A Scoping Review on Cue Reactivity in Methamphetamine Use Disorder
Abstract
1. Introduction
2. Materials and Methods
- Method of understanding the nature of methamphetamine dependence
- Predictor of relapse
- Method of studying treatment effects
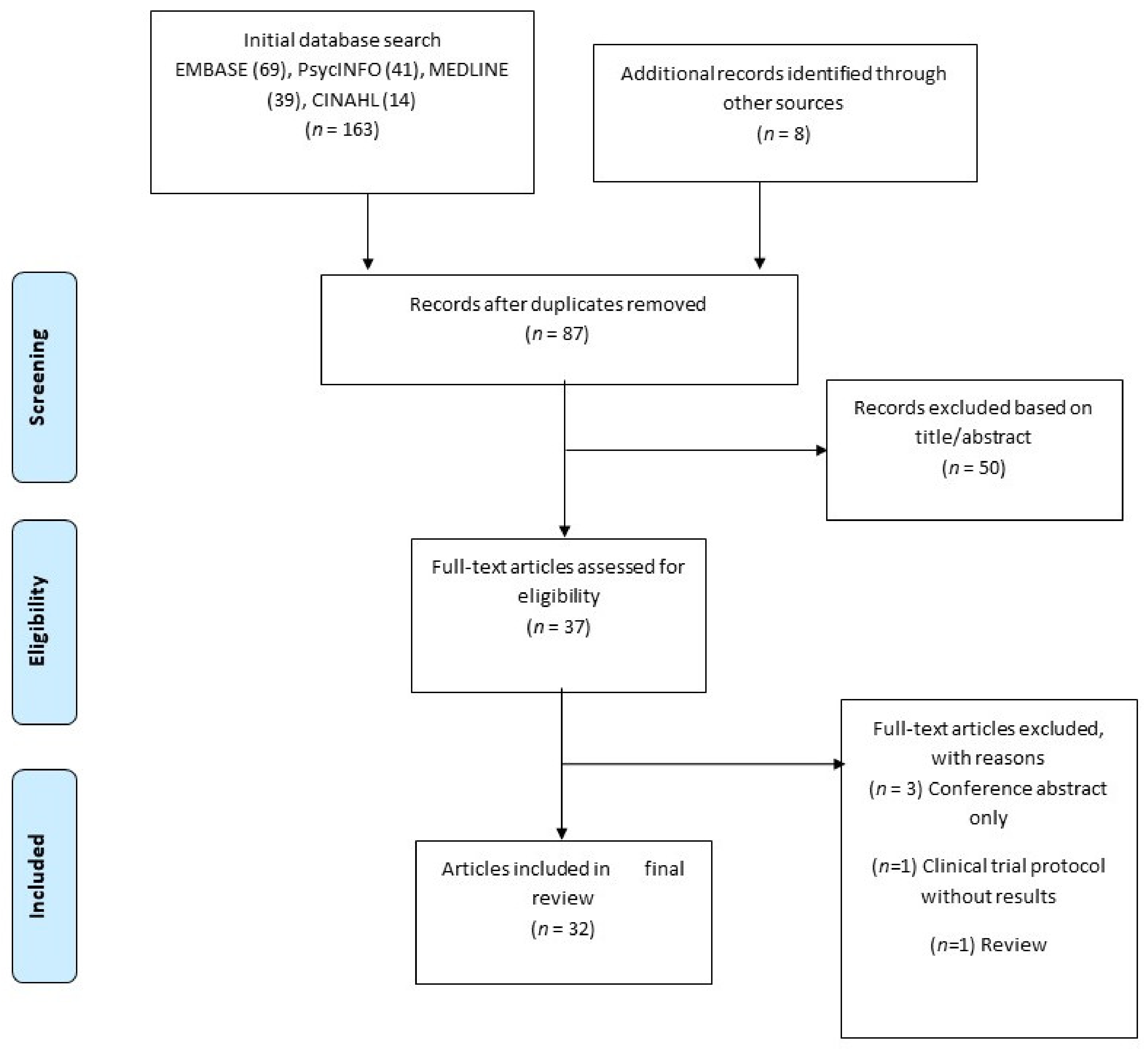
2.1. Search Strategy and Databases
2.2. Inclusion and Exclusion Criteria
2.3. Data Charting, Collating, Summarizing and Reporting
3. Results
3.1. Summary of Included Studies
3.2. Effects of Cue Exposure
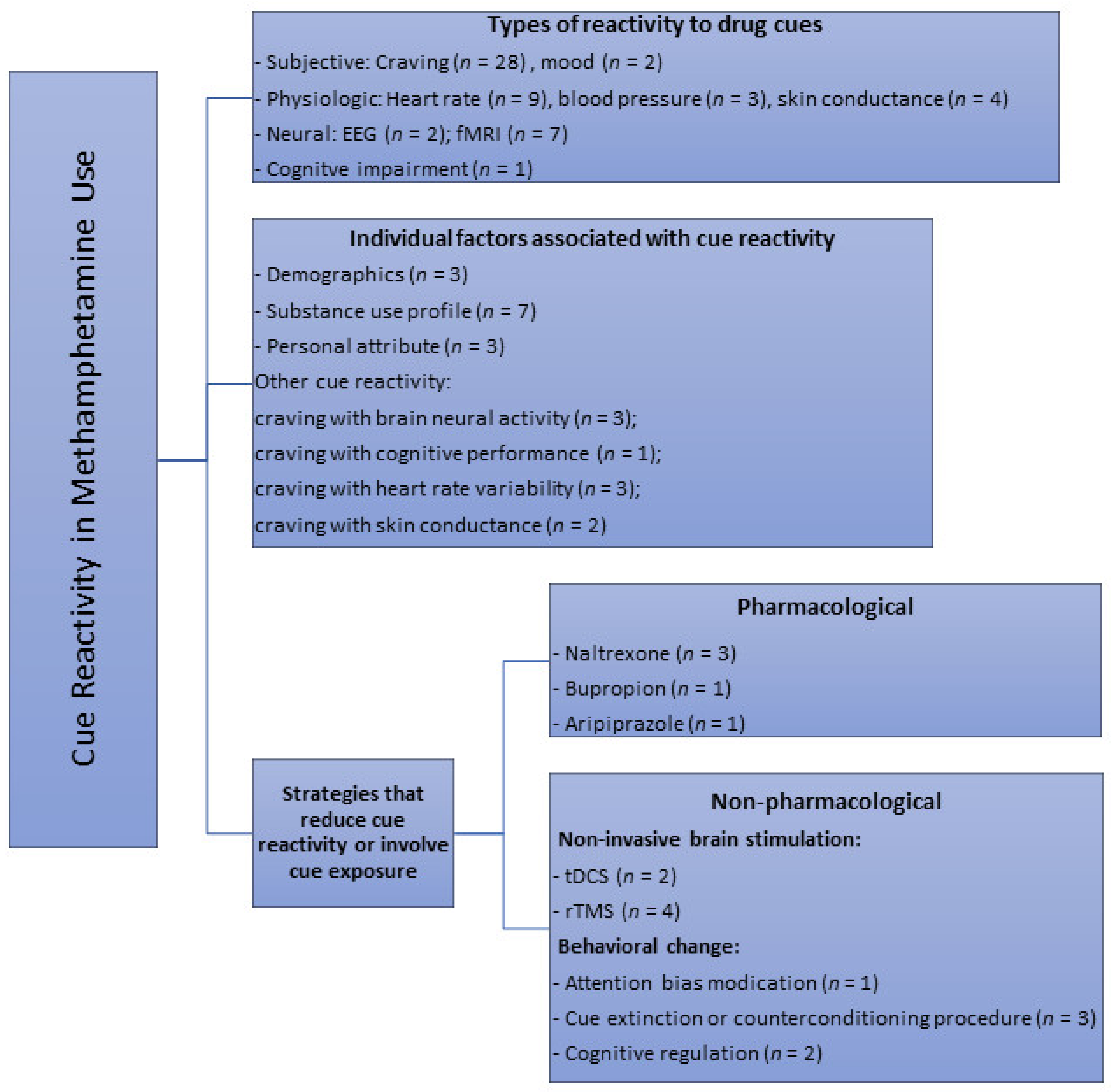
3.2.1. Subjective and Physiological Responses
3.2.2. Neural Reactivity
3.2.3. Cognitive Function
3.3. Factors Associated with Drug Cue Reactivity
3.3.1. Demographics
3.3.2. Substance Use Profile
3.3.3. Personality Attributes
3.3.4. Other Cue Reactivity Responses
3.4. Strategies or Interventions that Modulate Drug Cue Reactivity
3.4.1. Pharmacological Measures
3.4.2. Non-Pharmacological Measures
Non-Invasive Brain Stimulation
Behavioral Interventions
4. Discussion
Future Directions on Treatment Options
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tiffany, S.T. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol. Rev. 1990, 97, 147–168. [Google Scholar] [CrossRef]
- Drummond, D.C. Theories of drug craving, ancient and modern. Addiction 2001, 96, 33–46. [Google Scholar] [CrossRef]
- Childress, A.R.; McLellan, A.T.; O’Brien, C.P. Abstinent Opiate Abusers Exhibit Conditioned Craving, Conditioned Withdrawal and Reductions in both through Extinction. Br. J. Addict. 1986, 81, 655–660. [Google Scholar] [CrossRef]
- O’Brien, C.P.; Childress, A.R.; McLellan, A.T.; Ehrman, R. Classical conditioning in drug-dependent humans. Ann. N. Y. Acad. Sci. 1992, 654, 400–415. [Google Scholar] [CrossRef]
- Carter, B.L.; Tiffany, S.T. Meta-analysis of cue-reactivity in addiction research. Addiction 1999, 94, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Wikler, A. Conditioning factors in opiate addiction and relapse. J. Subst. Abus. Treat. 1984, 1, 279–285. [Google Scholar] [CrossRef]
