Characterization of Persistent Uncontrolled Asthma Symptoms in Community Members Exposed to World Trade Center Dust and Fumes
Abstract
1. Introduction
2. Methods
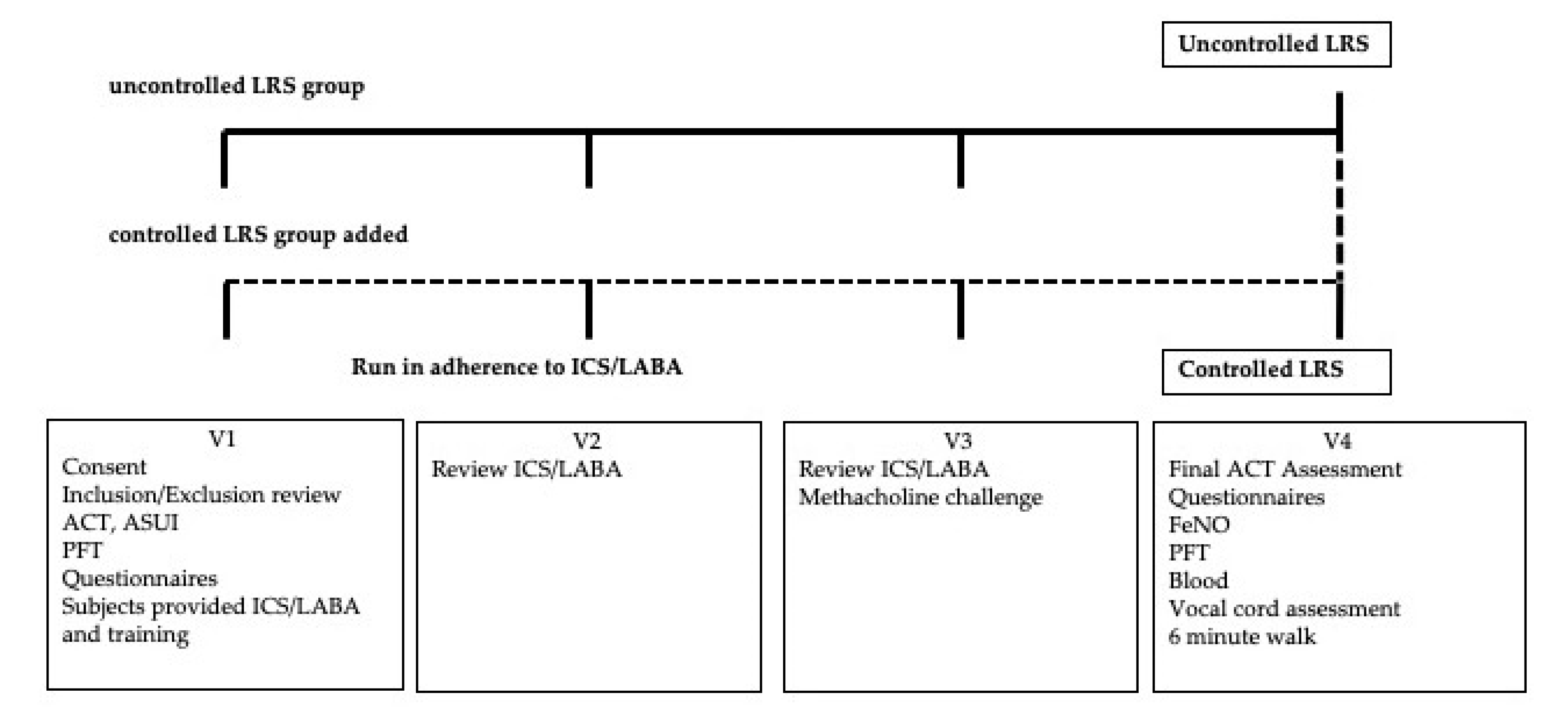
2.1. Study Design
2.2. Study Participants
2.3. Study Procedure
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Patient Recruitment
3.2. Upper Respiratory Symptoms
3.3. Lung Function: Spirometry and Small Airway Measures
3.4. Airway Hyper-Responsiveness
3.5. Inflammatory Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farfel, M.; DiGrande, L.; Brackbill, R.; Prann, A.; Cone, J.; Friedman, S.; Walker, D.J.; Pezeshki, G.; Thomas, P.; Galea, S.; et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J. Urban Health 2008, 85, 880–909. [Google Scholar] [CrossRef]
- Lin, S.; Reibman, J.; Bowers, J.A.; Hwang, S.A.; Hoerning, A.; Gomez, M.I.; Fitzgerald, E.F. Upper respiratory symptoms and other health effects among residents living near the World Trade Center site after September 11, 2001. Am. J. Epidemiol. 2005, 162, 499–507. [Google Scholar] [CrossRef]
- Reibman, J.; Levy-Carrick, N.; Miles, T.; Flynn, K.; Hughes, C.; Crane, M.; Lucchini, R.G. Destruction of the World Trade Center Towers. Lessons Learned from an Environmental Health Disaster. Ann. Am. Thorac Soc. 2016, 13, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Reibman, J.; Lin, S.; Hwang, S.A.; Gulati, M.; Bowers, J.A.; Rogers, L.; Berger, K.I.; Hoerning, A.; Gomez, M.; Fitzgerald, E.F. The World Trade Center residents’ respiratory health study: New-onset respiratory symptoms and pulmonary function. Environ. Health Perspect. 2005, 113, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Reibman, J.; Liu, M.; Cheng, Q.; Liautaud, S.; Rogers, L.; Lau, S.; Berger, K.I.; Goldring, R.M.; Marmor, M.; Fernandez-Beros, M.E.; et al. Characteristics of a residential and working community with diverse exposure to World Trade Center dust, gas, and fumes. J. Occup. Environ. Med. 2009, 51, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Lioy, P.J.; Weisel, C.P.; Millette, J.R.; Eisenreich, S.; Vallero, D.; Offenberg, J.; Buckley, B.; Turpin, B.; Zhong, M.; Cohen, M.D.; et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ. Health Perspect. 2002, 110, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Cohen, M.D.; Chen, L.C. Health effects of World Trade Center (WTC) Dust: An unprecedented disaster’s inadequate risk management. Crit. Rev. Toxicol. 2015, 45, 492–530. [Google Scholar] [CrossRef]
- Yiin, L.M.; Millette, J.R.; Vette, A.; Ilacqua, V.; Quan, C.; Gorczynski, J.; Kendall, M.; Chen, L.C.; Weisel, C.P.; Buckley, B.; et al. Comparisons of the dust/smoke particulate that settled inside the surrounding buildings and outside on the streets of southern New York City after the collapse of the World Trade Center, September 11, 2001. J. Air. Waste Manag. Assoc. 2004, 54, 515–528. [Google Scholar] [CrossRef]
