Animal-Assisted Therapy Improves Communication and Mobility among Institutionalized People with Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. General Procedures
2.3. Human and Animal Resources
2.4. Measurements
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Basal Scores
3.2. Evaluation of the Intervention
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvarado García, A.M.; Salazar Maya, A.M. Análisis del concepto de envejecimiento. Gerokomos 2014, 25, 57–62. [Google Scholar] [CrossRef]
- Langa, K.M. Is the risk of Alzheimer’s disease and dementia declining? Alzheimers Res. Ther. 2015, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Klimova, B.; Maresova, P. Computer-based training programs for older people with mild cognitive impairment and/or dementia. Front. Hum. Neurosci. 2017, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.M.; Broe, G.A.; Creasey, H.; Grayson, D.; Edelbrock, D.; O’toole, B. Neurological signs, aging, and the neurodegenerative syndromes. Arch. Neurol. 1996, 53, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Sèculi Sanchez, E.; Brugulat Guiteras, P.; March Llanes, J.; Medina Bustos, A.; Martines Beneyto, V.; Tresserras Gaju, R. Las caídas en los mayores de 65 años: Conocer para actuar. Aten. Primaria 2004, 34, 178–183. [Google Scholar] [CrossRef]
- Al-Moman, M.; Al-Momani, F.; Alghadir, A.H.; Alharethy, S.; Gabr, S.A. Factors related to gait and balance deficits in older adults. Clin. Interv. Aging 2016, 11, 1043–1049. [Google Scholar]
- Fuller, G.F. Falls in the Elderly. Am. Fam. Physician 2000, 61, 2159–2168. [Google Scholar]
- Machiels, M.; Metzelthin, S.F.; Hamers, J.P.; Zwakhalen, S.M. Interventions to improve communication between people with dementia and nursing staff during daily nursing care: A systematic review. Int. J. Nurs. Stud. 2017, 66, 37–46. [Google Scholar] [CrossRef]
- Bernstein, P.L.; Friedmann, E.; Malaspina, A. Animal-assisted therapy enhances resident social interaction and initiation in long-term care facilities. Anthrozoös 2000, 13, 213–224. [Google Scholar] [CrossRef]
- Olarazán, J.; Reisberg, B.; Clare, L.; Cruz, I.; Peña-Casanova, J.; Del Ser, T.; Woods, B.; Beck, C.; Auer, S.; Lai, C.; et al. Nonpharmacological therapies in Alzheimer´s Disease: A systematic review of efficacy. Dement. Geriatr. Cogn. Disord. 2010, 30, 161–178. [Google Scholar] [CrossRef]
- Nordgren, L.; Engström, G. Animal-assited intervention in dementia: Effects on quality of life. Clin. Nurs. Res. 2014, 23, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Perraud, F. Animals used in therapy for the wellbeing of elderly people. Soins. Gerontol. 2013, 99, 10–12. [Google Scholar] [CrossRef]
- Terminology. Pet Partners. 2020. Available online: https://petpartners.org/learn/terminology/ (accessed on 29 July 2020).