- Stewart, J.; de Wit, H.; Eikelboom, R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 1984, 91, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, P.I. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Ann. Neurosci. 2010, 17, 136–141. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Drummond, D.C. What does cue-reactivity have to offer clinical research? Addiction 2000, 95, S129–S144. [Google Scholar] [CrossRef]
- Modesto-Lowe, V.; Kranzler, H.R. Using cue reactivity to evaluate medications for treatment of cocaine dependence: A critical review. Addiction 1999, 94, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Mehrjerdi, Z.A.; Tasnim, S.; Ekhtiari, H. Measurement of cue-induced craving in human methamphetamine dependent subjects new methodological hopes for reliable assessment of treatment efficacy. Basic Clin. Neurosci. 2011, 2, 48–53. [Google Scholar]
- Barr, A.M.; Panenka, W.J.; MacEwan, G.W.; Thornton, A.E.; Lang, D.J.; Honer, W.G.; Lecomte, T. The need for speed: An update on methamphetamine addiction. J. Psychiatry Neurosci. 2006, 31, 301–313. [Google Scholar] [PubMed]
- Radfar, S.R.; Rawson, R.A. Current research on methamphetamine: Epidemiology, medical and psychiatric effects, treatment, and harm reduction efforts. Addict. Health 2014, 6, 146. [Google Scholar] [PubMed]
- Petit, A.; Karila, L.; Chalmin, F. Methamphetamine Addiction: A Review of the Literature. J. Addict. Res. Ther. 2012, 1. [Google Scholar] [CrossRef]
- The United Nations Office on Drugs and Crime: World Drug Report. 2019. Available online: https://wdr.unodc.org/wdr2019/ (accessed on 8 March 2020).
- Stoneberg, D.M.; Shukla, R.K.; Magness, M.B. Global methamphetamine trends: An evolving problem. Int. Crim. Justice Rev. 2018, 28, 136–161. [Google Scholar] [CrossRef]
- The United Nations Office on Drugs and Crime: Patterns and Trends of Amphetamine=Type Stimulants and other Drugs. Available online: http://www.unodc.org/documents/data-and-analysis/WDR2012/WDR_2012_web_small.pdf (accessed on 8 March 2020).
