounting, managerialism and sustainability: Is the planet safe in the hands of business and accounting? Adv. Environ. Account. Manag. 2000, 2, 1–44. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- McCune, B.; Grace, J.B. MjM Software Design; Analysis of Ecological Communities: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Ter Braak, C.J.F.; Looman, C.W.N. Biplots in Reduced-Rank Regression. Biom. J. 1994, 36, 983–1003. [Google Scholar] [CrossRef]
- Clarke, K.R.; Ainsworth, M. A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser. 1993, 92, 205–219. [Google Scholar] [CrossRef]
- Wooldridge, T. Estuarine Zooplankton Community Structure and Dynamics. In Estuaries of South Africa; Allanson, B.R., Baird, D., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 141–166. [Google Scholar]
- Colling, L.A.; Bemvenuti, C.E.; Gandra, M.S. Seasonal variability on the structure of sublittoral macrozoobenthic association in the Patos Lagoon estuary, southern Brazil. Inheringia. Ser. Zool. 2007, 97, 3. [Google Scholar] [CrossRef]
- Obolewski, K. Use of macrozoobenthos to assess the ecological status of the estuarial lake Jamno. Ochr. Sr. 2009, 31, 17–24. (In Polish) [Google Scholar]
- Dobrowolski, Z. Occurrence of macrobenthos in different littoral habitat of the polymictic Łebsko Lake. Ekol. Pol. 1994, 42, 19–40. [Google Scholar]
- Dobrowolski, Z. Species composition and co-occurrence of Chironomidae larvae in mid-lake benthos of several coastal Baltic lakes. Ekol. Pol. 1996, 44, 53–72. [Google Scholar]
- Dye, A.; Barros, F. Spatial patterns of macrofaunal assemblages in intermittently closed/open coastal lakes in New South Wales, Australia. Estuar. Coast. Shelf Sci. 2005, 64, 357–371. [Google Scholar] [CrossRef]
- Żmudziński, L.; Dobrowolski, Z.; Labuda, M.; Mudryk, Z.; Paturej, E.; Trojanowska, C. Variability of the Biocenoses of Three Polish Estuarine Lakes; 12th Baltic Marine Biologists Symposium: Helsingoer, Denmark, 1992; pp. 185–189. [Google Scholar]
- Kjerfve, B. Coastal Lagoons; Elsevier Oceanography Series: Amsterdam, The Netherlands, 1994; Volume 60, pp. 1–8. [Google Scholar] [CrossRef]
- Cieśliński, R. Short-term changes in specific conductivity in Polish coastal lakes (Baltic Sea basin). Oceanologia 2013, 55, 639–661. [Google Scholar] [CrossRef]