- Banauch, G.I.; Alleyne, D.; Sanchez, R.; Olender, K.; Cohen, H.W.; Weiden, M.; Kelly, K.J.; Prezant, D.J. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. Am. J. Respir. Crit. Care Med. 2003, 168, 54–62. [Google Scholar] [CrossRef]
- Banauch, G.I.; Dhala, A.; Alleyne, D.; Alva, R.; Santhyadka, G.; Krasko, A.; Weiden, M.; Kelly, K.J.; Prezant, D.J. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit. Care Med. 2005, 33, S102–S106. [Google Scholar] [CrossRef]
- Vandenplas, O.; Wiszniewska, M.; Raulf, M.; de Blay, F.; Gerth van Wijk, R.; Moscato, G.; Nemery, B.; Pala, G.; Quirce, S.; Sastre, J.; et al. EAACI position paper: Irritant-induced asthma. Allergy 2014, 69, 1141–1153. [Google Scholar] [CrossRef]
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allergy Clin. Immunol. 2007, 120, S94. [Google Scholar] [CrossRef]
- Global Initiative for Asthma Executive Committee. Global Strategy for Asthma Management and Prevention; NHLBI/WHO Workshop Report; GINA: Fontana, WI, USA, 2006. [Google Scholar]
- Brite, J.; Friedman, S.; de la Hoz, R.E.; Reibman, J.; Cone, J. Mental health, long-term medication adherence, and the control of asthma symptoms among persons exposed to the WTC 9/11 disaster. J. Asthma 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Caplan-Shaw, C.; Kazeros, A.; Pradhan, D.; Berger, K.; Goldring, R.; Zhao, S.; Liu, M.; Shao, Y.; Fernandez-Beros, M.E.; Marmor, M.; et al. Improvement in severe lower respiratory symptoms and small airway function in World Trade Center dust exposed community members. Am. J. Ind. Med. 2016, 59, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.T.; Friedman, S.M.; Reibman, J.; Goldring, R.M.; Miller Archie, S.A.; Ortega, F.; Alper, H.; Shao, Y.; Maslow, C.B.; Cone, J.E.; et al. Risk factors for persistence of lower respiratory symptoms among community members exposed to the 2001 World Trade Center terrorist attacks. Occup. Environ. Med. 2017, 74, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.T.; Stellman, S.D.; Reibman, J.; Farfel, M.R.; Brackbill, R.M.; Friedman, S.M.; Li, J.; Cone, J.E. Factors associated with poor control of 9/11-related asthma 10-11 years after the 2001 World Trade Center terrorist attacks. J. Asthma 2015, 52, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.K.; Pladevall, M.; Xi, H.; Peterson, E.L.; Joseph, C.; Lafata, J.E.; Ownby, D.R.; Johnson, C.C. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J. Allergy Clin. Immunol. 2004, 114, 1288–1293. [Google Scholar] [CrossRef]
- Ip, S.; Paulus, J.K.; Balk, E.M.; Dahabreh, I.J.; Avendano, E.E.; Lau, J. Role of Single Group Studies in Agency for Healthcare Research and Quality Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013.