- Vocci, F.J.; Appel, N.M. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction 2007, 102 (Suppl. 1), 96–106. [Google Scholar] [CrossRef]
- Rohsenow, D.J.; Niaura, R.S.; Childress, A.R.; Abrams, D.B.; Monti, P.M. Cue reactivity in addictive behaviors: Theoretical and treatment implications. Int. J. Addict. 1990, 25, 957–993. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Drummond, D.C.; Tiffany, S.T.; Glautier, S.; Remington, B. Cue exposure in understanding and treating addictive behaviours. In Addictive Behaviour: Cue Exposure Theory and Practice; John Wiley & Sons: Oxford, UK, 1995; pp. 1–17. [Google Scholar]
- Culbertson, C.; Nicolas, S.; Zaharovits, I.; London, E.D.; La Garza, R.D.; Brody, A.L.; Newton, T.F. Methamphetamine craving induced in an online virtual reality environment. Pharmacol. Biochem. Behav. 2010, 96, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Shen, Z.H.; Wu, X.C. Detection of patients with methamphetamine dependence with cue-elicited heart rate variability in a virtual social environment. Psychiatry Res. 2018, 270, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Liu, M.H.; Shen, Z.H. A virtual reality counterconditioning procedure to reduce methamphetamine cue-induced craving. J. Psychiatr. Res. 2019, 116, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chen, T.; Du, J.; Li, R.; Jiang, H.; Deng, C.-L.; Song, W.; Xu, D.; Zhao, M. Drug-related Virtual Reality Cue Reactivity is Associated with Gamma Activity in Reward and Executive Control Circuit in Methamphetamine Use Disorders. Arch. Med. Res. 2019, 50, 509–517. [Google Scholar] [CrossRef]
- Tolliver, B.K.; McRae-Clark, A.L.; Saladin, M.; Price, K.L.; Simpson, A.N.; DeSantis, S.M.; Baker, N.L.; Brady, K.T. Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am. J. Drug Alcohol Abus. 2010, 36, 106–113. [Google Scholar] [CrossRef]
- Grodin, E.N.; Courtney, K.E.; Ray, L.A. Drug-Induced Craving for Methamphetamine Is Associated with Neural Methamphetamine Cue Reactivity. J. Stud. Alcohol Drugs 2019, 80, 245–251. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Z.; Dai, Y.; Zhang, C.; Yang, C.; Fan, L.; Liu, J.; Hao, W.; Chen, H. Craving responses to methamphetamine and sexual visual cues in individuals with methamphetamine use disorder after long-term drug rehabilitation. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef]
- Malcolm, R.; Myrick, H.; Li, X.; Henderson, S.; Brady, K.T.; George, M.S.; See, R.E. Regional Brain Activity in Abstinent Methamphetamine Dependent Males Following Cue Exposure. J. Drug Abuse 2016, 2, 16. [Google Scholar] [CrossRef]
- Yin, J.J.; Ma, S.H.; Xu, K.; Wang, Z.X.; Le, H.B.; Huang, J.Z.; Fang, K.M.; Liao, L.M.; Cai, Z.L. Functional magnetic resonance imaging of methamphetamine craving. Clin. Imaging 2012, 36, 695–701. [Google Scholar] [CrossRef]
- Courtney, K.E.; Ghahremani, D.G.; Ray, L.A. The Effects of Pharmacological Opioid Blockade on Neural Measures of Drug Cue-Reactivity in Humans. Neuropsychopharmacology 2016, 41, 2872–2881. [Google Scholar] [CrossRef]
- Dean, A.C.; Nurmi, E.L.; Moeller, S.J.; Amir, N.; Rozenman, M.; Ghahremani, D.G.; Johnson, M.; Berberyan, R.; Hellemann, G.; Zhang, Z.; et al. No effect of attentional bias modification training in methamphetamine users receiving residential treatment. Psychopharmacology 2019, 236, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, S.; Yang, C.; Cai, W.; Chen, H.; Hao, W.; Liu, T.; Wang, X.; Worhunsky, P.D.; Potenza, M.N. Neurofunctional Differences Related to Methamphetamine and Sexual Cues in Men with Shorter and Longer Term Abstinence Methamphetamine Dependence. Int. J. Neuropsychopharmacol. 2019. [Google Scholar] [CrossRef]
- Shahmohammadi, F.; Golesorkhi, M.; Riahi Kashani, M.M.; Sangi, M.; Yoonessi, A.; Yoonessi, A. Neural Correlates of Craving in Methamphetamine Abuse. Basic Clin. Neurosci. 2016, 7, 221–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tolliver, B.K.; Price, K.L.; Baker, N.L.; LaRowe, S.D.; Simpson, A.N.; McRae-Clark, A.L.; Saladin, M.E.; DeSantis, S.M.; Chapman, E.; Garrett, M.; et al. Impaired cognitive performance in subjects with methamphetamine dependence during exposure to neutral versus methamphetamine-related cues. Am. J. Drug Alcohol Abuse 2012, 38, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ekhtiari, H.; Alam-Mehrjerdi, Z.; Nouri, M.; George, S.; Mokri, A. Designing and evaluation of reliability and validity of visual cue-induced craving assessment task for methamphetamine smokers. Basic Clin. Neurosci. 2010, 1, 34–37. [Google Scholar]
- Liang, Q.; Yuan, T.; Cao, X.; He, H.; Yang, J.; Yuan, J. Assessing the severity of methamphetamine use disorder beyond the subjective craving report: The role of an attention bias test. Gen. Psychiatry 2019, 32, e100019. [Google Scholar] [CrossRef]
- Su, H.; Zhong, N.; Gan, H.; Wang, J.; Han, H.; Chen, T.; Li, X.; Ruan, X.; Zhu, Y.; Jiang, H.; et al. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug Alcohol Depend. 2017, 84–91. [Google Scholar] [CrossRef]
- Lopez, R.B.; Onyemekwu, C.; Hart, C.L.; Ochsner, K.N.; Kober, H. Boundary conditions of methamphetamine craving. Exp. Clin. Psychopharmacol. 2015, 23, 436–444. [Google Scholar] [CrossRef]
- Wang, G.; Shi, J.; Chen, N.; Xu, L.; Li, J.; Li, P.; Sun, Y.; Lu, L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS ONE 2013, 8, e68791. [Google Scholar] [CrossRef]
- Saladin, M.; Santa Ana, E.; La Rowe, S.; Simpson, A.; Tolliver, B.; Price, K.; McRae-Clark, A.; Brady, K. Does Alexithymia Explain Variation in Cue-Elicited Craving Reported by Methamphetamine-Dependent Individuals? Am. J. Addict. 2012, 21, 130–135. [Google Scholar] [CrossRef]
- Ray, L.A.; Bujarski, S.; Courtney, K.E.; Moallem, N.R.; Lunny, K.; Roche, D.; Leventhal, A.M.; Shoptaw, S.; Heinzerling, K.; London, E.D.; et al. The Effects of Naltrexone on Subjective Response to Methamphetamine in a Clinical Sample: A Double-Blind, Placebo-Controlled Laboratory Study. Neuropsychopharmacology 2015, 40, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Roche, D.; Worley, M.; Ray, L. Naltrexone moderates the relationship between cue-induced craving and subjective response to methamphetamine in individuals with methamphetamine use disorder. Neuropsychopharmacology 2016, 41, S606–S607. [Google Scholar] [CrossRef] [PubMed]
- Newton, T.F.; Reid, M.S.; De La Garza, I.R.; Mahoney, I.J.J.; Abad, A.; Condos, R.; Palamar, J.; Halkitis, P.N.; Mojisak, J.; Anderson, A.; et al. Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. Int. J. Neuropsychopharmacol. 2008, 11, 1037–1045. [Google Scholar] [CrossRef]
- Newton, T.F.; Roache, J.D.; De La Garza, R., II; Fong, T.; Wallace, C.L.; Li, S.-H.; Elkashef, A.; Chiang, N.; Kahn, R. Bupropion Reduces Methamphetamine-Induced Subjective Effects and Cue-Induced Craving. Neuropsychopharmacology 2006, 31, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Malcolm, R.J.; Huebner, K.; Hanlon, C.A.; Taylor, J.J.; Brady, K.T.; George, M.S.; See, R.E. Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: A preliminary study. Drug Alcohol Depend. 2013, 133, 641–646. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, Y.; Cao, X.; Li, Y.; Chen, Y.; Yang, W.; Yuan, T.-F. Either at left or right, both high and low frequency rTMS of dorsolateral prefrontal cortex decreases cue induced craving for methamphetamine. Am. J. Addict. 2017, 26, 776–779. [Google Scholar] [CrossRef]