- Rodrigo-Claverol, M.; Casanova-Gonzalvo, C.; Malla-Clua, B.; Rodrigo-Claverol, E.; Jové-Naval, J.; Ortega-Bravo, M. Animal-Assisted Intervention Improves Pain Perception in Polymedicated Geriatric Patients with Chronic Joint Pain: A Clinical Trial. Int. J. Environ. Res. Public Health 2019, 16, 2843. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Carlsson, P.; Sjodahl, R.; Theodorsson, E.; Levin, L.A. Patient benefit of dog-assisted interventions in health care: A systematic review. BMC Complement. Altern. Med. 2017, 17, 358. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Redondo, E.; Pérez-Sáez, E.; González-Ingelmo, M.E. Intervención asistida con perros para personas con demencia. Boletín Digit. CRE Alzheimer Salamanca 2013, 3, 3. [Google Scholar]
- Gocheva, V.; Hund-Georgiadi, M.; Hediger, K. Effects of animal-assisted therapy on concentration and attention span in patients with acquired brain injury: A randomized controlled trial. Neuropsychology 2018, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Bunketorp-Kall, L.; Lundgren-Nilsson, Å.; Samuelsson, H.; Pekny, T.; Blomvé, K.; Pekna, M.; Pekny, M.; Blomstrand, C.; Nilsson, M. Long-term improvements after multimodal rehabilitation in late phase after stroke: A randomized controlled trial. Stroke 2017, 48, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Kim, S.G.; Yong, M.S. Efects of hippotherapy on recovery of gait and balance ability in patients with stroke. J. Phys. Ther. Sci 2014, 26, 309–311. [Google Scholar] [CrossRef]
- Machová, K.; Procházková, R.; Ríha, M.; Svobodová, I. The Effect of Animal-Assisted Therapy on the State of Patients’ Health after a Stroke: A Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 3272. [Google Scholar] [CrossRef]
- Macauley, B.L. Animal-assisted therapy for persons with aphasia: A pilot study. J. Rehabil. Res. Dev. 2006, 43, 357–366. [Google Scholar] [CrossRef]
- Sunwoo, H.; Chang, W.H.; Kwon, J.Y.; Kim, T.W.; Lee, J.Y.; Kim, Y.H. Hippotherapy in adult patients with chronic brain disorders: A pilot study. Ann. Rehabil. Med. 2012, 36, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, L.; Corriveau, H.; Bier, N.; Camden, C.; Champagne, N.; Dion, C. Effectiveness of a rehabilitation dog in fostering gait retraining for adults with a recent stroke: A multiple single-case study. NeuroRehabilitation 2010, 27, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Fine, A. Nuestros Fieles Compañeros. Explorando la Esencia de Nuestra Relación con los Animales, Kns ed.; SC (Spanish version): A Coruña, Spain, 2015; ISBN 978-84-941852-9-8. [Google Scholar]
- Yakimicki, M.L.; Edwards, N.E.; Richards, E.; Beck, A.M. Animal-assisted intervention and dementia: A systematic review. Clin. Nurs. Res. 2018, 28, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Pope, W.S.; Hunt, C.; Ellison, K. Animal assisted therapy for elderly residents of a skilled nursing facility. J. Nurs. Educ. Pract. 2016, 6, 56–62. [Google Scholar] [CrossRef]
- Wesenberg, S.; Mueller, C.; Nestmann, F.; Holthoff-Detto, V. Effects of an animal-assisted intervention on social behaviour, emotions, and behavioural and psychological symptoms in nursing home residents with dementia. Psychogeriatrics 2018, 19. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; De Leon, M.D.; Crook, T. The global deterioration scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar]