- Garcia-Arberas, L.; Rallo, A. Life cycle, demography and secondary production of the polychaete Hediste diversicolor in a non-polluted estuary in the Bay of Biscay. Mar. Ecol. 2002, 23, 237–251. [Google Scholar] [CrossRef]
- Virgilio, M.; Fauvelot, C.; Costantin, F.; Abbiati, M.; Backeljau, T. Phylogeography of the common rag worm Hediste diversicolor (Polychaeta: Nereididae) reveal scryptic diversity and multiple colonization event sacrossits distribution. Mol. Ecol. 2009, 18, 1980–1994. [Google Scholar] [CrossRef]
- Maltagliati, F.; Massaro, L.; Cossu, P.; Castelli, A. Morphological differentiation in the rag worm, Hediste diversicolor (Polychaeta, Nereididae), as revealed by variation of paragnath number and distribution. Ital. J. Zool. 2006, 73, 255–262. [Google Scholar] [CrossRef]
- Adamiak-Brud, Ż.; Jabłońska-Barn, I.; Bielecki, A.; Kobak, J. Factors shaping leech (Clitellata, Hirudinida) assemblages on artificial and natural substrata in urban water bodies. Limnologica 2018, 69, 125–134. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Belanger, R.R. The controversies of silicon’s role in plant biology. New. Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Davies-Vollum, K.S.; Zhang, Z.; Agyekumhene, A. Impacts of lagoon opening and implications for coastal management: Case study from Muni-Pomadze lagoon, Ghana. J. Coast. Conserv. 2019, 23, 293–301. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control: Sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Saidi, I.; Said, O.; Ben, B.; Abdelmalek, J.; Jouili, S.; Chicharo, L.; Beyrem, H. Impact of heavy metals of industrial plant wastewater on benthic communities of Bizerte Lagoon (Tunisia). Chem. Ecol. 2019, 35, 746–774. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the contiental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Hazarika, J.; Ghosh, U.; Kalamdhad, A.S.; Kwairakpam, M.; Singh, J. Transformation of elemental toxic metal sinto immobile fractions in paper mill sludge through rotary drum compositing. Ecol. Eng. 2017, 101, 185–192. [Google Scholar] [CrossRef]
- Fu, D.; Gong, W.; Xu, Y.; Singh, R.P.; Surampalli, R.Y.; Zhang, T.C. Nutrient mitigation capacity of agricultural drainage ditches in Tai lake basin. Ecol. Eng. 2014, 71, 101–107. [Google Scholar] [CrossRef]