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- WTC Health Program. Available online: https://www.cdc.gov/wtc/ppm.html (accessed on 1 September 2020).
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef]
- Bime, C.; Wei, C.Y.; Holbrook, J.T.; Sockrider, M.M.; Revicki, D.A.; Wise, R.A. Asthma symptom utility index: Reliability, validity, responsiveness, and the minimal important difference in adult asthmatic patients. J. Allergy Clin. Immunol. 2012, 130, 1078–1084. [Google Scholar] [CrossRef]
- Revicki, D.; Weiss, K.B. Clinical assessment of asthma symptom control: Review of current assessment instruments. J. Asthma 2006, 43, 481–487. [Google Scholar] [CrossRef]
- Schilling, R.S.; Hughes, J.P.; Dingwall-Fordyce, I. Disagreement between observers in an epidemiological study of respiratory disease. Br. Med. J. 1955, 1, 65–68. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Kennedy, D.W. Quantification for staging sinusitis. The Staging and Therapy Group. Ann. Otol. Rhinol. Laryngol. Suppl. 1995, 167, 17–21. [Google Scholar] [CrossRef]
- Walker, F.D.; White, P.S. Sinus symptom scores: What is the range in healthy individuals? Clin. Otolaryngol. Allied Sci. 2000, 25, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Birring, S.S.; Prudon, B.; Carr, A.J.; Singh, S.J.; Morgan, M.D.; Pavord, I.D. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003, 58, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.A.; Lee, A.S.; Osborne, J.; Zullo, T.; Murry, T. Development and validation of the voice handicap index-10. Laryngoscope 2004, 114, 1549–1556. [Google Scholar] [CrossRef]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef]
- Goldman, M.D.; Saadeh, C.; Ross, D. Clinical applications of forced oscillation to assess peripheral airway function. Respir. Physiol. Neurobiol. 2005, 148, 179–194. [Google Scholar] [CrossRef]
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farre, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef]
- Pradhan, D.; Xu, N.; Reibman, J.; Goldring, R.M.; Shao, Y.; Liu, M.; Berger, K.I. Bronchodilator Response Predicts Longitudinal Improvement in Small Airway Function in World Trade Center Dust Exposed Community Members. Int. J. Environ. Res. Public Health 2019, 16, 1421. [Google Scholar] [CrossRef]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; Hankinson, J.L.; Irvin, C.G.; MacIntyre, N.R.; McKay, R.T.; Wanger, J.S.; Anderson, S.D.; et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am. J. Respir. Crit. Care Med. 2000, 161, 309–329. [Google Scholar] [CrossRef]
- Forrest, L.A.; Husein, T.; Husein, O. Paradoxical vocal cord motion: Classification and treatment. Laryngoscope 2012, 122, 844–853. [Google Scholar] [CrossRef]
- Halvorsen, T.; Walsted, E.S.; Bucca, C.; Bush, A.; Cantarella, G.; Friedrich, G.; Herth, F.J.F.; Hull, J.H.; Jung, H.; Maat, R.; et al. Inducible laryngeal obstruction: An official joint European Respiratory Society and European Laryngological Society statement. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Barr, R.G.; Bleecker, E.; Christenson, S.A.; Couper, D.; Curtis, J.L.; Gouskova, N.A.; Hansel, N.N.; Hoffman, E.A.; Kanner, R.E.; et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N. Engl. J. Med. 2016, 374, 1811–1821. [Google Scholar] [CrossRef]