- Tinetti, M.E.; Ginten, S.F. Identifying Mobility Dysfunctions in Elderly Patients Standard Neuromuscular Examination on Direct Assessment? JAMA 1988, 259, 1190–1193. [Google Scholar] [CrossRef]
- Grisha Stewart, M.A. Manual de Ahimsa Dog Traininig. In Una Guía Práctica Para la Solución de Problemas y la Educación Canina Sin Violencia; Empowered Animals, LLC.: Anchorage, AK, USA, 2014; ISBN 13978-1494459192. [Google Scholar]
- Laurence, K. Learning about Dogs. In Entrenamiento con Clícker—La Base Perfecta; Trudango, S.L., Ed.; Spanish versión; Dogalia: Madrid, Spain, 2012; ISBN 978-84-939535-0-8. [Google Scholar]
- Signes Llopis, M.A. Manual de Educación y Adiestramiento de Perros de Terapia; Psylicom Ed.: Valencia, Spain, 2016; ISBN 978-84-944975-5-1. [Google Scholar]
- Holden, R.R.; Mendonca, J.D.; Mazmanian, D.; Reddon, J.R. Clinical construct validity of the Holden Psychological Screening Inventory (HPSI). J. Clin. Psychol. 1992, 48, 627–633. [Google Scholar] [CrossRef]
- Strøm, B.S.; Engedal, K.; Šaltyte Benth, J.; Grov, E.K. Psychometric evaluation of the Holden Communication Scale (HCS) for persons with dementia. BMJ Open 2016, 6, e013447. [Google Scholar] [CrossRef]
- Rodríguez Guevara, C.; Helena Lugo, L. Validez y confiabilidad de la Escala de Tinetti para población colombiana. Rev. Colomb. Reumatol. 2012, 19, 218–233. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bono, A.V.; BenVenuti, C.; Buzzi, M.; Ciatti, R.; Relli, V.C.; Chiambretto, P.; Relli, C.M.; CiRoli, M.P.; Pini, A.; Prestigiacomo, T.; et al. Effects of animal assisted therapy (AAT) carried out with dogs on the evolution of mild cognitive impairment. J. Gerontol. Geriatr. 2015, 1, 32–36. [Google Scholar]
- Friedmann, E.; Galik, E.; Thomas, S.A.; Hall, P.S.; Chung, S.Y.; McCune, S. Evaluation of a pet-assisted living intervention for improving functional status in assisted living residents with mild to moderate cognitive impairment: A pilot study. Am. J. Alzheimers Dis. Other Demen. 2015, 30, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.S.; Cohen-Mansfield, J.; Regier, N.G.; Dakheel-Ali, M.; Srihari, A.; Thein, K. The Impact of Different Dog-Related Stimuli on Engagement of Persons with Dementia. Am. J. Alzheimers Dis. Other Demen. 2010, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, J.; Cooper, M.; DiGiovanni, N.M.; Litchkofski, A.; Nichols, A.L.; Ramsey, A. Dog-Assisted Therapy for Residents of Long-Term Care Facilities: An Evidence-Based Review with Implications for Occupational Therapy. Phys. Occup. Ther. Geriatr. 2013, 31, 214–240. [Google Scholar] [CrossRef]
- Wood, W.; Fields, B.; Rose, M.; McLure, M. Animal-assisted therapies and dementia: A systematic mapping review using the lived environment life quality (LELQ) model. Am. J. Occup. Ter. 2017, 71, 7105190030. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, P.; Leng, M.; Li, C. Animal-assisted intervention for individuals with cognitive impairment: A meta-analysis of randomized controlled trials and quasi-randomized controlled trials. Psychiatry Res. 2017, 260, 418–427. [Google Scholar] [CrossRef]
- Majic, T.; Gutzmann, H.; Heinz, A.; Lang, U.E.; Rapp, M.A. Animal-assisted therapy and agitation and depression in nursing home residents with dementia: A matched case-control trial. Am. J. Geriatr. Psychiatry 2013, 21, 1052–1059. [Google Scholar] [CrossRef]