- Manno, E.; Varrica, D.; Dongarra, G. Metal distribution in road dust samples collected in an urban area close to a petrochemical plant at Gela, Sicily. Atmospheric. Environ. 2006, 40, 5929–5941. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Shojaei, N.; Sorooshian, A.; Soltani, N.; Delshab, H. Geochemistry and environmental effects of potentially toxic elements, polycyclic aromatic hydrocarbons and microplastics in coastal sediments of the Persian Gulf. Environ. Earth Sci. 2019, 78, 492. [Google Scholar] [CrossRef]
- Turner, A.; Cabon, A.; Glegg, G.A.; Fisher, A.S. Sediment–water interactions of thallium under simulated estuarine conditions. Geochim. Cosmochim. Acta 2010, 74, 6779–6787. [Google Scholar] [CrossRef]
- Boyle, J. Redox remobilization and the heavy metal record in lake sediments: A modelling approach. J. Paleolimnol. 2001, 26, 423–431. [Google Scholar] [CrossRef]
- Tylmann, W.; Łysek, K.; Kinder, M.; Pempkowiak, J. Regional pattern of heavy metal content in lake sediments in Northeastern Poland. Water Air Soil Pollut. 2011, 216, 217–228. [Google Scholar] [CrossRef]
- Journal of Laws: 1187 Regulation of the Minister of the Environment on the Method of Classifying the State of Surface Water Bodies and Environmental Quality Standards for Priority Substances; Minister of Environment, Republic of Poland: Warsaw, Poland, 2016. (In Polish)
- Oliver, B.G. Dihaloacetonitriles in drinking water: Algae and fulvic acid as precursors. Environ. Sci. Technol. 1983, 17, 80–83. [Google Scholar] [CrossRef]
- Wiederholm, T. The ecology and aquatic insects. In Response of Aquatic Insects to Environmental Pollution; Res, V.H., Rosenberg, D.M., Eds.; Proger Publishers: New York, NY, USA, 1984; pp. 508–557. [Google Scholar]
- Lencioni, V.; Lazzara, M. Subfossil chironomids (Diptera, Chironomidae) from Lake Tovel (Trentino, central-eastern Alps): A view from the previous 400 years. Sci. Nat. Acta Biol. 2006, 81, 155–165. [Google Scholar]
- Bian, B.; Zhou, Y.; Fang, B.B. Distribution of heavy metals and benthic macroinvertebrates: Impact from typical inflow river sediments in the Taihu Basin, China. Ecol. Indic. 2016, 69, 348–359. [Google Scholar] [CrossRef]
