The Effect of Strict State Measures on the Epidemiologic Curve of COVID-19 Infection in the Context of a Developing Country: A Simulation from Jordan
Abstract
1. Introduction
2. Materials and Methods
2.1. Simulated Model and Modelling Software
2.2. Model Parameterization
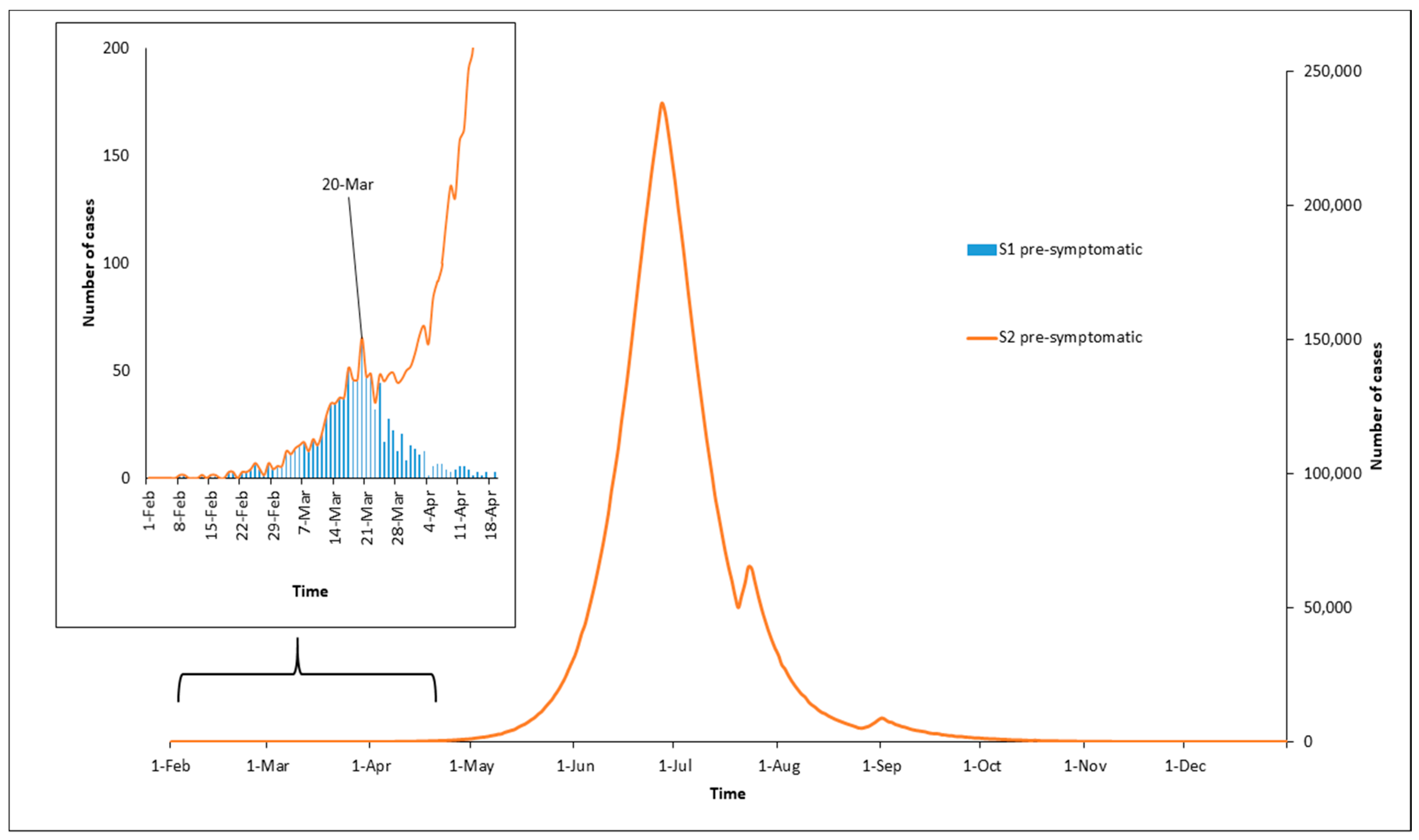
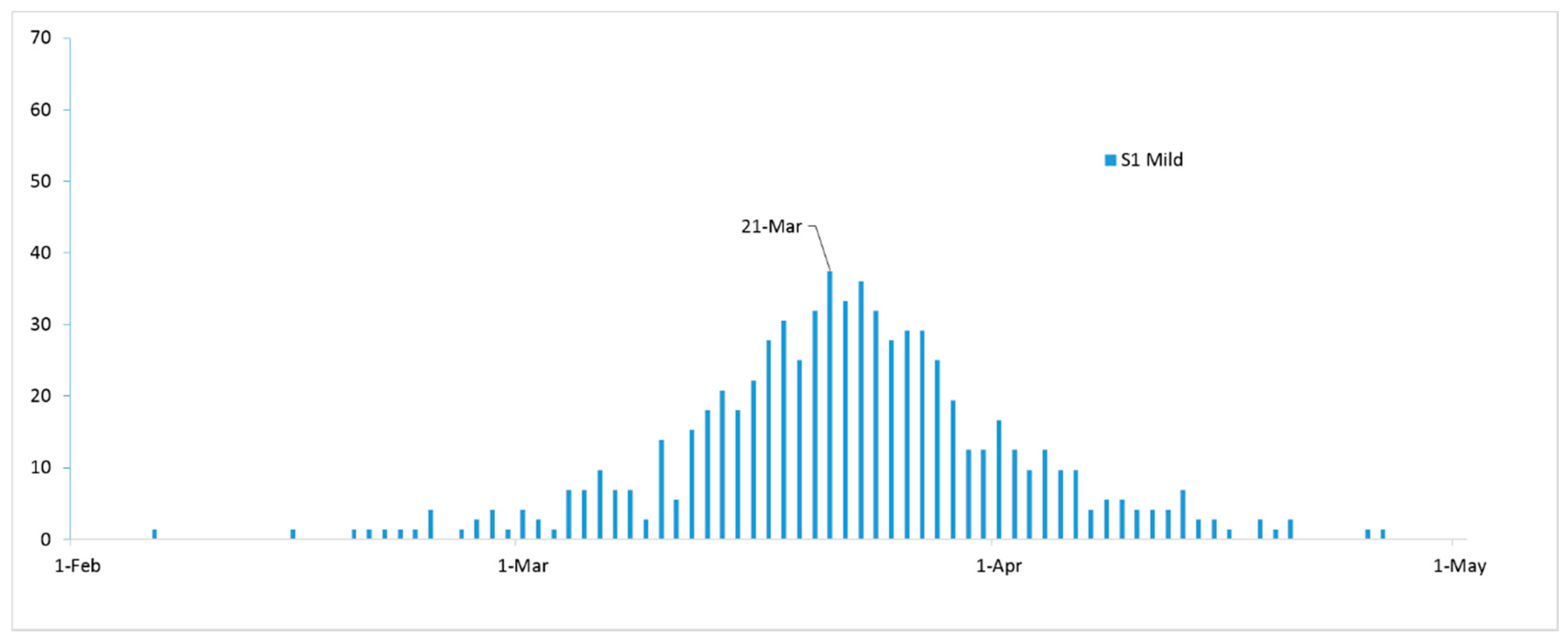
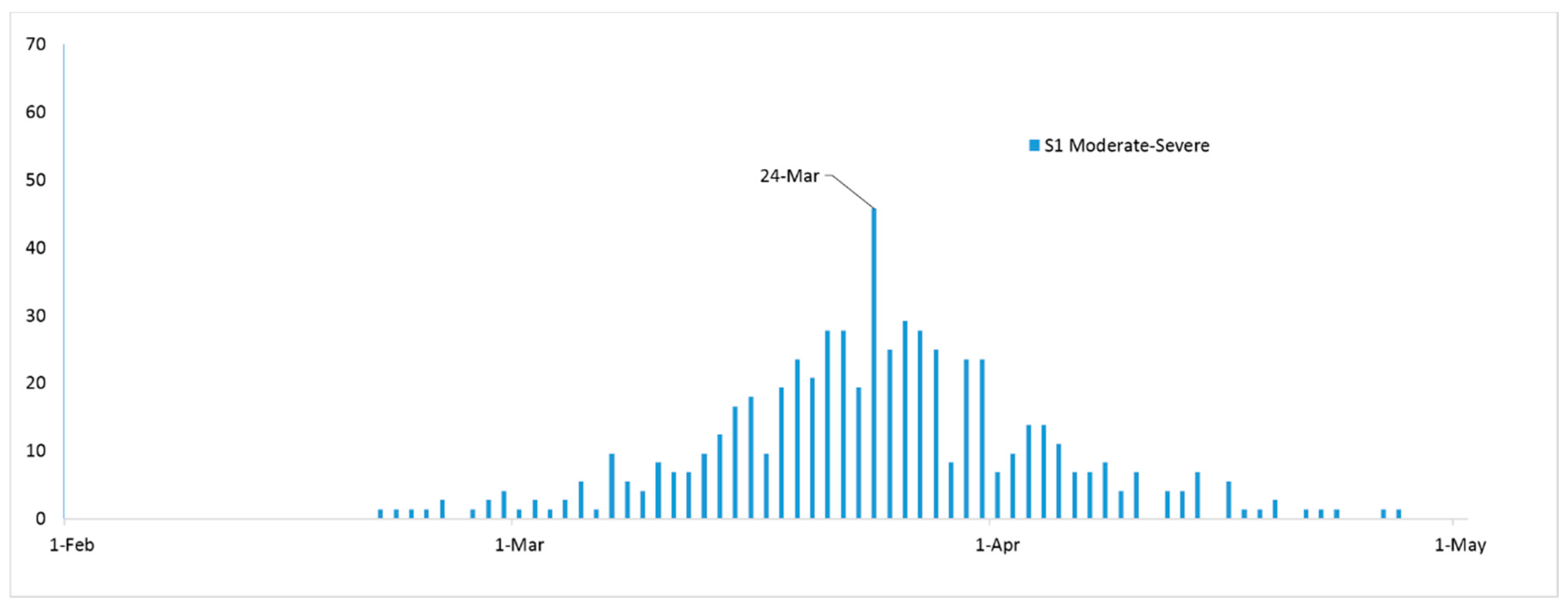
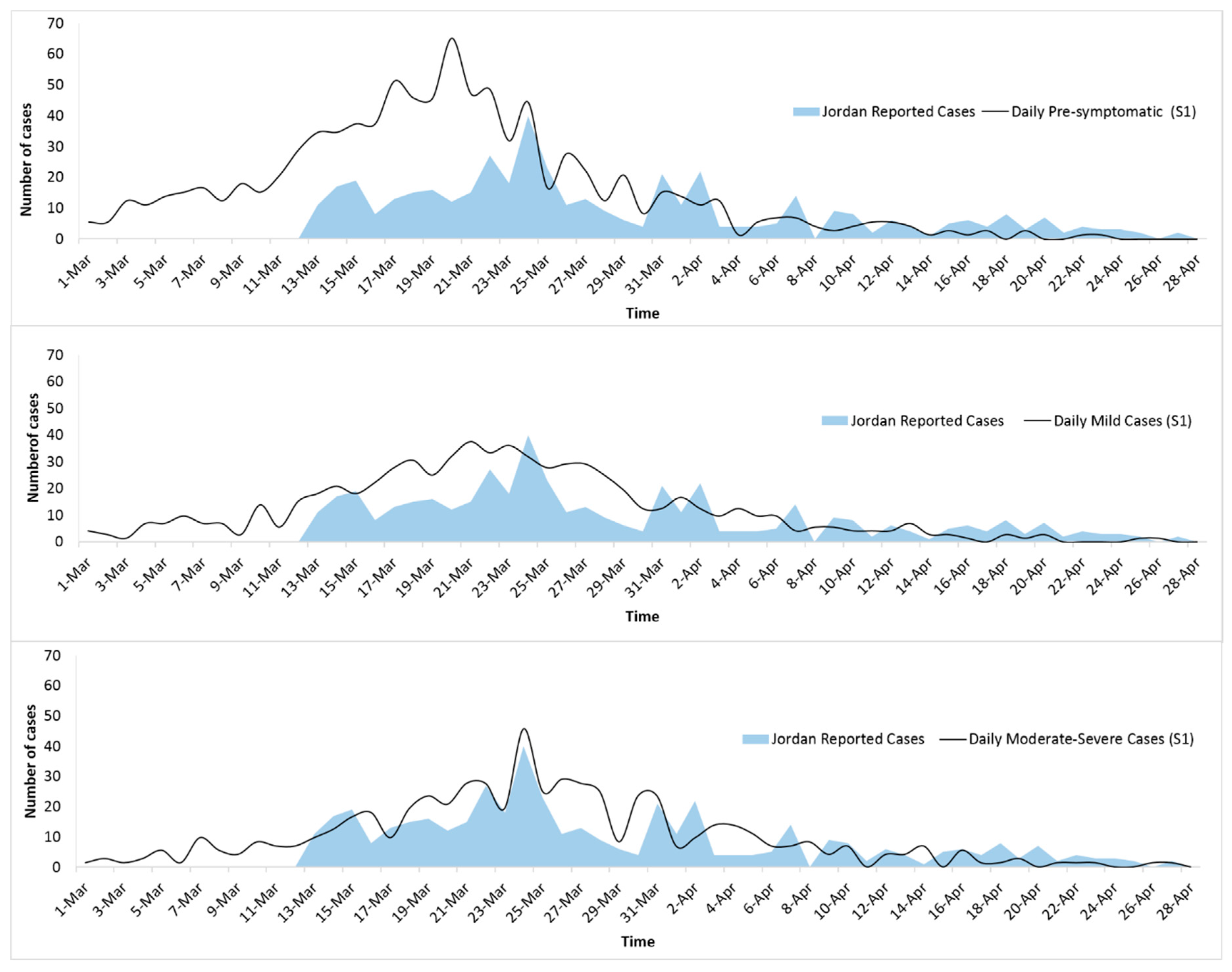
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, A.C.-P.; Li, X.; Lau, S.K.P.; Woo, P.C.Y. Global Epidemiology of Bat Coronaviruses. Viruses 2019, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Phan, T. Novel coronavirus: From discovery to clinical diagnostics. Infect. Genet. Evol. 2020, 79, 104211. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Du, L.; Ojcius, D.M.; Pan, C.; Jiang, S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020, 22, 74–79. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2019 Novel Coronavirus (2019-nCoV): Strategic Preparedness and Response Plan; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report—154; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Battegay, M.; Kuehl, R.; Tschudin-Sutter, S.; Hirsch, H.H.; Widmer, A.F.; Neher, R.A. 2019-Novel Coronavirus (2019-nCoV): Estimating the case fatality rate—A word of caution. Swiss Med. Wkly. 2020, 150, w20203. [Google Scholar] [CrossRef]
- Tuite, A.; Bogoch, I.I.; Sherbo, R.; Watts, A.; Fisman, D.; Khan, K. Estimation of Coronavirus Disease 2019 (COVID-19) Burden and Potential for International Dissemination of Infection from Iran. Ann. Intern. Med. 2020, 172, 699–701. [Google Scholar] [CrossRef]