- Javelot, H.; Antoine-Bernard, E.; Garat, J.; Javelot, T.; Weiner, L.; Mervelay, V. Snoezelen and animal-assisted therapy in dementia patients. Soins. Gerontol. 2012, 94, 11–14. [Google Scholar] [CrossRef]
- Nordgren, L.; Engström, G. Effects of dog-assisted intervention on behavioural and psychological symptoms of dementia. Nurs. Older People 2014, 26, 31–38. [Google Scholar] [CrossRef]
- Mossello, E.; Ridolfi, A.; Mello, A.M.; Lorenzini, G.; Mugnai, F.; Piccini, C.; Barone, D.; Peruzzi, A.; Masotti, G.; Marchionni, N. Animal-assisted activity and emotional status of patients with Alzheimer’s disease in daycare. Int. Psychogeriatr. 2011, 23, 899–905. [Google Scholar] [CrossRef]
- Cherniack, E.P.; Cherniack, A.R. The benefit of pets and animal-assisted therapy to the health of older individuals. Curr. Gerontol. Geriatr. Res. 2014, 2014, 623203. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.; Pedersen, I.; Bergland, A.; Enders-Slegers, M.J.; Ihlebaek, C. Effect of animal-assisted activity on balance and quality of life in home-dwelling persons with dementia. Geriatr. Nurs. 2016, 37, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Klimova, B.; Toman, J.; Kuca, K. Effectiveness of the dog therapy for patients with dementia—A systematic review. BMC Psychiatry 2019, 19, 276. [Google Scholar] [CrossRef] [PubMed]
- Hediger, K.; Thommen, S.; Wagner, C.; Gaab, J.; Hund-Georgiadis, M. Effects of animal-assisted therapy on social behaviour in patients with acquired brain injury: A randomized controlled trial. Sci. Rep. 2019, 9, 5831. [Google Scholar] [CrossRef] [PubMed]
- Swall, A.; Ebbeskog, B.; LundhHagelin, C.; Fagerberg, I. Can therapy dogs evoke awareness of one’s past and present life in persons with Alzheimer’s disease? Int. J. Older People Nurs. 2015, 10, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, H.; Okada, S.; Tsutani, K.; Park, H.; Okuizumi, H.; Handa, S.; Oshio, T.; Park, S.J.; Kitayuguchi, J.; Abe, T.; et al. Effectiveness of animal-assisted therapy: A systematic review of randomized controlled trials. Complement. Ther. Med. 2014, 22, 371–390. [Google Scholar] [CrossRef]
- Lange, A.M.; Cox, J.A.; Bernert, D.J.; Jenkins, C.D. Is counseling going to the dogs? An exploratory study related to the inclusion of an animal in group counseling with adolescents. J. Creat. Ment. Health 2007, 2, 17–31. [Google Scholar] [CrossRef]
- Snipelisky, D.; Burton, M.C. Canine-assisted therapy in the inpatient setting. South. Med. J. 2014, 107, 265–273. [Google Scholar] [CrossRef]
- Menna, L.F.; Santaniello, A.; Gerardi, F.; Di Maggio, A.; Milan, G. Evaluation of the efficacy of animal-assisted therapy based on the reality orientation therapy protocol in Alzheimer’s disease patients: A pilot study. Psychogeriatrics 2016, 16, 240–246. [Google Scholar] [CrossRef]
- Travers, C.; Perkins, J.; Rand, J.; Bartlett, H.; Morton, J. An Evaluation of Dog-Assisted Therapy for Residents of Aged Care Facilities with Dementia. Anthrozoös 2013, 26, 213–225. [Google Scholar] [CrossRef]