- Berezina, N.A. Tolerance of freshwater invertebrates to changes in water salinity. Russ. J. Ecol. 2003, 34, 261–266. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Han, J.; Li, P. The current status of heavy metal in lake sediments from China: Pollution and ecological risk assessment. Ecol. Evol. 2017, 7, 545–5466. [Google Scholar] [CrossRef]
- Pastorino, P.; Pizzul, E.; Bertoli, M.; Perillis, S.; Brizio, P.; Salvi, G.; Esposito, G.; Abete, M.C.; Prearo, M.; Squadrone, S. Macrobrnthic invertebrates as bioindicators of trace elements in high-mountain lakes. Environ. Sci. Pollut. Res. 2019, 27, 5958–5970. [Google Scholar] [CrossRef]
- Costas, N.; Pardo, I.; Mendez-Fernandez, L.; Martinez-Madrid, M.; Rodriguez, P. Sensitivity of macroinvertebrate indicator taxa to metal gradients in mining areas in Northern. Spain Ecol. Ind. 2018, 93, 207–218. [Google Scholar] [CrossRef]
- Ryu, J.; Khim, J.S.; Kang, S.-G.; Kang, D.; Lee, C.-H.; Koh, C.-H. The impact of heavy metal pollution gradients in sediment on benthic macrofauna at population and community levels. Environ. Pollut. 2011, 159, 2622–2629. [Google Scholar] [CrossRef]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]
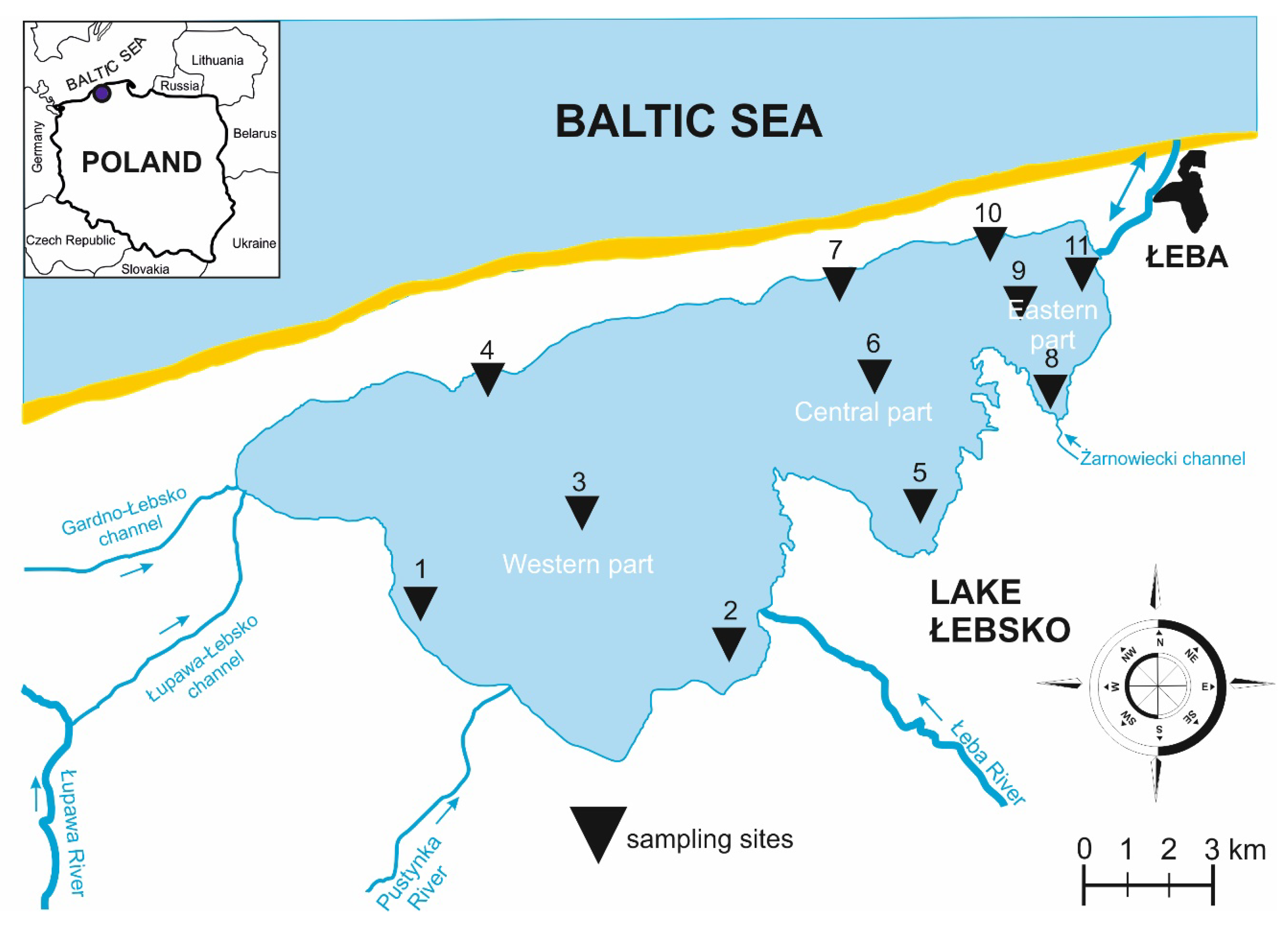
| Geographic Coordinates | Area (ha) | Mean Depth (m) | Capacity (106 m3) | Level of Salinity | Hydrological Connectivity | SB | Type of Habitat |
|---|---|---|---|---|---|---|---|
| 54°43′ N, 17°25′ E | 7020 | 1.6 | 113.5 | β-oligohalinity | Permanently connected, seawater enters it by canal of Łeba River | 276 | Brackish |
| Statistics | T | pH | EC | DO | Sal | Cl− | SO42- | Na+ | Mg2+ | Ca2+ | K+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | - | μS cm−1 | % | PSU | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | mg·L−1 | |
| Western part (inter region) | |||||||||||
| Min | 12.8 | 8.18 | 2980 | 62.2 | 1.57 | 830.3 | 103.2 | 30.5 | 2.0 | 33.2 | 5.0 |
| Max | 26.1 | 8.86 | 6336 | 126.8 | 3.49 | 1511.9 | 225.7 | 881.2 | 105.3 | 96.2 | 34.3 |
| Mean | 16.0 | 8.50 | 4652 | 88.8 | 2.50 * | 1216.5* | 178.4* | 633.4* | 69.9 | 50.3 | 24.3 |
| Central part | |||||||||||
| Min | 10.5 | 8.10 | 1747 | 54.0 | 0.93 | 944.1 | 118.7 | 539.9 | 52.2 | 33.2 | 16.6 |
| Max | 25.9 | 8.80 | 6395 | 114.3 | 4.32 | 1707.9 | 254.1 | 1099.1 | 116.8 | 97.8 | 42.4 |