- Weston, S.; Frieman, M.B. COVID-19: Knowns, Unknowns, and Questions. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Imai, N.D.I.; Cori, A.; Donnelly, C.; Riley, S.; Ferguson, N.M. Report 2: Estimating the Potential Total Number of Novel Coronavirus Cases in Wuhan City, China; Imperial College London: London, UK, 2020. [Google Scholar]
- Ferguson, N.M.; Laydon, D.; Nedjati-Gilani, G.; Imai, N.; Ainslie, K.; Baguelin, M.; Bhatia, S.; Boonyasiri, A.; Cucunubá, Z.; Cuomo-Dannenburg, G.; et al. Impact of Non-Pharmaceutical Interventions (NPIs) to Reduce COVID-19 Mortality and Healthcare Demand; Imperial College: London, UK, 2020. [Google Scholar]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef]
- Choi, S.C.; Ki, M. Estimating the reproductive number and the outbreak size of COVID-19 in Korea. Epidemiol. Health 2020, 42, e2020011. [Google Scholar] [CrossRef]
- Davies, N.G.; Kucharski, A.J.; Eggo, R.M.; Gimma, A.; Edmunds, W.J. The effect of non-pharmaceutical interventions on COVID-19 cases, deaths and demand for hospital services in the UK: A modelling study. Lancet Public Health 2020, 5, e375–e385. [Google Scholar] [CrossRef]
- Chinazzi, M.; Davis, J.T.; Ajelli, M.; Gioannini, C.; Litvinova, M.; Merler, S.; Piontti, A.P.Y.; Mu, K.; Rossi, L.; Sun, K.; et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 2020, 368, 395–400. [Google Scholar] [CrossRef]
- Kucharski, A.J.; Russell, T.W.; Diamond, C.; Liu, Y.; Edmunds, J.; Funk, S.; Eggo, R.M.; Sun, F.; Jit, M.; Munday, J.D.; et al. Early dynamics of transmission and control of COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 553–558. [Google Scholar] [CrossRef]