- Kårefjärd, A.; Nordgren, L. Effects of dog-assisted intervention on quality of life in nursing home residents with dementia. Scand. J. Occup. Ther. 2019, 26, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.; Martin, K.; Christian, H.; Nathan, A.; Lauritsen, C.; Houghton, S.; McCune, S. The pet factor-companion animals as a conduit for getting to know people, friendship formation and social support. PLoS ONE 2015, 10, e0122085. [Google Scholar] [CrossRef] [PubMed]
- Briones, M.A.; Pardo-García, I.; Escribano-Sotos, F. Effectiveness of a Dog-Assisted Therapy Program to Enhance Quality of Life in Institutionalized Dementia Patients. Clin. Nurs. Res. 2019, 6, 1054773819867250. [Google Scholar] [CrossRef] [PubMed]
- Filan, S.L.; Llewellyn-Jones, R.H. Animal-assisted therapy for dementia: A review of the literature. Int. Psycogeriatr. 2006, 18, 597–611. [Google Scholar] [CrossRef] [PubMed]
- McGilton, K.S.; Rochon, E.; Sidani, S.; Shan, A.; Ben-David, B.M.; Saragosa, M.; Boscart, V.M.; Wilson, R.; Galimidi-Epstein, K.K.; Pichora-Fuller, M.K. Can we help care providers communicate more effectively with persons having dementia living in long-term care homes? Am. J. Alzheimers Dis. Other Demen. 2017, 32, 41–50. [Google Scholar] [CrossRef]
- Bernabei, V.; De Ronchi, D.; La Ferla, T.; Moretti, F.; Tonelli, L.; Ferrari, B.; Forlani, M.; Atti, A.R. Animal-assisted interventions for elderly patients affected by dementia or psychiatric disorders: A review. J. Psychiatr. Res. 2013, 47, 762–773. [Google Scholar] [CrossRef]
- Püllen, R.; Coy, M.; Hunger, B.; Koetter, G.; Spate, M.; Richter, A. Animal-assisted therapy for demented patients in acute care hospitals. Z. Gerontol. Geriatr. 2013, 46, 233–236. [Google Scholar] [CrossRef]
- LaFrance, C.; Garcia, L.J.; Labreche, J. The effect of a therapy dog on the communication skills of and adult with aphasia. J. Commun. Disord. 2007, 40, 215–224. [Google Scholar] [CrossRef]
- Hammar, L.M.; Emami, A.; Gotell, E.; Engström, G. The impact of caregivers’ singing on expressions of emotion and resistance during morning care situations in persons with dementia: An intervention in dementia care. J. Clin. Nurs. 2011, 20, 969–978. [Google Scholar] [CrossRef]
- Pérez-Sáez, E.; Pérez-Redondo, E.; González-Ingelmo, E. Effects of Dog-Assisted Therapy on Social Behaviors and Emotional Expressions: A Single-Case Experimental Design in 3 People with Dementia. J. Geriatr. Psychiatry Neurol. 2019, 33, 109–119. [Google Scholar] [CrossRef]
- Vrbanac, Z.; Zecevic, I.; Ljubic, M.; Melic, M.; Stani, D.; Bottegaro, N.B.; Jurki, G.; Skrlin, B.; Bedrica, L.; Zubcic, D. Animal assisted therapy and perception of loneliness in geriatric nursing home residents. Coll. Antropol. 2013, 37, 973–976. [Google Scholar] [PubMed]
- Swall, A.; Ebbeskog, B.; Hagelin, C.L.; Fagerberg, I. Stepping out of the shadows of Alzheimer’s disease: A phenomenological hermeneutic study of older people with Alzheimer’s disease caring for a therapy dog. Int. J. Qual. Stud. Health Well Being 2017, 12, 1347013. [Google Scholar] [CrossRef] [PubMed]
| ALL PARTICIPANTS | GDS 2–4 | GDS 5–6 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total | Control | Experimental | Statistic | p-value | Total | Control | Experimental | Statistic | p-value | Total | Control | Experimental | Statistic | p-value |
| N = 46 | N = 23 | N = 23 | N = 15 | N = 6 | N = 9 | N = 31 | N = 17 | N = 14 | |||||||
| Age (years), mean (IQR) | 85.0 (80.2; 87.0) | 86.0 (80.5; 87.5) | 83.0 (80.5; 86.5) | 296 | 0.488 | 86.0 (80.5; 90.5) | 90.0 (87.0; 92.2) | 81.0 (80.0; 86.0) | 42 | 0.076 | 83.0 (80.5; 86.5) | 84.0 (80.0; 86.0) | 83.0 (81.2; 86.8) | 108.5 | 0.676 |