| Mean | 15.6 | 8.42 | 5421 | 89.1 | 2.95 | 1363.3 | 194.9 | 748.9 | 82.6 | 53.0 | 28.2 |
| Eastern part (outer region) | |||||||||||
| Min | 11.3 | 7.54 | 3132 | 29.0 | 1.64 | 1099.0 | 163.2 | 28.4 | 2.0 | 32.1 | 4.5 |
| Max | 25.2 | 8.61 | 14615 | 124.9 | 8.53 | 6777.2 | 630.1 | 2270.1 | 279.6 | 98.9 | 83.2 |
| Mean | 16.0 | 8.18 | 7626 | 92.2 | 4.23* | 2166.4* | 284.8* | 980.1* | 98.3 | 54.7 | 32.5 |
| ANOVA (p) | 0.58 | 0.00 | 0.00 | 0.62 | 0.00 | 0.01 | 0.00 | 0.01 | 0.06 | 0.71 | 0.05 |
| Statistics | Al | Cr | Ni | Pb | Cu |
|---|---|---|---|---|---|
| Western part (inflow region) | |||||
| Min | 0.161 | 0.002 | 0.007 | 0.003 | 0.006 |
| Max | 2.974 | 0.057 | 0.078 | 0.349 | 0.040 |
| Mean | 0.995 | 0.018 | 0.034 | 0.049 | 0.017 |
| Central part | |||||
| Min | 0.115 | 0.002 | 0.006 | 0.003 | 0.008 |
| Max | 3.928 | 0.046 | 0.147 | 0.159 | 0.058 |
| Mean | 0.943 | 0.016 | 0.090 | 0.040 | 0.027 |
| Eastern part (outflow region) | |||||
| Min | 0.125 | 0.001 | 0.005 | 0.002 | 0.010 |
| Max | 4.234 | 0.034 | 0.060 | 0.088 | 0.037 |
| Mean | 0.864 | 0.014 | 0.055 | 0.035 | 0.019 |
| ANOVA (p) | 0.49 | 0.30 | 0.76 | 0.88 | 0.58 |
| Statistics | Al | Cr | Ni | Pb | Cu |
|---|---|---|---|---|---|
| Western part (inflow region) | |||||
| Min | 176.26 | 10.30 | 8.34 | 2.15 | 3.40 |
| Max | 9946.06 | 61.62 | 86.44 | 91.73 | 94.32 |
| Mean | 5283.28 * | 28.64 | 36.17 | 30.29 * | 46.52 * |
| Central part | |||||
| Min | 412.26 | 0.48 | 13.72 | 9.48 | 6.69 |
| Max | 10216.40 | 51.81 | 184.43 | 99.84 | 84.83 |
| Mean | 4692.69 | 30.48 * | 44.85* | 33.50 | 44.19 |
| Eastern part (outflow region) | |||||
| Min | 238.48 | 10.52 | 5.95 | 2.22 | 11.52 |
| Max | 6010.93 | 91.53 | 76.36 | 225.53 | 63.63 |
| Mean | 2595.96 * | 26.98* | 33.02 * | 29.31 * | 36.90 * |
| ANOVA (p) | 0.01 | 0.00 | 0.05 | 0.00 | 0.05 |
| Taxa and Indices | Western | Central | Eastern | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| 1-OLIGOCHAETA | 1096.3 | 3866.6 | 1111.1 | 800.0 | 592.5 | 488.9 | 2133.4 | 992.6 | 429.5 | 518.7 | 415.5 | 0.041 |
| 2-POLYCHAETA | 44.4 | 29.6 | 14.8 | 177.8 | 0.191 | |||||||
| Hediste diversicoilor | 44.4 | 163.0 | ||||||||||
| Pygospio elegans | ||||||||||||
| Mysis mixta | 29.6 | 14.8 | 14.8 | |||||||||