- Shahbabaie, A.; Golesorkhi, M.; Zamanian, B.; Ebrahimpoor, M.; Keshvari, F.; Nejati, V.; Fregni, F.; Ekhtiari, H. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int. J. Neuropsychopharmacol. 2014, 17, 1591–1598. [Google Scholar] [CrossRef]
- Rohani Anaraki, M.; Dolatshahi, B.; Nosratabadi, M.; Nouri Yalghouzaghaji, M.; Rezaei Mashhadi, S. Repeated Transcranial Direct Current Stimulation (tDCS) on Methamphetamine Craving: A Randomized, Sham-controlled Study. Iran. Rehabil. J. 2019, 17, 385–394. [Google Scholar] [CrossRef]
- Su, H.; Chen, T.; Jiang, H.; Zhong, N.; Du, J.; Xiao, K.; Xu, D.; Song, W.; Zhao, M. Intermittent theta burst transcranial magnetic stimulation for methamphetamine addiction: A randomized clinical trial. Eur. Neuropsychopharmacol. 2020, 31, 158–161. [Google Scholar] [CrossRef]
- Kirton, A.; Chen, R.; Friefeld, S.; Gunraj, C.; Pontigon, A.M.; Deveber, G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: A randomised trial. Lancet Neurol. 2008, 7, 507–513. [Google Scholar] [CrossRef]
- Desantis, S.M.; Bandyopadhyay, D.; Back, S.E.; Brady, K.T.; DeSantis, S.M.; Bandyopadhyay, D.; Back, S.E.; Brady, K.T. Non-treatment laboratory stress- and cue-reactivity studies are associated with decreased substance use among drug-dependent individuals. Drug Alcohol Depend. 2009, 105, 227–233. [Google Scholar] [CrossRef][Green Version]
- Bruehl, A.M.; Lende, D.H.; Schwartz, M.; Sterk, C.E.; Elifson, K. Craving and control: Methamphetamine users’ narratives. J. Psychoact. Drugs 2006, 38 (Suppl. 3), 385–392. [Google Scholar] [CrossRef]
- Price, K.L.; Saladin, M.E.; Baker, N.L.; Tolliver, B.K.; DeSantis, S.M.; McRae-Clark, A.L.; Brady, K.T. Extinction of drug cue reactivity in methamphetamine-dependent individuals. Behav. Res. Ther. 2010, 48, 860–865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jasinska, A.J.; Stein, E.A.; Kaiser, J.; Naumer, M.J.; Yalachkov, Y. Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci. Biobehav. Rev. 2014, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hormes, J.M. The clinical significance of craving across the addictive behaviors: A review. Curr. Addict. Rep. 2017, 4, 132–141. [Google Scholar] [CrossRef]
- Karila, L.; Weinstein, A.; Aubin, H.J.; Benyamina, A.; Reynaud, M.; Batki, S.L. Pharmacological approaches to methamphetamine dependence: A focused review. Br. J. Clin. Pharmacol. 2010, 69, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.; De Wit, H. A multidimensional approach to studying responses to a methamphetamine-associated contextual cue in healthy, non-dependent humans. Neuropsychopharmacology 2014, 39, S516. [Google Scholar] [CrossRef][Green Version]
- Cavallo, J.S.; Mayo, L.M.; de Wit, H. Acquisition of Conditioning between Methamphetamine and Cues in Healthy Humans. PLoS ONE 2016, 11, e0161541. [Google Scholar] [CrossRef]
- Conklin, C.A.; Tiffany, S.T. Applying extinction research and theory to cue-exposure addiction treatments. Addiction 2002, 97, 155–167. [Google Scholar] [CrossRef]
- Hone-Blanchet, A.; Wensing, T.; Fecteau, S. The use of virtual reality in craving assessment and cue-exposure therapy in substance use disorders. Front. Hum. Neurosci. 2014, 8, 844. [Google Scholar] [CrossRef]
- Tunstall, B.J.; Verendeev, A.; Kearns, D.N. A comparison of therapies for the treatment of drug cues: Counterconditioning vs. extinction in male rats. Exp. Clin. Psychopharmacol. 2012, 20, 447. [Google Scholar] [CrossRef] [PubMed]
- Newall, C.; Watson, T.; Grant, K.-A.; Richardson, R. The relative effectiveness of extinction and counter-conditioning in diminishing children’s fear. Behav. Res. Ther. 2017, 95, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ciketic, S.; Hayatbakhsh, M.R.; Doran, C.M.; Najman, J.M.; McKetin, R. A review of psychological and pharmacological treatment options for methamphetamine dependence. J. Subst. Use 2012, 17, 363–383. [Google Scholar] [CrossRef]
| Authors (Year) | Study Sample & Context | Drug Cue(s) | Methods | Outcome(s) of Interest | Main Findings |
|---|---|---|---|---|---|
| Culbertson et al. (2010) | 17 non-treatment seeking METH users; USA | METH virtual reality (METH-VR) of virtual avatars and drug-use animations of smoking, injecting, snorting) created using online gaming platform; METH-video that included professional actors/actresses administering METH followed by in vivo mock METH paraphernalia | Participants were asked to complete four test sessions: (1) METH-VR, (2) neutral-VR, (3) METH-video and (4) neutral-video in a counter-balanced fashion. | - Subjective ratings of urges to use METH, mood, and physical state on a VAS (1-100, “none” to “very much”) prior to (time = 0), during (time = 5), after (time = 10) and following (time = 15) each cue condition - Heart rate variability (HRV) recorded over 10 min interval | - METH-VR cue condition elicited greater increases in subjective cravings (VAS) compared to all neutral cue conditions. - Participants also reported higher increase in “anxiety” (VAS) to METH-VR compared to neutral VR. No effect of cue condition on HRV measures was found. - “high craving” and ‘low craving” participants tend to display more high and low frequency cardiovascular activity (HRV), respectively during the cue conditions. |
| Ekhtiari et al. (2010) | 50 outpatients who met DSM-IV-TR criteria for METH dependence in the past 6 months; Iran | Cues were classified into 4 main themes (drug, instruments 1, accompanying cues 2 and act of abuse) and photos were taken for each cue 1 refers to drug paraphernalia 2 refers for example to candies, beverages, money etc. | 50 photos with high levels of evocative potency (CICT 50) and 10 photos with the most evocative potency (CICT 10) out of 72 cues (60 active evocative photos + 10 neutral photos) were rated by participants. | - Self-reported craving intensity on VAS (0-100) when presented with cues | - Differences in cue-induced craving (VAS) in CICT 50 and CICT 10 were not associated with age, education, income, marital status, employment and sexual activity in the past 30 days prior to study entry. - Family living condition was marginally correlated with higher scores in CICT 50. Age of onset for (opioids, cocaine and methamphetamine) was negatively correlated with CICT 50 and CICT 10 and age of first opiate use was negatively correlated with CICT 50. |
| Tolliver et al. (2010) | 43 treatment and non-treatment seeking participants who met DSM-IV criteria for METH dependence in the past 6 months; USA | (1) 30–35 still photographs of individuals procuring and using METH, (2) a 7–8 min video depicting METH use in a variety of settings, and (3) in vivo paraphernalia and simulated METH placed in front of participants for 5 minutes | Participants were exposed to the three cue modalities in a counter-balanced fashion. Clinical and demographic correlates of METH craving were also explored. | - Subjective craving on WSRS using 100 mm VAS anchored with adjectival modifiers (not at all. mildly, moderately, extremely) - Physiological responses such as heart rate and skin conductance - All measures were obtained during and immediately after exposure to each cue modality - Baseline measures were collected 20-min and 5-min prior to the cue exposure | - Relative to baseline, subjective craving (WSRS-VAS) was increased by all three cue modalities to a similar extent. - Physiological cue reactivity correlated poorly with cue induced craving. - Differences in cue-induced craving (WSRS- VAS) were not associated with age, sex, education, employment, treatment status, or number of days using METH in the 60 days prior to study entry. |