- Berkhof, F.F.; Boom, L.N.; ten Hertog, N.E.; Uil, S.M.; Kerstjens, H.A.; van den Berg, J.W. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual. Life Outcomes 2012, 10, 4. [Google Scholar] [CrossRef]
- Arffa, R.E.; Krishna, P.; Gartner-Schmidt, J.; Rosen, C.A. Normative values for the Voice Handicap Index-10. J. Voice 2012, 26, 462–465. [Google Scholar] [CrossRef]
- Berger, K.I.; Reibman, J.; Oppenheimer, B.W.; Vlahos, I.; Harrison, D.; Goldring, R.M. Lessons from the World Trade Center disaster: Airway disease presenting as restrictive dysfunction. Chest 2013, 144, 249–257. [Google Scholar] [CrossRef]
- Friedman, S.M.; Maslow, C.B.; Reibman, J.; Pillai, P.S.; Goldring, R.M.; Farfel, M.R.; Stellman, S.D.; Berger, K.I. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am. J. Respir. Crit. Care Med. 2011, 184, 582–589. [Google Scholar] [CrossRef]
- Oppenheimer, B.W.; Goldring, R.M.; Herberg, M.E.; Hofer, I.S.; Reyfman, P.A.; Liautaud, S.; Rom, W.N.; Reibman, J.; Berger, K.I. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007, 132, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Brightling, C.; Baldi, S.; Van den Berge, M.; Fabbri, L.M.; Gagnatelli, A.; Papi, A.; Van der Molen, T.; Rabe, K.F.; Siddiqui, S.; et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): Baseline data from a prospective cohort study. Lancet Respir. Med. 2019, 7, 402–416. [Google Scholar] [CrossRef]
- Aldrich, T.K.; Weakley, J.; Dhar, S.; Hall, C.B.; Crosse, T.; Banauch, G.I.; Weiden, M.D.; Izbicki, G.; Cohen, H.W.; Gupta, A.; et al. Bronchial Reactivity and Lung Function After World Trade Center Exposure. Chest 2016, 150, 1333–1340. [Google Scholar] [CrossRef]
- Israel, E.; Reddel, H.K. Severe and Difficult-to-Treat Asthma in Adults. N. Engl. J. Med. 2017, 377, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Kazeros, A.; Maa, M.T.; Patrawalla, P.; Liu, M.; Shao, Y.; Qian, M.; Turetz, M.; Parsia, S.; Caplan-Shaw, C.; Berger, K.I.; et al. Elevated peripheral eosinophils are associated with new-onset and persistent wheeze and airflow obstruction in world trade center-exposed individuals. J. Asthma 2013, 50, 25–32. [Google Scholar] [CrossRef]
- Nolan, A.; Naveed, B.; Comfort, A.L.; Ferrier, N.; Hall, C.B.; Kwon, S.; Kasturiarachchi, K.J.; Cohen, H.W.; Zeig-Owens, R.; Glaser, M.S.; et al. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest 2012, 142, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Kazeros, A.; Zhang, E.; Cheng, X.; Shao, Y.; Liu, M.; Qian, M.; Caplan-Shaw, C.; Berger, K.I.; Goldring, R.M.; Ghumman, M.; et al. Systemic Inflammation Associated with World Trade Center Dust Exposures and Airway Abnormalities in the Local Community. J. Occup. Environ. Med. 2015, 57, 610–616. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Simpson, J.L.; Powell, H.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin. Exp. Allergy 2014, 44, 1137–1145. [Google Scholar] [CrossRef]