- Prem, K.; Cook, A.R.; Jit, M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Boil. 2017, 13, e1005697. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, K.E.; Walters, C.E.; Fu, H.; Bhatia, S.; Wang, H.; Xi, X. Report 11: Evidence of Initial Success for China Exiting COVID-19 Social Distancing Policy after Achieving Containment; Imperial College London: London, UK, 2020. [Google Scholar]
- Flaxman, S.; Mishra, S.; Gandy, A. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef]
- Petropoulos, F.; Makridakis, S. Forecasting the novel coronavirus COVID-19. PLoS ONE 2020, 15, e0231236. [Google Scholar] [CrossRef] [PubMed]
- Panovska-Griffiths, J. Can mathematical modelling solve the current Covid-19 crisis? BMC Public Health 2020, 20, 551. [Google Scholar] [CrossRef]
- Brand, S.P.C.; Aziza, R.; Kombe, I.K.; Agoti, C.N.; Hilton, J.; Rock, K.S.; Parisi, A.; Nokes, D.J.; Keeling, M.; Barasa, E. Forecasting the scale of the COVID-19 epidemic in Kenya. medRxiv 2020. [Google Scholar] [CrossRef]
- Zia, K.; Farooq, U. COVID-19 Outbreak in Oman: Model-Driven Impact Analysis and Challenges. medRxiv 2020. [Google Scholar] [CrossRef]
- Stehle, J.; Voirin, N.; Barrat, A.; Cattuto, C.; Colizza, V.; Isella, L.; Regis, C.; Pinton, J.F.; Khanafer, N.; Van den Broeck, W.; et al. Simulation of an SEIR infectious disease model on the dynamic contact network of conference attendees. BMC Med. 2011, 9, 87. [Google Scholar] [CrossRef]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020, 41, 145–151. [Google Scholar] [CrossRef]
- Balcan, D.; Hu, H.; Goncalves, B.; Bajardi, P.; Poletto, C.; Ramasco, J.J.; Paolotti, D.; Perra, N.; Tizzoni, M.; Van den Broeck, W.; et al. Seasonal transmission potential and activity peaks of the new influenza A(H1N1): A Monte Carlo likelihood analysis based on human mobility. BMC Med. 2009, 7, 45. [Google Scholar] [CrossRef]