| Saladin et al. (2012) | 40 treatment and non-treatment seeking participants who met DSM-IV criteria for METH dependence; USA | (1) 30–35 still photographs of individuals procuring and using METH, (2) a 7–8 min video depicting METH use in a variety of settings, and (3) in vivo paraphernalia and simulated METH placed in front of participants for 5 minutes | The relationship between alexithymia and baseline and cue-elicited craving was examined. | - METH craving on WSRS using 100 mm VAS anchored with adjectival modifiers (not at all, mildly, moderately, extremely) after each cue presentation | - Toronto Alexithymia Scale factor 1 (a measure of difficulty identifying feelings) scores measured at baseline were found to positively associate with cue-elicited craving (WSRS-VAS). |
| Tolliver et al. (2012) | 30 participants who met DSM-IV criteria for METH dependence in the past 6 months and 30 controls; USA | Public domain video footage with sequential 15–30 s segments of individuals or actors manufacturing, procuring, or using METH through various routes of administration | Participants were instructed to perform an auditory dual task cognitive test while viewing METH-related and neutral video cues in a counter-balanced fashion. | - Subjective craving on WSRS recorded before and after each video cue presentation - Reaction time, response errors, and inhibition errors on the auditory Go–No Go task | - Both response errors and inhibition errors increased significantly in METH participants while control participants exhibited only slightly increased rates of response errors upon exposure to cues. - Only response error rates, during exposure, were significantly associated with craving scores (WSRS) in METH participants. |
| Yin et al. (2012) | 26 METH users who had not received recent treatment and had not taken the drug at least 24 h before the experiment and 26 gender-matched controls; China | Not clearly described | Participants viewed METH cues vs neutral cues vs happy and sad (control cues) stimuli based on subjective evaluations of the emotional valence of the pictures via a block design in a balanced order. | - Activation in specific affect-related regions of the brain (ACC and frontal gyrus) were recorded with fMRI while exposure to picture cues | - Robust activation of the ACC gyrus was evident in patients watching METH cues, but not in those watching sad or happy pictures or in healthy participants under any condition. - In contrast, patients showed less activation than healthy participants during the METH-cue pictures in areas of the frontal lobes. |
| Wang et al. (2013) | 139 inpatients who had a history of DSM-IV METH dependence with varying abstinence period: 6 d (n = 24), 14 d (n = 26), 1 m (n = 19), 3 m (n = 20), 6 m (n = 20) and 1 y (n = 29); China | (1) 32 photographs of individuals procuring and using METH, with each presented for 7 s in a slide show, (2) a 5 min video in which METH abusers made drug paraphernalia and used METH, and (3) paraphernalia placed directly on the table in front of the participants | Participants underwent a cue session where either neutral cues or METH cues were presented first in a randomized manner. | - Subjective craving on VAS (1–10, “not at all” to “extremely high”)- Physiological responses such as heart rate and blood pressure- All measures were recorded before and after each cue presentation | - Cue-induced craving (VAS) increased until 3 months of abstinence and decreased at 6 months and 1 year of abstinence. - The effect of length of abstinence on cue-induced physiological measures did not differ significantly. |
| Lopez et al. (2015); Study 1 | 21 participants who met criteria for METH abuse or dependence as assessed by SCID-IV; USA | 84 images and 42 short videos from online sources, documentaries and feature films depicting METH use | Participants were grouped by their preferred route of administration (intranasal vs. smoking) and were shown visual stimuli- food (control cues) vs. people smoking METH vs. people snorting METH vs. ’substance only’ with no specific route of administration. | - Level of craving on a single-item scale (1–5 rating scale, “not at all” to “very much”) assessed after presentation of picture cues | - Participants who preferred to smoke METH reported significantly stronger craving for smoking stimuli, whereas those who preferred the intranasal route reported stronger craving for intranasal stimuli. - Meth users reported significantly higher craving for all METH stimuli to food stimuli. METH smokers and intranasal users did not differ in reported craving for food. |
| Malcolm et al. (2016) | 9 non-treatment seeking males who met DSM-IV-TR criteria for current METH dependence and 9 gender-, race- and alcohol use-matched controls; USA | Sequence of slides consisting of meth use (IV, nasal, smoking) pictures | Participants viewed visual cues of METH, neutral objects (matched for color and hue) and rest (crosshair on a neutral background) conditions in a randomized presentation. | - Activation of brain circuitry recorded with fMRI while presented with cues - Current urge to use METH (0–4 scale) after each blocks of stimulus categories | - METH participants rated their craving for METH cues significantly higher and had increased brain activation in the ventral striatum and medial frontal cortex compared to controls in response to METH cues (vs. neutral cues). - The ventral striatum activation was found to correlate significantly and negatively with the days since the last use of METH among the dependents. |
| Shahmohammadi et al. (2016) | 10 pure METH users for at least 6 months and 10 age- and gender-matched controls; Iran | Color photographs depicted on a black background containing drug associated cues and drugs (including with face and hand) | Participants viewed a series of images with neutral and METH-related content presented in fixed pre-randomized order | - Event-related potentials recordings while presented with cues | - Drug abusers exhibited significant positive activities in response to METH-related cues, which are most pronounced as P300 peaks in time range from about 300 to 600 ms and were maximal in channels FP1, FPz, FP2 and F8. |
| Huang et al. (2018) | 28 participants who met criteria for METH dependence as assessed by SCID-IV after long term (>16 months) drug rehabilitation and 27 age-matched controls; China | 30 images that fall into METH sample, drug paraphernalia and simulation scenarios of drug use shot by researchers | Participants viewed visual cues of METH, sexual (control cues), and neutral cues with order of images, blocks within epoch, and the epochs all randomly presented. | - Patterns of cortical activation recorded with fMRI while presented with cues - Subjective drug craving on VAS (0–10, “weakest craving” to “strongest craving”) assessed prior to and immediately following each MRI scan | - Elevated activity in the bilateral mPFC and right lateral posterior cingulate cortex in response to METH cues was observed among those with METH use disorder compared to controls. - Activation of the anterior cingulate region of mPFC was positively correlated with change in craving scores (VAS) and previous drug use frequency. - Compared to METH cues, those with METH use disorder had increased activation in the occipital lobe when exposed to sexual cues. |