- Kipen, H.M.; Blume, R.; Hutt, D. Asthma experience in an occupational and environmental medicine clinic. Low-dose reactive airways dysfunction syndrome. J. Occup. Med. 1994, 36, 1133–1137. [Google Scholar] [CrossRef]
- Malo, J.L.; Tarlo, S.M.; Sastre, J.; Martin, J.; Jeebhay, M.F.; Le Moual, N.; Heederik, D.; Platts-Mills, T.; Blanc, P.D.; Vandenplas, O.; et al. An official American Thoracic Society Workshop Report: Presentations and discussion of the fifth Jack Pepys Workshop on Asthma in the Workplace. Comparisons between asthma in the workplace and non-work-related asthma. Ann. Am. Thorac Soc. 2015, 12, S99–S110. [Google Scholar] [CrossRef]
- Rojano, B.; West, E.; Ferdermann, E.; Markowitz, S.; Harrison, D.; Crowley, L.; Busse, P.; Federman, A.D.; Wisnivesky, J.P. Allergen Sensitization and Asthma Outcomes among World Trade Center Rescue and Recovery Workers. Int. J. Environ. Res. Public Health 2019, 16, 737. [Google Scholar] [CrossRef] [PubMed]
- Rojano, B.; West, E.; Goodman, E.; Weiss, J.J.; de la Hoz, R.E.; Crane, M.; Crowley, L.; Harrison, D.; Markowitz, S.; Wisnivesky, J.P. Self-management behaviors in World Trade Center rescue and recovery workers with asthma. J. Asthma 2019, 56, 411–421. [Google Scholar] [CrossRef] [PubMed]
- van Boven, J.F.M.; Koponen, M.; Lalic, S.; George, J.; Bell, J.S.; Hew, M.; Ilomaki, J. Trajectory Analyses of Adherence Patterns in a Real-Life Moderate to Severe Asthma Population. J. Allergy Clin. Immunol. Pract. 2020, 8, 1961–1969.e6. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.C.; Butler, M.G.; Li, L.; Fung, V.; Kharbanda, E.O.; Larkin, E.K.; Vollmer, W.M.; Miroshnik, I.; Davis, R.L.; Lieu, T.A.; et al. Primary adherence to controller medications for asthma is poor. Ann. Am. Thorac Soc. 2015, 12, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Taylor, D.R.; Bateman, E.D.; Boulet, L.P.; Boushey, H.A.; Busse, W.W.; Casale, T.B.; Chanez, P.; Enright, P.L.; Gibson, P.G.; et al. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009, 180, 59–99. [Google Scholar] [CrossRef]
- Banks, D.E.; Shi, R.; McLarty, J.; Cowl, C.T.; Smith, D.; Tarlo, S.M.; Daroowalla, F.; Balmes, J.; Baumann, M. American College of Chest Physicians consensus statement on the respiratory health effects of asbestos. Results of a Delphi study. Chest 2009, 135, 1619–1627. [Google Scholar] [CrossRef]
- Tarlo, S.M.; Balmes, J.; Balkissoon, R.; Beach, J.; Beckett, W.; Bernstein, D.; Blanc, P.D.; Brooks, S.M.; Cowl, C.T.; Daroowalla, F.; et al. Diagnosis and management of work-related asthma: American College of Chest Physicians Consensus Statement. Chest 2008, 134, 1S–41S. [Google Scholar] [CrossRef]
- Borak, J.; Lefkowitz, R.Y. Bronchial hyperresponsiveness. Occup. Med. 2016, 66, 95–105. [Google Scholar] [CrossRef]
- Jayet, P.Y.; Schindler, C.; Kunzli, N.; Zellweger, J.P.; Brandli, O.; Perruchoud, A.P.; Keller, R.; Schwartz, J.; Ackermann-Liebrich, U.; Leuenberger, P.; et al. Reference values for methacholine reactivity (SAPALDIA study). Respir. Res. 2005, 6, 131. [Google Scholar] [CrossRef][Green Version]
- Leynaert, B.; Bousquet, J.; Henry, C.; Liard, R.; Neukirch, F. Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am. J. Respir. Crit. Care Med. 1997, 156, 1413–1420. [Google Scholar] [CrossRef]