| 3-CRUSTACEA | 14.8 | 102.9 | 103.7 | 44.4 | 74.0 | 251.8 | 178.1 | 177.7 | 148.1 | 0.506 | ||
| Asellusa quaticus | 74.1 | 44.4 | ||||||||||
| Gammarus duebeni | 14.1 | 74.1 | 74.0 | 251.8 | 59.6 | 133.3 | 88.9 | |||||
| Gammarus oceanicus | 44.4 | 14.8 | 14.8 | |||||||||
| Neomysis integer | 14.8 | 88.8 | 29.6 | 44.4 | 29.6 | - | ||||||
| 4-HIRUDINEA | 14.8 | 0.170 | ||||||||||
| Pisicola geometra | ||||||||||||
| 5-DIPTERA LARVAE | 918.4 | 3733.2 | 1688.7 | 5526.0 | 1436.9 | 3229.5 | 1288.8 | 755.5 | 1703.7 | 1644.5 | 133.3 | 0.019 |
| Chironomus sp. | 399.9 | 3525.9 | 1348.1 | 5466.8 | 1140.7 | 2977.7 | 888.9 | 651.9 | 1392.6 | 325.9 | 14.8 | |
| Chironomidae n.det. | 29.6 | 29.6 | 59.2 | 14.8 | 29.6 | 14.8 | 14.8 | 88.9 | ||||
| Dicrochironomus sp. | 29.6 | 29.6 | 14.8 | |||||||||
| Procladius sp. | 14.8 | 222.2 | 222.2 | 14.8 | 14.8 | |||||||
| Polypedilum sp. | 14.8 | 29.6 | 133.3 | |||||||||
| Psectrocladius sp. | 14.8 | 14.8 | ||||||||||
| Bezzia sp. | 29.6 | 148.1 | 118.4 | 29.6 | 74.1 | 44.4 | ||||||
| Microtendipes sp. | 74.1 | |||||||||||
| Sergentia sp. | 414.9 | 281.5 | 948.2 | 103.7 | ||||||||
| Einfeldia sp. | 29.6 | 14.8 | 74.0 | 311.1 | 163.0 | |||||||
| Clunio sp. | 14.8 | |||||||||||
| 6-MOLLUSCA | 14.8 | 74.0 | 14.8 | 29.6 | 29.6 | |||||||
| A-GASTROPODA | 14.8 | 44.4 | 0.0 | 29.6 | 29.6 | 0.280 | ||||||
| Bithynia tentaculata | 29.6 | |||||||||||
| Valvata piscinalis | 14.8 | |||||||||||
| Theodoxus fluviatilis | 29.6 | |||||||||||
| Potamopyrgus jenkinsi | 14.8 | |||||||||||
| Hydrobia ulvae | 29.6 | |||||||||||
| B-BIVALVIA | 29.6 | 14.8 | 0.511 | |||||||||
| Dreissena polymorpha | 14.8 | |||||||||||
| Anodonta anatina | 29.6 | |||||||||||
| N, ind. m−2 | 345.7 | 1286.3 | 496.3 | 1064.2 | 355.5 | 661.7 | 575.3 | 291.4 | 390.2 | 392.6 | 148.3 | |
| S, species m−2 | 9 | 9 | 9 | 5 | 8 | 7 | 9 | 5 | 7 | 12 | 10 | |
| N, ind. m−2 per part | 798.1 | 530.8 | 366.7 | 0.018 | ||||||||
| S, species m−2 per part | 17 | 12 | 15 | 0.310 | ||||||||
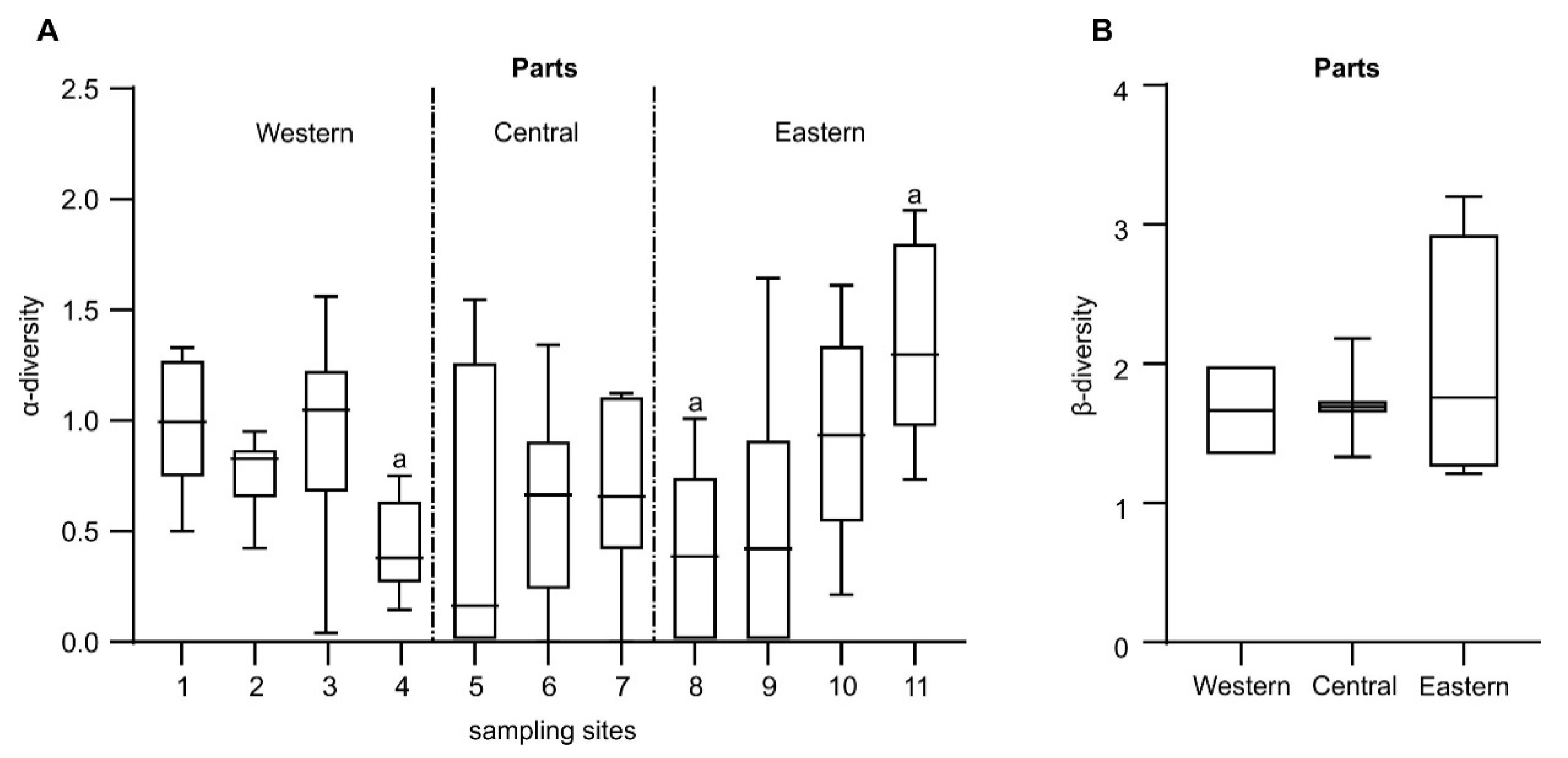
| α-diversity (H’) | 0.781 | 0.606 | 0.804 | 0.011 | ||||||||
| Evenness (J’) | 0.654 | 0.477 | 0.717 | 0.411 | ||||||||
| Variance | Sources of Variation | df | SS | MS | Pseudo F Values | p (MC) |
|---|---|---|---|---|---|---|