| Wang, Shen and Wu. (2018) | 61 male patients who met DSM-IV criteria for METH dependence and had completed more than 1 month of forced detoxification and 45 age-matched male controls; China | A METH-related virtual social environment created VR video depicting a real-life story of men/women who are using METH together and who invite the observers to take METH | Participants first went through 8-min resting state, followed by 8-min viewing of the METH-cue video. | - Cue-induced cravings on VAS (0-10, “no craving at all” to “extremely strong craving”) assessed immediately after the cue - Heart rate variability (HRV) recorded with ECG | - Cue-induced condition elicited a larger HRV in patients with METH dependence, whereas a reverse pattern of HRV change was observed in the controls. - Among the patient group, subjective craving scores were associated with HRV changes. |
| Grodin, Courtney and Ray. (2019) | 15 non-treatment seeking participants with current METH use disorder; USA | 32 METH cue pictures consisting of drug, drug pipes, and drug use | Participants completed METH Infusion in the laboratory before completing two runs of cue task, which included 4 blocks of METH cues and 4 blocks of neutral cues presented pseudo randomly. Relationship between METH-induced craving and neural responses to METH cues was explored. | - Neural responses or patterns of brain activation recorded by fMRI while presented with cues | - METH cues activated a widespread set of regions, including mesocorticolimbic regions, such as the ventral and dorsal striatum, and ventromedial prefrontal cortex, compared to neutral cues. - Higher activation to METH cues was also observed in the precuneus, insula, anterior and posterior cingulate, and occipital lobe. - Peak ratings of METH-induced craving was associated positively with neural responses in the precuneus, putamen, and ventromedial prefrontal cortex. |
| Liang et al. (2019) | 52 males who met DSM-5 criteria for METH use disorder; China | METH use-related pictures consisting of intake utensils, tools and the scenarios of intake | Participants were exposed to six METH-related images presented in a block-wise method for 24 s (4 s each). The reliability of cue-induced craving as an indicator for addiction severity was examined. | - Cue-induced craving on VAS (0 -100, “not at all” to “extremely intense”) assessed after viewing picture cues | - 24 of the 52 METH users rated non-zero increase in subjective craving (VAS) upon exposure to cues. - Those who rated non-zero were not distinct from users who rated zero in terms of age, impulsiveness, emotion stability and clinical characteristics of addiction severity including MA use duration, maximum amount and weekly amount. |
| Tan et al. (2019) | 60 males who met DSM-5 criteria for METH use disorder; China | A METH-related virtual social environment designed based on results of focus group, depicting a video in which four persons played with paraphernalia, smoked and talked about the quality of METH crystals | Participants were exposed to neutral environment (5 min) and drug cue environment (5 min) presented in VR headset sequentially. | - Self-reported craving on VAS (0-10, “no craving” to “most craving ever experienced for METH”) assessed after presentation of cue) - Physiological responses such as skin conductance and heart rate variability (HRV) recorded while presented with cues - Brain electrophysiological response (gamma activity) recorded with EEG while presented with cues | - Self-reported craving (VAS) and skin conductance level increased in response to VR drug cues compared with neutral cues. - HRV was only marginally increased but not significant. - Gamma activity in mPFC/OFC and right DLPFC were decreased after cue exposure and predicted the ski conductance level changes. - Self-reported craving (VAS) was not associated with electrophysiological or physiological responses. |
| Chen et al. (2019) | 99 males who met criteria for METH dependence as assessed by SCID-IV; of which 49 men had short term (1–3 months) while 50 had long term (16–40 months) abstinence and 47 controls; China | 30 images of METH itself, people who were smoking METH, or the instruments they used to smoke METH | Participants viewed visual cues of METH, sexual (control cues), and neutral cues with order of images, blocks within epoch, and the epochs all pseudo-randomly presented. Relationships between regional activations and baseline methamphetamine use and impulsivity were also explored. | - Regional brain activations recorded with fMRI while presented with cues | - Greater METH cue–related activation in the ventral mPFC was observed in METH-using participants relative to healthy controls. - METH users also displayed greater sexual cue-related anterior insula activation compared to METH and neutral cues, with no difference reported between short- and long- term abstinence groups in anterior insula responses. - In short-term METH abstinence participants, both attentional and nonplanning impulsivity scores negatively correlated with METH cue–related superior frontal cortex activation. |
| Authors (Year) | Study Sample & Context | Study Design | Drug Cue(s) | Methods | Outcome(s) of Interest | Main Findings |
|---|---|---|---|---|---|---|
| Pharmacological methods | ||||||
| Newton et al. (2006) | 20 non- treatment seeking participants who met DSM-IV-TR criteria for METH abuse or dependence; USA | Double-blind, placebo controlled, parallel group design | Videotaped cues showing actors using METH | Following baseline METH dosing, participants received a second identical series of METH doses 6 days after initiation of twice- daily oral 150 mg bupropion (n = 10) or placebo (n = 10) before cue exposure session. | - Cue induced cravings on GCS and WSRS - Both scales administrated twice before randomization and twice after randomization | - Treatment with bupropion, compared to placebo, was associated with significantly reduced cue-induced craving on the GCS Total Score and in the Behavioral Intention subscale of the GCS. - Similar results were obtained for WSRS ‘likely to use’ but not WSRS ‘feel like using’. |
| Newton et al. (2008) | 16 non-treatment seeking participants who met DSM-IV-TR criteria for METH dependence; USA | Double-blind, placebo controlled, parallel group design | 5-min of METH paraphernalia (pipe stems, a lighter, and a small plastic bag containing white powder) viewing and handling followed by 10-min of video (actors using METH) viewing | Following baseline METH dosing, participants received repeated METH dosing after 2-week treatment with oral 15 mg aripiprazole (n = 8) or placebo (n = 8) before cue-exposure session. | - Cue-induced craving on BSCS before and after cue presentation - Subjective effects: “desire for METH”, “depressed”, “anxious”, “stimulated”, “likely to use METH” and “METH-like effect” measured on VAS before and after cue presentation - Physiological responses such as blood pressure and heart rate assessed in 5-min intervals before, during and after cue presentation | - No significant effects of aripiprazoletreatment on cue-induced METH craving (BSCS) was observed, although exposure to METH cues induced moderate increases in craving. - VAS measures on “anxious”, “nervous” and “irritable” were higher in group receiving aripiprazole both pre- and post- cue exposure. - There was no effect of aripiprazole treatment, or cue exposure on blood pressure and heart rate. |
