Urinary Acrylonitrile Metabolite Concentrations Before and after Smoked, Vaporized, and Oral Cannabis in Frequent and Occasional Cannabis Users
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Exposures and Sample Collection
2.4. 2CYEMA Analysis
2.5. Statistical Analysis
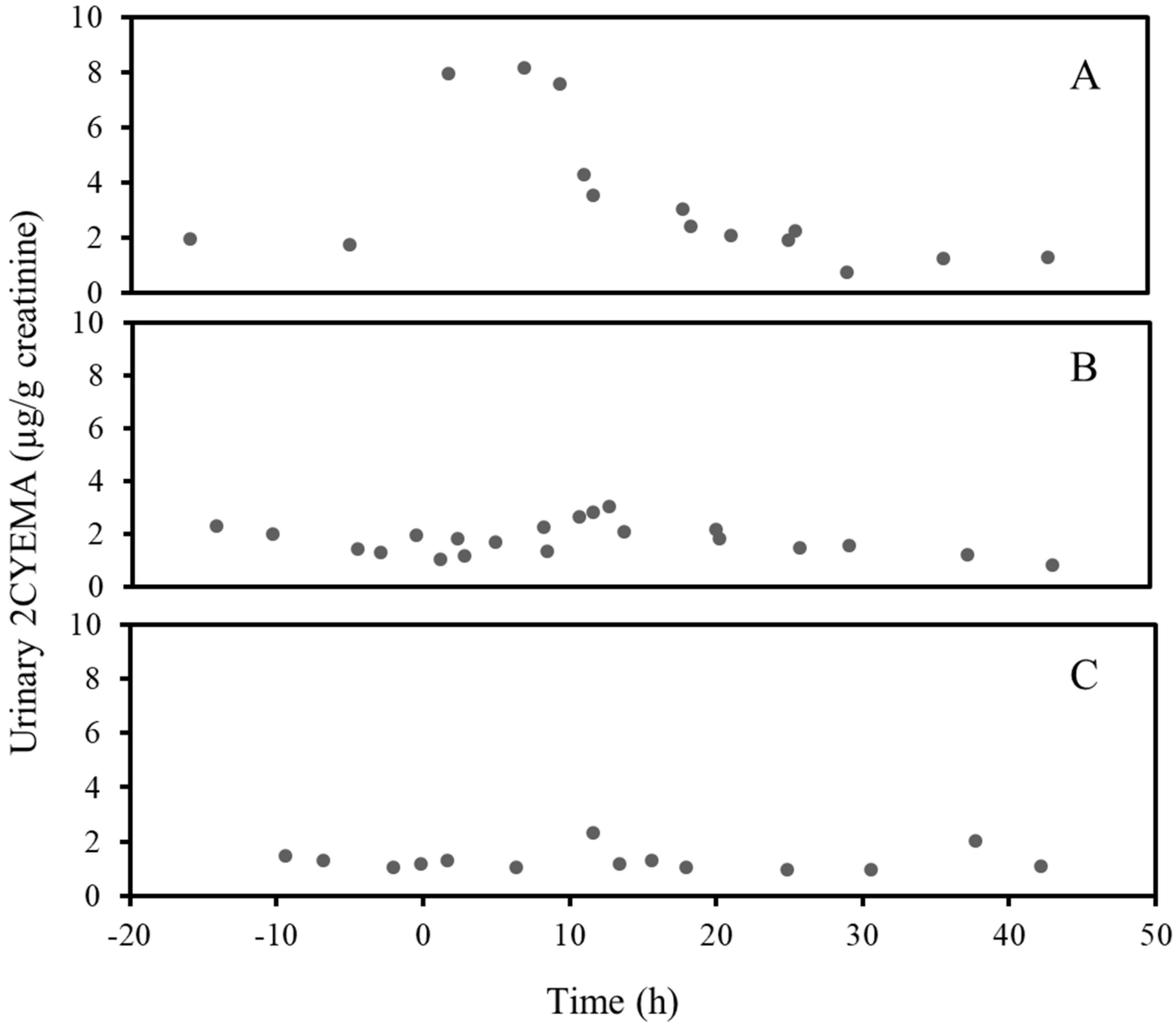
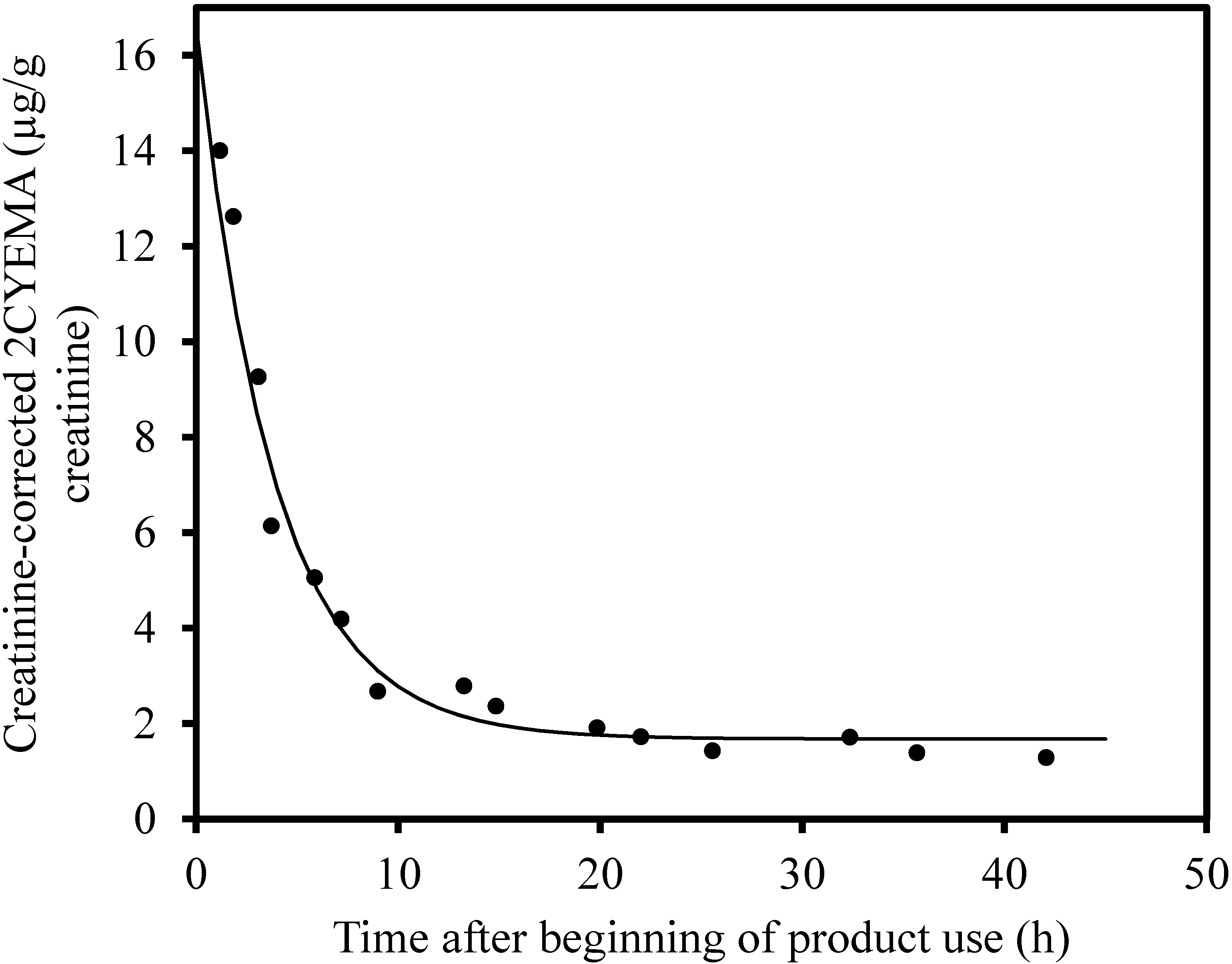
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkinson, S.T.; Yarnell, S.; Radhakrishnan, R.; Ball, S.A.; D’Souza, D.C. Cannabis legalization, Impact on physicians and public health. Ann. Rev. Med. 2016, 67, 453–466. [Google Scholar] [CrossRef]
- National Conference of State Legislatures. Marijuana Overview. Available online: https://www.ncsl.org/research/civil-and-criminal-justice/marijuana-overview.aspx (accessed on 27 July 2020).