- Greenwood, P.E.; Gordillo, L.F. Stochastic epidemic modeling. In Mathematical and Statistical Estimation Approaches in Epidemiology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 31–52. [Google Scholar]
- Tang, B.; Bragazzi, N.L.; Li, Q.; Tang, S.; Xiao, Y.; Wu, J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov). Infect. Dis. Model. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Hilton, J.; Keeling, M.J. Estimation of country-level basic reproductive ratios for novel Coronavirus (COVID-19) using synthetic contact matrices. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Castorina, P.; Iorio, A.; Lanteri, D. Data analysis on Coronavirus spreading by macroscopic growth laws. Int. J. Mod. Phys. C 2020, 31, 2050103. [Google Scholar] [CrossRef]
- Linton, N.M.; Kobayashi, T.; Yang, Y.; Hayashi, K.; Akhmetzhanov, A.R.; Jung, S.M.; Yuan, B.; Kinoshita, R.; Nishiura, H. Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: A Statistical Analysis of Publicly Available Case Data. J. Clin. Med. 2020, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Siwiak, M.M.; Szczesny, P.; Siwiak, M.P. From a single host to global spread. The global mobility based modelling of the COVID-19 pandemic implies higher infection and lower detection rates than current estimates. medRxiv 2020. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.; Whittaker, C.; Watson, O.; Baguelin, M.; Ainslie, K.; Bhatia, S. The Global Impact of COVID-19 and Strategies for Mitigation and Suppression; Imperial College London: London, UK, 2020. [Google Scholar]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklov, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef]