- Sumino, K.; Sugar, E.A.; Irvin, C.G.; Kaminsky, D.A.; Shade, D.; Wei, C.Y.; Holbrook, J.T.; Wise, R.A.; Castro, M.; Writing Committee for American Lung Association Asthma Clinical Research, C. Variability of methacholine bronchoprovocation and the effect of inhaled corticosteroids in mild asthma. Ann. Allergy Asthma Immunol. 2014, 112, 354–360.e1. [Google Scholar] [CrossRef]
- Sumino, K.; Sugar, E.A.; Irvin, C.G.; Kaminsky, D.A.; Shade, D.; Wei, C.Y.; Holbrook, J.T.; Wise, R.A.; Castro, M.; American Lung Association Asthma Clinical Research, C. Methacholine challenge test: Diagnostic characteristics in asthmatic patients receiving controller medications. J. Allergy Clin. Immunol. 2012, 130, 69–75.e6. [Google Scholar] [CrossRef] [PubMed]
- Vertigan, A.E.; Bone, S.L.; Gibson, P.G. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology 2013, 18, 948–956. [Google Scholar] [CrossRef]
- Berger, K.I.; Turetz, M.; Liu, M.; Shao, Y.; Kazeros, A.; Parsia, S.; Caplan-Shaw, C.; Friedman, S.M.; Maslow, C.B.; Marmor, M.; et al. Oscillometry complements spirometry in evaluation of subjects following toxic inhalation. ERJ Open Res. 2015, 1. [Google Scholar] [CrossRef]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef]
- Malo, J.L.; L’Archeveque, J.; Castellanos, L.; Lavoie, K.; Ghezzo, H.; Maghni, K. Long-term outcomes of acute irritant-induced asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Bigler, J.; Boedigheimer, M.; Schofield, J.P.R.; Skipp, P.J.; Corfield, J.; Rowe, A.; Sousa, A.R.; Timour, M.; Twehues, L.; Hu, X.; et al. A Severe Asthma Disease Signature from Gene Expression Profiling of Peripheral Blood from U-BIOPRED Cohorts. Am. J. Respir. Crit. Care Med. 2017, 195, 1311–1320. [Google Scholar] [CrossRef]
- Citron, J.; Willcocks, E.; Crowley, G.; Kwon, S.; Nolan, A. Genomics of Particulate Matter Exposure Associated Cardiopulmonary Disease: A Narrative Review. Int. J. Environ. Res. Public Health 2019, 16, 4335. [Google Scholar] [CrossRef]
- Ho, S.-M. Environmental epigenetics of asthma: An update. J. Allergy Clin. Immunol. 2010, 126, 453–465. [Google Scholar] [CrossRef]
- Sunil, V.R.; Vayas, K.N.; Fang, M.; Zarbl, H.; Massa, C.; Gow, A.J.; Cervelli, J.A.; Kipen, H.; Laumbach, R.J.; Lioy, P.J.; et al. World Trade Center (WTC) dust exposure in mice is associated with inflammation, oxidative stress and epigenetic changes in the lung. Exp. Mol. Pathol. 2017, 102, 50–58. [Google Scholar] [CrossRef]
- Arslan, A.A.; Tuminello, S.; Yang, L.; Zhang, Y.; Durmus, N.; Snuderl, M.; Heguy, A.; Zeleniuch-Jacquotte, A.; Shao, Y.; Reibman, J. Genome-Wide DNA Methylation Profiles in Community Members Exposed to the World Trade Center Disaster. Int. J. Environ. Res. Public Health 2020, 17, 5493. [Google Scholar] [CrossRef]
- Kuan, P.F.; Mi, Z.; Georgopoulos, P.; Hashim, D.; Luft, B.J.; Boffetta, P. Enhanced exposure assessment and genome-wide DNA methylation in World Trade Center disaster responders. Eur. J. Cancer Prev. 2019, 28, 225–233. [Google Scholar] [CrossRef]