- Schulenberg, J.E.; Johnston, L.D.; O’Malley, P.M.; Bachman, J.G.; Meich, R.A.; Patrick, M.E. Monitoring the Future National Survey Results on Drug Use, 1975–2017, Volume II, College Students and Adults Ages 19–55; The University of Michigan: Ann Arbor, MI, USA, 2017. [Google Scholar]
- Knapp, A.A.; Lee, D.C.; Borodovsky, J.T.; Auty, S.G.; Gabrielli, J.; Budney, A.J. Emerging trends in cannabis administration among adolescent cannabis users. J. Adolesc. Health. 2018, 64, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.N.; Bae, D.; Barrington-Trimis, J.L.; Jarvis, B.P.; Leventhal, A.M. Prevalence and sociodemographic correlates of adolescent use and polyuse of combustible, vaporized, and edible cannabis. JAMA. Netw. Open 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Schauer, G.L.; King, B.A.; Bunnell, R.E.; Promoff, G.; McAfee, T.A. Toking, vaping, and eating for health or fun: Cannabis use patterns in adults, U.S., 2014. Am. J. Prev. Med. 2016, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Azofeifa, A.; Mattson, M.E.; Schauer, G.; McAfee, T.; Grant, A.; Lyerla, R. National estimates of cannabis use and related indicators—National Survey on Drug Use and Health, United States, 2002–2014. MMWR Surveill. Summ. 2016, 65, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S. US Epidemiology of cannabis use and associated problems. Neuropsychopharmacology 2018, 43, 195–212. [Google Scholar] [CrossRef]
- Rogeberg, O.; Elvik, R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction 2016, 111, 1348–1359. [Google Scholar] [CrossRef]
- Curran, H.V.; Freeman, T.P.; Mokrysz, C.; Lewis, D.A.; Morgan, C.J.; Parsons, L.H. Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 2016, 17, 293–306. [Google Scholar] [CrossRef]
- Monte, A.A.; Zane, R.D.; Heard, K.J. The implications of cannabis legalization in Colorado. JAMA 2015, 313, 241–242. [Google Scholar] [CrossRef]
- Albertson, T.E.; Chenoweth, J.A.; Colby, D.K.; Sutter, M.E. The changing drug culture: Medical and recreational cannabis. FP Essent 2016, 441, 11–17. [Google Scholar]
- Russell, C.; Rueda, S.; Room, R.; Tyndall, M.; Fischer, B. Routes of administration for cannabis use—basic prevalence and related health outcomes: A scoping review and synthesis. Int. J. Drug Policy 2018, 52, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.L.; Novotny, M.; Bartle, K.D. Gas chromatography/mass spectrometric and nuclear magnetic resonance spectrometric studies of carcinogenic polynuclear aromatic hydrocarbons in tobacco and cannabis smoke condensates. Anal. Chem. 1976, 48, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Budney, A.J.; Lynskey, M.T. The co-occurring use and misuse of cannabis and tobacco: A review. Addiction 2012, 107, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Moir, D.; Rickert, W.S.; Levasseur, G.; Larose, Y.; Maertens, R.; White, P.; Desjardins, S. A comparison of mainstream and sidestream cannabis and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008, 21, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Bloor, R.N.; Wang, T.S.; Spanel, P.; Smith, D. Ammonia release from heated ‘street’ cannabis leaf and its potential toxic effects on cannabis users. Addiction 2008, 103, 1671–1677. [Google Scholar] [CrossRef]
- Rickert, W.S.; Robinson, J.C.; Rogers, B. A comparison of tar, carbon monoxide and pH levels in smoke from marihuana and tobacco cigarettes. Can. J. Public Health 1982, 73, 386–391. [Google Scholar]
- Cheng, Y.C.; Reyes-Guzman, C.M.; Christensen, C.H.; Rostron, B.L.; Edwards, K.C.; Wang, L.; Feng, J.; Jarrett, J.M.; Ward, C.D.; Xia, B.; et al. Biomarkers of exposure among adult smokeless tobacco users in the Population Assessment of Tobacco and Health Study (Wave 1, 2013–14). Cancer Epidemiol. Biomarkers Prev. 2020, 29, 659–667. [Google Scholar] [CrossRef]
- Abulseoud, O.A.; Zuccoli, M.L.; Zhang, L.; Barnes, A.; Huestis, M.A.; Lin, D.T. The acute effect of cannabis on plasma, liver and brain ammonia dynamics, a translational study. Eur. Neuropsychopharmacol. 2017, 27, 679–690. [Google Scholar] [CrossRef]
- Wu, T.C.; Tashkin, D.P.; Djahed, B.; Rose, J.E. Pulmonary hazards of smoking cannabis as compared with tobacco. N. Engl. J. Med. 1988, 318, 347–351. [Google Scholar] [CrossRef]
- Wei, B.; Alwis, K.U.; Li, Z.; Wang, L.; Valentin-Blasini, L.; Sosnoff, C.S.; Xia, Y.; Conway, K.P.; Blount, B.C. Urinary concentrations of PAH and VOC metabolites in cannabis users. Environ. Int. 2016, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; O’Connor, R.J.; Wei, B.; Travers, M.; Hyland, A.; Goniewicz, M.L. Nicotine and Toxicant Exposure Among Concurrent Users (Co-Users) of Tobacco and Cannabis. Nicotine. Tob. Res. 2020, 22, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Acrylonitrile. Monograph 71–7. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono71-7.pdf (accessed on 27 July 2020).