- Tang, B.; Wang, X.; Li, Q.; Bragazzi, N.L.; Tang, S.; Xiao, Y.; Wu, J. Estimation of the Transmission Risk of the 2019-nCoV and Its Implication for Public Health Interventions. J. Clin. Med. 2020, 9, 462. [Google Scholar] [CrossRef]
- Sanche, S.; Lin, Y.T.; Xu, C.; Romero-Severson, E.; Hengartner, N.; Ke, R. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg. Infect. Dis. 2020, 26, 1470–1477. [Google Scholar] [CrossRef]
- Saidan, M.; Shbool, M.; Arabeyyat, O.S.; Al-Shihabi, S.T.; Al Abdallat, Y.; Barghash, M.A.; Saidan, H. Estimation of the probable outbreak size of novel coronavirus (COVID-19) in social gathering events and industrial activities. Int. J. Infect. Dis. 2020, 98, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Al Zobbi, M.; Alsinglawi, B.; Mubin, O.; Alnajjar, F. Measurement Method for Evaluating the Lockdown Policies during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 5574. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M.E.; Rozhnova, G.; van Boven, M.E. Isolation and contact tracing can tip the scale to containment of COVID-19 in populations with social distancing. medRxiv 2020. [Google Scholar] [CrossRef]
| Parameter and Symbols | Description | Scenario 1 Values |
|---|---|---|
| β (beta) | Describes the transmission rate | February 1 to March 17 = 0.37 March 18 to April 24 = 0.06 April 25 to May 15 = 0.20 After May 15 = 0.37 |
| α (alpha) | Reduction in transmission rate. (Moderate to Severe) | 0.5 |
| ε (epsilon) | The incubation period from the state of exposure to the disease to become infectious | 1/5.2 |