- Rakowski, E.; Zhao, S.; Liu, M.; Ahuja, S.; Durmus, N.; Grunig, G.; Curotto de Lafaille, M.; Wu, Y.; Reibman, J. Variability of blood eosinophils in patients in a clinic for severe asthma. Clin. Exp. Allergy 2019, 49, 163–170. [Google Scholar] [CrossRef]
| Level | Total | Uncontrolled | Controlled | p | |
|---|---|---|---|---|---|
| n | 60 | 49 | 11 | ||
| Sex, n (%) | F | 42 (70) | 32 (65) | 10 (91) | 0.15 |
| M | 18 (30) | 17 (35) | 1 (9) | ||
| Age, median [IQR] | 56.5 [47.8, 62] | 57.0 [48.0, 62.0] | 56.0 [47.0, 62.0] | 0.92 | |
| Race/Ethnicity, n (%) | Hispanic | 30 (50) | 26 (53) | 4 (36) | 0.04 |
| NH-White | 11 (18) | 10 (20) | 1 (9) | ||
| NH-Black | 15 (25) | 12 (24) | 3 (27) | ||
| Asian and Other | 4 (7) | 1 (2) | 3 (27) | ||
| BMI. median [IQR] | 29.4 [26.7, 33] | 29.5 [27.1, 32.9] | 27.8 [26.0, 34.4] | 0.58 | |
| BMI, n (%) | Normal (<25) | 6 (10) | 4 (8) | 2 (18) | 0.57 |
| Overweight (25-30) | 27 (45) | 22 (45) | 5 (45) | ||
| Obese (≥30) | 27 (45) | 23 (47) | 4 (36) | ||
| Dust cloud, n (%) | No | 28 (47) | 21 (43) | 7 (64) | 0.32 |
| Yes | 32 (53) | 28 (57) | 4 (36) | ||
| Exposure category, n (%) | Clean-up Worker | 14 (23) | 11 (22) | 3 (27) | 0.36 |
| Other | 5 (8) | 3 (6) | 2 (18) | ||
| Resident | 7 (12) | 7 (14) | 0 (0) | ||
| Local Worker | 34 (57) | 28 (57) | 6 (55) | ||
| ASUI Score, median [IQR] | 0.6 [0.5, 0.8] | 0.6 [0.4, 0.7] | 0.8 [0.7, 0.9] | 0.001 | |
| MMRC, n (%) | (<3) | 49 (82) | 39 (80) | 10 (91) | 0.44 |
| (3) | 11 (18) | 10 (20) | 1 (9) | ||
| 6MWT, Median distance, [IQR] | 388.8 [345.3, 447.7] | 392.7 [344.4, 452.9] | 369.0 [358.9, 434.0] | >0.99 |
| Total | Uncontrolled | Controlled | p Value | |
|---|---|---|---|---|
| ICSD, median [IQR] | 31.0 [19.0, 41.0] | 35.0 [24.0, 42.5] | 26.0 [18.2, 29.5] | 0.027 |
| LCQ, median [IQR] | 12.7 [9.0, 16.2] | 12.0 [8.9, 15.4] | 15.6 [12.7, 20.2] | 0.047 |
| VHI, median [ IQR] | 7.0 [0.5, 16.5] | 7.0 [1.0, 17.0] | 1.0 [0.0, 9.8] | 0.18 |
| Level | Total | Uncontrolled | Controlled | p-Value for % Pred | |
|---|---|---|---|---|---|
| SPIROMETRY | |||||
| Pre BD | |||||
| FVC,L (% of predicted, median) | 3.2 (98) | 3.2 (97) | 3.3 (100) | 0.59 | |
| FEV1,L (% of predicted, median) | 2.5 (93) | 2.5 (92) | 2.3 (96.5) | 0.24 | |
| FEV1/FVC, median | 78.5 [73.2, 82.4] | 78.5 [72.4, 82.4] | 81.2 [75.3,83.4] | 0.34 | |
| Post BD | |||||
| FVC,L (% of pred, median) | 3.1 (99) | 3.2 (98.5) | 3.1 (100) | 0.57 | |
| FEV1,L (% of pred, median) | 2.5 (96) | 2.6 (96) | 2.4 (96.5) | 0.81 | |
| FEV1/FVC, median | 78.3 [75.1, 82.3] | 78.3 [75.0, 82.2] | 77.7 [75.4,81.1] | 0.98 | |
| FOT Measurements | |||||
| Pre BD | |||||
| R5 median [IQR] | 4.3 [3.4, 5.7] | 4.4 [3.4,5.7] | 3.7 [3.3, 5.7] | 0.82 | |
| R5–20 median [IQR] | 0.9 [0.4, 1.5] | 1.0 [0.5,1.5] | 0.6 [0.4, 1.4] | 0.62 | |
| Post BD | |||||
| R5 median [IQR] | 4.1 [3.4, 5.4] | 4.1 [3.5,5.3] | 4.0 [3.4, 5.7] | 0.84 | |
| R5–20 median [IQR] | 0.8 [0.4, 1.2] | 0.8 [0.4,1.2] | 0.6 [0.4, 1.1] | 0.73 | |
| Level | Total | n (% Controlled) | p Value | |
|---|---|---|---|---|
| BHR (at dose ≤16mg) | Negative | 24 | 3 (12.5) | 0.46 * |
| Positive | 24 | 6 (25) | ||
| NA | 12 | |||
| Any PVFM | Negative | 29 | 5 (17) | 0.74 * |