- Tashkin, D.P.; Gliederer, F.; Rose, J.; Change, P.; Hui, K.K.; Yu, J.L.; Wu, T.C. Effects of varying cannabis smoking profile on deposition of tar and absorption of CO and delta-9-THC. Pharmacol. Biochem. Behav. 1991, 40, 651–656. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Havel, C.M.; Peng, M.W.; Jacob, P., 3rd; Dempsey, D.; Yu, L.; Zielinska-Danch, W.; Koszowski, B.; Czogala, J.; Sobczak, A.; et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol. Biomarkers. Prev. 2009, 18, 3421–3425. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Geiger, L.E.; Hogy, L.L.; Wright, P.L. In vitro metabolism of acrylonitrile to 2-cyanoethylene oxide, reaction with glutathione, and irreversible binding to proteins and nucleic acids. Cancer. Res. 1981, 41, 4925–4933. [Google Scholar]
- Newmeyer, M.N.; Swortwood, M.J.; Barnes, A.J.; Abulseoud, O.A.; Scheidweiler, K.B.; Huestis, M.A. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: Identification of recent cannabis Intake. Clin. Chem. 2016, 62, 1579–1592. [Google Scholar] [CrossRef]
- Swortwood, M.J.; Newmeyer, M.N.; Abulseoud, O.A.; Scheidweiler, K.B.; Huestis, M.A. Cannabinoid disposition in oral fluid after controlled smoked, vaporized and oral cannabis administration. Drug Testing Anal. 2017, 9, 905–915. [Google Scholar] [CrossRef]
- Alwis, K.U.; Blount, B.C.; Britt, A.S.; Patel, D.; Ashley, D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 2012, 750, 152–160. [Google Scholar] [CrossRef]
- Wei, B.; Feng, J.; Rehmani, I.J.; Miller, S.; McGuffey, J.E.; Blount, B.C.; Wang, L. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin. Chim. Acta 2014, 436, 290–297. [Google Scholar] [CrossRef]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002, 4, 149–159. [Google Scholar] [CrossRef]
- Talih, S.; Balhas, Z.; Eissenberg, T.; Salman, R.; Karaoghlanian, N.; El Hellani, A.; Baalbaki, R.; Saliba, N.; Shihadeh, A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield, Measurements and model predictions. Nicotine Tob. Res. 2015, 17, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Vansickel, A.R.; Eissenberg, T. Electronic cigarettes: Effective nicotine delivery after acute administration. Nicotine Tob. Res. 2013, 15, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, M.; Linhart, I.; Pielas, G.; Kopecky, J. 2-Cyanoethylmercapturic acid (CEMA) in the urine as a possible indicator of exposure to acrylonitrile. Br. J. Ind. Med. 1987, 44, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, P.J.; van Sittert, N.J. Suitability of S-phenyl mercapturic acid and trans-trans-muconic acid as biomarkers for exposure to low concentrations of benzene. Environ. Health Perspect. 1996, 104, 1151–1157. [Google Scholar] [PubMed]
- Fuhr, U.; Boettcher, M.I.; Kinzig-Schippers, M.; Weyer, A.; Jetter, A.; Lazar, A.; Taubert, D.; Tomalik-Scharte, D.; Pournara, P.; Jakob, V.; et al. Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 266–271. [Google Scholar] [CrossRef]