| Ps | Probability of developing severe SAR-CoV-2 symptoms | 0.01 |
| µ (mu) | Recovery rate | 1/14 days |
| R0 | Basic Reproduction number | 5.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheirallah, K.A.; Alsinglawi, B.; Alzoubi, A.; Saidan, M.N.; Mubin, O.; Alorjani, M.S.; Mzayek, F. The Effect of Strict State Measures on the Epidemiologic Curve of COVID-19 Infection in the Context of a Developing Country: A Simulation from Jordan. Int. J. Environ. Res. Public Health 2020, 17, 6530. https://doi.org/10.3390/ijerph17186530
Kheirallah KA, Alsinglawi B, Alzoubi A, Saidan MN, Mubin O, Alorjani MS, Mzayek F. The Effect of Strict State Measures on the Epidemiologic Curve of COVID-19 Infection in the Context of a Developing Country: A Simulation from Jordan. International Journal of Environmental Research and Public Health. 2020; 17(18):6530. https://doi.org/10.3390/ijerph17186530
Chicago/Turabian StyleKheirallah, Khalid A., Belal Alsinglawi, Abdallah Alzoubi, Motasem N. Saidan, Omar Mubin, Mohammed S. Alorjani, and Fawaz Mzayek. 2020. "The Effect of Strict State Measures on the Epidemiologic Curve of COVID-19 Infection in the Context of a Developing Country: A Simulation from Jordan" International Journal of Environmental Research and Public Health 17, no. 18: 6530. https://doi.org/10.3390/ijerph17186530
APA StyleKheirallah, K. A., Alsinglawi, B., Alzoubi, A., Saidan, M. N., Mubin, O., Alorjani, M. S., & Mzayek, F. (2020). The Effect of Strict State Measures on the Epidemiologic Curve of COVID-19 Infection in the Context of a Developing Country: A Simulation from Jordan. International Journal of Environmental Research and Public Health, 17(18), 6530. https://doi.org/10.3390/ijerph17186530