| Ray et al. (2015) | 30 non-treatment seeking participants who met DSM-IV criteria for METH abuse or dependence; USA | Double-blind, randomized, crossover, placebo-controlled | Audiotaped script that induced sensory and emotional memories related to METH use and the handling of METH paraphernalia (e.g., glass pipe) at various times of exposure | Participants completed two separate 5-day inpatient stays. During each admission, participants completed testing sessions comprised of METH cue reactivity and intravenous 30 mg METH administration after receiving oral 50 mg Naltrexone or placebo for 4 days. | - Cue-induced craving on MAUQ assessed after each standardized exposure - Physiological responses such as heart rate and blood pressure assessed before and after cue administration | - Naltrexone was found to reduce cue-induced craving (MAUQ), as compared with placebo. - Significant increase in heart rate and diastolic blood pressure during the METH cue compared to control cue was reported in the placebo condition, but these effects were not significant in the Naltrexone condition. |
| Courtney, Ghahremani and Ray. (2016) | 23 non-treatment seeking participants who met DSM-5 criteria for METH use disorder; USA | Randomized, placebo controlled, within-subject design | 4 blocks of METH cue pictures, with each block consisting of four pictures, presented for 5 s each | Participants underwent a cue reactivity task during two fMRI sessions following 3 days of 50 mg naltrexone administration and matched time for placebo. | - Blood-oxygen-level dependent activation and functional connectivity recorded with fMRI while presented with cues - Subjective craving on a urge scale (1–4, “no urge” to “high urge”) assessed following each block of picture cues | - Administration of naltrexone reduced cue reactivity in sensorimotor areas and occipital regions and was associated with altered functional connectivity of dorsal striatum, ventral tegmental area, and precuneus with frontal, visual, sensory, and motor-related regions. - Naltrexone weakened the associations between subjective craving and functional connectivity with sensorimotor regions but strengthened its associations with dorsal striatum and frontal regions connectivity, thus engaging greater frontal regulation over salience attribution. |
| Roche et al. (2016) | 30 non-treatment seeking participants who met criteria for METH abuse of dependence as assessed by SCID-IV; USA | Randomized, counter-balanced, and double-blind | Audiotaped script that induced sensory and emotional memories related to METH use and the handling of METH paraphernalia (e.g., glass pipe) at various times of exposure | Participants completed two 4-day medication regimens of oral 50 mg naltrexone or placebo. On day 4 of each medication regimen, they completed a cue reactivity paradigm followed by intravenous METH administration. | - Cue-induced craving on MAUQ assessed after each standardized exposure | - Cue-induced craving for METH (MAUQ) was positively associated with post-infusion subjective METH effects, including positive, negative and craving-related responses. - Naltrexone (vs. placebo) significantly reduced the association between cue-induced craving (MAUQ) and positive subjective response to METH. |
| Non-Pharmacological methods | ||||||
| Bruehl et al. (2006) | 82 active METH users; Georgia | Qualitative | Not applicable | In-depth interviewing | - Narrative responses that corresponded with three types of craving (cue-, drug- and withdrawal-induced) | - Traditional cues, drugs and withdrawal states may lead to craving but do not necessarily provoke it. - Users described being able to overcome craving through personalized methods of control. |
| DeSantis et al. (2009) | 40 participants who met DSM-IV criteria for METH abuse and dependence in the past six months but maintained abstinence on test day; USA | Longitudinal | (1) 30–35 still photographs of individuals procuring and using METH, (2) a 7–8 min video depicting METH use in a variety of settings, and (3) in vivo paraphernalia and simulated METH placed in front of participants for 5 min | Participants underwent a human laboratory cue exposure procedures. | - Subjective reports of craving (unclear on the scale used) - Physiological responses such as skin conductance and heart rate - All measures were assessed before, during and immediately after exposure to each cue modality. - Dollar value and frequency of METH use for 90 days prior and 14 days following the study as assessed by TLFB (not cue reactivity). | - Participation in cue reactivity paradigm decreased the odds (OR = 0.39) of remaining in or transitioning to the high use state, though not significant. - None of the 25 participants who for whom follow-up data were available used METH in the two weeks after participation in the study. *Results on cue reactivity were not reported. |
| Price et al. (2010) | 24 participants who met DSM-IV criteria for METH dependence in the past six months; USA | Pre-post design | Pictures and video of individuals procuring and using METH and “in vivo” paraphernalia and simulated METH | Participant underwent 20-min sequences of multi-modal METH cue exposure over each of two one-hour sessions (total of six cue sequences), with multi-modal METH cues counter-balanced for presentation order. | - Cue-induced craving on modified WSRS-VAS (0–10) - Physiological responses such as heart rate and skin conductance - All measures were collected 20-min and 5-min prior to initial cue exposure for each session and subsequently during each cue sequence | - METH cue-elicited craving (WSRS-VAS) was extinguished during two sessions of repeated within-session exposures to multi-modal cues, with no evidence of spontaneous recovery between sessions. - No significant changes were identified for heart rate and skin conductance patterns. - A greater decrease in conditioned craving was observed in the group with longer (4–7 days) inter-session intervals, compared to those with ≤3 days. |
| Li et al. (2013) | 10 non-treatment seeking participants who met DSM-IV-TR criteria for METH dependence and 8 gender-, race-, and other biographical characteristics-matched controls; USA | Single-blind, crossover, sham-controlled | 40 METH-related pictures (drug, paraphernalia, or persons using the drug) | Participants were randomized to receive 15 min of sham and real (1 Hz) DLPFC rTMS in two experimental sessions separated by 1 h. | - Cue-induced craving on VAS (100 mm lines with anchoring statements at both ends, “no craving at all” to “the most craving I have ever had”) at baseline and during stimulation | - Real rTMS over the left DLPFC increased self-reported craving (VAS) as compared to sham stimulation in METH users, but no effect on craving in control group was observed. |
| Shahbabaie et al. (2014) | 32 male who met DSM-IV criteria for METH dependence for a history of at least 12 months and were abstinent from any drug use for a least a week prior to experiment (mean abstinence duration= 73.33 days); Iran | Double-blind, crossover, sham-controlled | Computerized cue-induced craving assessment task comprising of two series of 20 drug related images each | 20 min ‘anodal’ tDCS (2 mA) or ‘sham’ tDCS was applied over right DLPFC in a random sequence while participants performed a craving task starting after 10 min of stimulation. | - Self-reported craving on VAS (0–100 scale) before tDCS, after 10 min of tDCS, and after tDCS termination | - Active prefrontal tDCS increased craving (VAS) upon meth-related cue exposure. - The more provocative picture cues (drugs> drug use process> instruments> associated cues) induced significantly more craving (VAS) in the active condition in comparison to the sham condition. |