| Subject | Smoked Cannabis | Vaped Cannabis | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Exposure | Cmax Post Exposure (µg/g Creatinine) | % Difference | Tmax (h) | Pre-Exposure | Cmax Post Exposure (µg/g Creatinine) | % Difference | Tmax (h) | |
| Occasional Users | ||||||||
| K | 1.7 | 8.2 | 382 | 7.1 | 2.0 | 3.2 | 60.0 | 12.8 |
| P * | 29.2 | 69.0 | 136 | 2.2 | 15.5 | 15.6 | 0.6 | 0.7 |
| U | 7.4 | 11.5 | 55.4 | 2.4 | 1.1 | 1.5 | 36.4 | 9.8 |
| W | 3.5 | 23.7 | 577 | 3.8 | 3.2 | 2.8 | −12.5 | 5.0 |
| X | 0.84 | 14.0 | 1570 | 1.2 | 1.2 | 2.1 | 75.0 | 3.9 |
| Frequent Users | ||||||||
| D * | 23.3 | 30.0 | 28.8 | 3.8 | 34.2 | 34.7 | 1.5 | 2.4 |
| G * | 65.2 | 42.0 | −35.6 | 6.6 | 44.5 | 28.5 | −36.0 | 20.0 |
| L * | 25.9 | 52.0 | 101 | 3.8 | 26.6 | 24.3 | −8.6 | 1.2 |
| T * | 204 | 166 | −18.6 | 0.4 | 140 | 114 | −18.6 | 0.6 |
| Y * | 57.9 | 144 | 148 | 1.3 | 23.4 | 24.9 | 6.4 | 3.1 |
| Occasional Users | Time 0 Increase above Baseline (µg/g Creatinine) | Baseline (µg/g Creatinine) | Half-Life (h) | Pr > F |
|---|---|---|---|---|
| K | 23.2 | 1.4 | 6.0 | 0.0005 |
| P * | 101 | 19.8 | 3.1 | <0.0001 |
| U | 13.0 | 2.3 | 9.0 | 0.0001 |
| W | 32.9 | 3.1 | 7.4 | <0.0001 |
| X | 14.9 | 1.7 | 3.8 | <0.0001 |
| Frequent Users | ||||
| D * | Did not converge ** | |||
| G * | 163 | 25.0 | 3.9 | 0.020 |
| L * | 33.0 | 26.8 | 10.1 | 0.14 |
| T * | 87.4 | 93.0 | 2.5 | 0.30 |
| Y * | 309 | 33.4 | 2.5 | 0.0007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashley, D.L.; De Jesús, V.R.; Abulseoud, O.A.; Huestis, M.A.; Milan, D.F.; Blount, B.C. Urinary Acrylonitrile Metabolite Concentrations Before and after Smoked, Vaporized, and Oral Cannabis in Frequent and Occasional Cannabis Users. Int. J. Environ. Res. Public Health 2020, 17, 6438. https://doi.org/10.3390/ijerph17186438
Ashley DL, De Jesús VR, Abulseoud OA, Huestis MA, Milan DF, Blount BC. Urinary Acrylonitrile Metabolite Concentrations Before and after Smoked, Vaporized, and Oral Cannabis in Frequent and Occasional Cannabis Users. International Journal of Environmental Research and Public Health. 2020; 17(18):6438. https://doi.org/10.3390/ijerph17186438
Chicago/Turabian StyleAshley, David L., Víctor R. De Jesús, Osama A. Abulseoud, Marilyn A. Huestis, Daniel F. Milan, and Benjamin C. Blount. 2020. "Urinary Acrylonitrile Metabolite Concentrations Before and after Smoked, Vaporized, and Oral Cannabis in Frequent and Occasional Cannabis Users" International Journal of Environmental Research and Public Health 17, no. 18: 6438. https://doi.org/10.3390/ijerph17186438
APA StyleAshley, D. L., De Jesús, V. R., Abulseoud, O. A., Huestis, M. A., Milan, D. F., & Blount, B. C. (2020). Urinary Acrylonitrile Metabolite Concentrations Before and after Smoked, Vaporized, and Oral Cannabis in Frequent and Occasional Cannabis Users. International Journal of Environmental Research and Public Health, 17(18), 6438. https://doi.org/10.3390/ijerph17186438