| Positive | 27 | 6 (22) | ||
| NA | 4 | |||
| PVFM unprovoked | Negative | 50 | 10 (20) | >0.99 * |
| Positive | 5 | 1 (20) | ||
| NA | 5 | |||
| PVFM provoked | Negative | 29 | 5 (17) | 0.74 * |
| Positive | 27 | 6 (22) | ||
| NA | 4 | |||
| Any PVFM and BHR | -PVFM, -BHR | 9 | 0 (0) | 0.02 ** |
| -PVFM, +BHR | 14 | 3 (21) | ||
| +PVFM, -BHR | 14 | 3 (21) | ||
| +PVFM,+BHR | 9 | 3 (33) | ||
| NA | 14 |
| Total | Uncontrolled | Controlled | p | |
|---|---|---|---|---|
| n | 60 | 49 | 11 | |
| FeNO, ppb median [IQR] | 16.5 [13.1, 21.8] | 17.8 [14.0, 22.2] | 13.0 [10.2, 13.9] | 0.01 |
| Blood markers | ||||
| White blood cells *, Median [IQR] | 7.2 [5.2, 8.5] | 7.1 [5.0, 8.6] | 7.2 [6.5, 8.1] | 0.70 |
| Eosinophils *, Median [IQR] | 0.10 [0.10, 0.20] | 0.10 [0.10, 0.20] | 0.10 [0.10, 0.10] | 0.34 |
| Neutrophils *, Median [IQR] | 3.8 [3.0, 5.1] | 3.7 [2.7, 5.1] | 4.1 [3.7, 4.8] | 0.37 |
| Lymphocytes *, Median [IQR] | 2.0 [1.6, 2.8] | 2.0 [1.5, 2.9] | 2.2 [1.8, 2.5] | 0.65 |
| ELR, Median [IQR] | 0.06 [0.04, 0.11] | 0.1 [0.0, 0.1] | 0.1 [0.0, 0.1] | 0.39 |
| ENR, Median [IQR] | 0.03 [0.02, 0.06] | 0.0 [0.0, 0.1] | 0.0 [0.0, 0.0] | 0.18 |
| NLR, Median [IQR] | 1.78 [1.30, 2.47] | 1.8 [1.2, 2.5] | 1.8 [1.5, 2.3] | 0.76 |
| Total IgE, median [IQR] | 37 [19.8, 115.5] | 41.0 [20.0, 135.0] | 31.0 [21.5, 57.0] | 0.52 |
| Any allergen-specific IgE, n (%) | 26 (43.8) | 23 (47) | 3 (27) | 0.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reibman, J.; Caplan-Shaw, C.; Wu, Y.; Liu, M.; Amin, M.R.; Berger, K.I.; Cotrina-Vidal, M.L.; Kazeros, A.; Durmus, N.; Fernandez-Beros, M.-E.; et al. Characterization of Persistent Uncontrolled Asthma Symptoms in Community Members Exposed to World Trade Center Dust and Fumes. Int. J. Environ. Res. Public Health 2020, 17, 6645. https://doi.org/10.3390/ijerph17186645
Reibman J, Caplan-Shaw C, Wu Y, Liu M, Amin MR, Berger KI, Cotrina-Vidal ML, Kazeros A, Durmus N, Fernandez-Beros M-E, et al. Characterization of Persistent Uncontrolled Asthma Symptoms in Community Members Exposed to World Trade Center Dust and Fumes. International Journal of Environmental Research and Public Health. 2020; 17(18):6645. https://doi.org/10.3390/ijerph17186645
Chicago/Turabian StyleReibman, Joan, Caralee Caplan-Shaw, Yinxiang Wu, Mengling Liu, Milan R. Amin, Kenneth I. Berger, Maria L. Cotrina-Vidal, Angeliki Kazeros, Nedim Durmus, Maria-Elena Fernandez-Beros, and et al. 2020. "Characterization of Persistent Uncontrolled Asthma Symptoms in Community Members Exposed to World Trade Center Dust and Fumes" International Journal of Environmental Research and Public Health 17, no. 18: 6645. https://doi.org/10.3390/ijerph17186645
APA StyleReibman, J., Caplan-Shaw, C., Wu, Y., Liu, M., Amin, M. R., Berger, K. I., Cotrina-Vidal, M. L., Kazeros, A., Durmus, N., Fernandez-Beros, M.-E., Goldring, R. M., Rosen, R., & Shao, Y. (2020). Characterization of Persistent Uncontrolled Asthma Symptoms in Community Members Exposed to World Trade Center Dust and Fumes. International Journal of Environmental Research and Public Health, 17(18), 6645. https://doi.org/10.3390/ijerph17186645