| Density | Parts | 2 | 2267984 | 113399 | 4.276 | 0.018 |
| Sites | 10 | 1365870 | 236541 | 3.145 | 0.021 | |
| Residual | 63 | 1670603 | 265175 | |||
| Pair-wise tests | ||||||
| Compared of parts | ||||||
| West vs Central | 2.75 | 0.23 | ||||
| Central vs East | 18.10 | 0.007 | ||||
| West vs East | 7.66 | 0.57 | ||||
| Total α-diversity | Parts | 2 | 0.620 | 3.1000 | 4.806 | 0.01 |
| Sites | 10 | 0.555 | 2.9574 | 3.587 | 0.02 | |
| Residual | 63 | 4.064 | 0.6454 | |||
| Pair-wise tests | ||||||
| Compared of parts | ||||||
| West vs Central | 243.40 | 0.07 | ||||
| Central vs East | 482.20 | 0.01 | ||||
| West vs East | 206.80 | 0.67 | ||||
| Diptera density | Parts | 2 | 0.0098 | 0.0049 | 0.0781 | 0.02 |
| Sites | 10 | 0.0147 | 0.0258 | 0.1487 | 0.001 | |
| Residual | 63 | 4.4960 | 0.0624 | |||
| Pair-wise tests | ||||||
| Compared of parts | ||||||
| West vs Central | 264.60 | 0.01 | ||||
| Central vs East | 320.80 | 0.004 | ||||
| West vs East | 669.90 | 0.03 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrozińska, N.; Bąkowska, M. Effects of Heavy Metals in Lake Water and Sediments on Bottom Invertebrates Inhabiting the Brackish Coastal Lake Łebsko on the Southern Baltic Coast. Int. J. Environ. Res. Public Health 2020, 17, 6848. https://doi.org/10.3390/ijerph17186848
Mrozińska N, Bąkowska M. Effects of Heavy Metals in Lake Water and Sediments on Bottom Invertebrates Inhabiting the Brackish Coastal Lake Łebsko on the Southern Baltic Coast. International Journal of Environmental Research and Public Health. 2020; 17(18):6848. https://doi.org/10.3390/ijerph17186848
Chicago/Turabian StyleMrozińska, Natalia, and Martyna Bąkowska. 2020. "Effects of Heavy Metals in Lake Water and Sediments on Bottom Invertebrates Inhabiting the Brackish Coastal Lake Łebsko on the Southern Baltic Coast" International Journal of Environmental Research and Public Health 17, no. 18: 6848. https://doi.org/10.3390/ijerph17186848
APA StyleMrozińska, N., & Bąkowska, M. (2020). Effects of Heavy Metals in Lake Water and Sediments on Bottom Invertebrates Inhabiting the Brackish Coastal Lake Łebsko on the Southern Baltic Coast. International Journal of Environmental Research and Public Health, 17(18), 6848. https://doi.org/10.3390/ijerph17186848