| Lopez et al. (2015); Study 2 | 13 METH smokers who met criteria for METH abuse of dependence as assessed by SCID-IV; USA | Within subject design | 54 METH stimuli that were rated at least 3.5 on a 1 to 5 scale on their effectiveness in eliciting craving | METH smokers implemented cognitive regulation (either focusing on positive or negative consequences or no regulation) while viewing photographs depicting METH smoking. | - Level of craving on a single-item scale (0–10, “not at all” to “very much”) at the end of each trial | - Participants reported significantly lower craving when focusing on the negative consequences associated with METH use. |
| Liu et al. (2017) | 50 male pure METH abusers; China | Randomized, sham-controlled | Handling with tools of drug use and faked METH for 5 min | Participants were randomized to receive five modes of rTMS stimulation: 10 Hz left P3 (sham), 10 Hz L-DLPFC, 10 Hz R-DLPFC, 1 Hz L-DLPFC, 1 Hz R-DLPFC. | -Cue-induced craving on a 0–100 scale prior to rTMS stimulation, 30 min after rTMS on day 1, and 30 min after on day 5 | - Both high and low frequency rTMS at either left or right DLPFC decreased cue-induced craving score immediately and after 5 days of continuous treatment; while no such effect was observed for active rTMS stimulation at P3 point. |
| Su et al. (2017) | 30 males who met DSM-5 criteria for moderate or severe METH use disorders; China | Randomized, double blind and controlled clinical trial | 80 MA-related (drug-use materials, person and situation) pictures and recalling of last use of METH | Participants were randomized to receive 5 sessions of 8 min sham (n = 15) or 10 Hz rTMS (n = 15) to the left DLPFC. | - Cue-induced craving on VAS (0–100 mm, “no craving” to “most craving ever experienced for MA”) before and after real rTMS or sham stimulation as well as pre experiment baseline | - Real rTMS over the left DLPFC reduced craving (VAS) significantly after 5 sessions of rTMS as compared to sham stimulation. - Changes in craving ratings (VAS) were also significantly predicted positively by age and negatively by education. |
| Rohani Anaraki et al. (2019) | 30 male who met DSM-5 criteria for methamphetamine use disorder and were abstinent from any drug use for at least one week before treatment (mean abstinence duration = 57.46 days); Iran | Randomized, double-blind, sham-controlled | Verbal induction where participants were asked to describe 3 previous situations that had led to craving and drug use | Participants underwent 5 sessions of 20 min bilateral real (n = 15) or sham (n = 15) 2 mA tDCS (anode right/cathode left) of DLPFC. | - Cue-induced craving on VAS (0–100 mm, “I absolutely don’t have a craving” to “It is the strongest craving I have ever had”) at pretest and posttest | - Cue-induced craving (VAS) was reduced significantly in tDCS related to sham condition. |
| Dean et al. (2019) | 17 participants who met DSM-IV criteria for current METH dependence who were receiving residential treatment; USA | Randomized, single blind controlled trial | METH-related images consisting of glass pipes, METH in crystallized or powered form, people smoking METH (without faces shown), or any combination of these | Participants were randomly assigned to either receive 12 sessions of computerized attentional bias modification (ABM) training (train attention away from METH stimuli 100% of the time) (n = 8) or an attentional control condition (away from METH stimuli 50% of the time) (n = 9). | - Cue-induced craving on a Likert-type scale (0–4, “not at all” to “very much”) following cue presentation- Brain activation recorded by fMRI when presented with cues | - Cue-induced cravings and activation in the ventromedial prefrontal cortex was reduced over time, but ABM training did not influence these effects. |
| Wang, Liu and Shen. (2019) | Study 1: 61 male patients who met DSM-IV criteria for METH dependence; China Study 2: 1008 abstinent participants with a history of METH dependence recruited from 4 detoxification centres; China | Randomized controlled trial | VR METH-cue model comprising of 8-min VR video, which simulates a real METH-related social context including various METH-related cues For counterconditioning procedure, participants also viewed the characters in the videos experience a distinct adverse consequence caused by METH use. | Study 1: Participants were randomly assigned to either the intervention group (n = 31) who received VRCP or the waiting list group (n = 29) who did not receive VRCP. Study 2: Participants were assigned into intervention group (n = 643) and waiting-list group (n = 305). The former group received the computerized VRCP, while the latter did not. | Study 1: - Cue-induced ECG assessed concurrently under the exposure to VR cues - Three subjective scores on the extent of how participants crave Meth right now, find Meth pleasant/unpleasant and are likely to use Meth if they have access on VAS (0–10) after VR cue Study 2: - Cue-induced ECG assessed concurrently under the exposure to VR cues | - Study 1: Those who received VRCP showed a significantly larger decrease on the score of METH-craving and METH-liking from baseline to follow-up assessments, compared to those who did not received VRCP. - Study 1 and 2: Participant in the intervention group (those who received VRCP_ showed a significantly larger decrease in HRV indexes on time and non-linear domains from baseline to follow-up assessments upon exposure to VR cues, compared to those in waiting-list group. |
| Su et al. (2020) | 126 treatment seeking participants who met DSM-5 criteria for severe METH use disorder; China | Randomized, double-blind, sham-controlled | 5-min view of METH-related pictures | Participants were randomized to receive either intermittent theta burst stimulation (iTBS; n = 70) or sham (n = 56) over the DLPFC for four weeks (20 daily sessions, 900 pulses per day). | - Cue-induced craving on VAS (0–100, “no craving” to “highest craving for METH”) at baseline and each of the 5 study sessions (Week 1–4) | - After four weeks of intervention, cue-induced craving rating (VAS) showed a significant time × group interaction effect and a significant difference for time. - iTBS reduced cue-induced craving (VAS) whereas sham did not. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seow, L.S.E.; Ong, W.J.; Hombali, A.; AshaRani, P.V.; Subramaniam, M. A Scoping Review on Cue Reactivity in Methamphetamine Use Disorder. Int. J. Environ. Res. Public Health 2020, 17, 6504. https://doi.org/10.3390/ijerph17186504
Seow LSE, Ong WJ, Hombali A, AshaRani PV, Subramaniam M. A Scoping Review on Cue Reactivity in Methamphetamine Use Disorder. International Journal of Environmental Research and Public Health. 2020; 17(18):6504. https://doi.org/10.3390/ijerph17186504
Chicago/Turabian StyleSeow, Lee Seng Esmond, Wei Jie Ong, Aditi Hombali, P. V. AshaRani, and Mythily Subramaniam. 2020. "A Scoping Review on Cue Reactivity in Methamphetamine Use Disorder" International Journal of Environmental Research and Public Health 17, no. 18: 6504. https://doi.org/10.3390/ijerph17186504
APA StyleSeow, L. S. E., Ong, W. J., Hombali, A., AshaRani, P. V., & Subramaniam, M. (2020). A Scoping Review on Cue Reactivity in Methamphetamine Use Disorder. International Journal of Environmental Research and Public Health, 17(18), 6504. https://doi.org/10.3390/ijerph17186504