| Gender, N (%) | 0.48 | 0.489 | 0.51 | 0.604 | 0.52 | 0.671 | |||||||||
| Women | 35 (76.1) | 19 (82.6) | 16 (69.6) | 11 (73.3) | 5 (83.3) | 6 (66.7) | 24 (77.4) | 14 (82.4) | 10 (71.4) | ||||||
| Men | 11 (23.9) | 4 (17.4) | 7 (30.4) | 4 (26.7) | 1 (16.7) | 3 (33.3) | 7 (22.6) | 3 (17.6) | 4 (28.6) | ||||||
| GDS, N (%) | 2.02 | 0.762 | 0.63 | 0.82 | 0.53 | 0.707 | |||||||||
| 2 | 2 (4.35) | 1 (4.35) | 3 (13.0) | 4 (26.7) | 1 (16.7) | 3 (33.3) | - | - | - | ||||||
| 3 | 6 (13.0) | 3 (13.0) | 3 (13.0) | 6 (40.0) | 3 (50.0) | 3 (33.3) | - | - | - | ||||||
| 4 | 5 (10.9) | 2 (8.70) | 3 (13.0) | 5 (33.3) | 2 (33.3) | 3 (33.3) | - | - | - | ||||||
| 5 | 11 (23.9) | 7 (30.4) | 4 (17.4) | - | - | - | 11 (35.5) | 7 (41.2) | 4 (28.6) | ||||||
| 6 | 20 (43.5) | 10 (43.5) | 10 (43.5) | - | - | - | 20 (64.5) | 10 (58.8) | 10 (71.4) | ||||||
| Outcome variables, median (IQR) | |||||||||||||||
| Holden scale | 11.0 (7.00; 17.0) | 10.0 (7.50; 16.5) | 13.0 (7.00; 18.0) | 264.5 | 1 | 7.00 (4.00; 9.00) | 6.50 (4.50; 8.50) | 7.00 (4.00; 9.00) | 26.5 | 0.953 | 16.0 (9.00; 21.0) | 14.0 (9.00; 17.0) | 16.5 (9.25; 24.5) | 103.5 | 0.538 |
| Tinetti scale | |||||||||||||||
| Total score | 14.5 (11.0; 18.8) | 16.0 (13.0; 20.0) | 13.0 (9.00; 17.0) | 353 | 0.051 | 13.0 (10.5; 14.5) | 14.5 (13.2; 18.8) | 11.0 (8.00; 14.0) | 44 | 0.044 * | 16.0 (11.0; 19.0) | 16.0 (13.0; 20.0) | 15.0 (11.0; 18.0) | 140.5 | 0.392 |
| Gait score | 5.50 (3.00; 8.00) | 6.00 (4.00; 9.00) | 5.00 (3.00; 7.00) | 334.5 | 0.122 | 5.00 (3.00; 6.50) | 5.50 (4.25; 7.50) | 3.00 (3.00; 6.00) | 40 | 0.123 | 6.00 (3.50; 9.00) | 7.00 (4.00; 9.00) | 5.50 (3.25; 8.50) | 138 | 0.448 |
| Balance score | 9.00 (7.00; 10.0) | 10.0 (9.00; 11.0) | 8.00 (5.50; 10.0) | 356.5 | 0.041 * | 9.00 (6.50; 9.00) | 9.00 (9.00; 11.2) | 7.00 (5.00; 8.00) | 48 | 0.012 * | 10.0 (7.50; 10.5) | 10.0 (8.00; 11.0) | 9.00 (7.00; 10.0) | 138.5 | 0.433 |
| TOTAL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome variables | Control (N = 23) | Experimental (N = 23) | ||||||||
| Preintervention, median (IQR) | Postintervention, median (IQR) | Difference | U statistic | p-value | Pre-intervention, median (IQR) | Postintervention, median (IQR) | Difference | U statistic | p-value | |
| Holden scale | 10.0 (7.50; 16.5) | 9.00 (6.00; 15.5) | −1.00 (−2.00; 0.00) | 10 | 0.004 * | 13.0 (7.00; 18.0) | 7.00 (3.50; 13.5) | −3.00 (−5.00; −2.00) | 0 | <0.001 * |
| Tinetti scale | ||||||||||
| Total score | 16.0 (13.0; 20.0) | 18.0 (14.0; 22.0) | 1.00 (1.00; 4.00) | 171 | <0.001 * | 13.0 (9.00; 17.0) | 17.0 (11.5; 19.0) | 3.00 (2.00; 3.50) | 231 | <0.001 * |
| Gait score | 6.00 (4.00; 9.00) | 6.00 (4.50; 9.00) | 0.00 (0.00; 0.50) | 21 | 0.026 * | 5.00 (3.00; 7.00) | 6.00 (3.50; 7.50) | 1.00 (0.00; 1.00) | 85.5 | 0.004 * |
| Balance score | 10.0 (9.00; 11.0) | 11.0 (10.0; 13.0) | 1.00 (1.00; 3.50) | 171 | <0.001 * | 8.00 (5.50; 10.0) | 10.0 (8.00; 12.0) | 2.00 (1.00; 3.00) | 253 | <0.001 * |
| GDS 2–4 | ||||||||||
| Control (N = 6) | Experimental (N = 9) | |||||||||
| Holden scale | 6.5 (4.5; 8.5) | 6 (4.5; 6.75) | −1 (−1.75; −0.25) | 0 | 0.098 | 7 (4; 9) | 4 (2; 5) | −3 (−6; −2) | 0 | 0.009 * |
| Tinetti scale | ||||||||||
| Total score | 14.5 (13.25; 18.75) | 19 (16.5; 23.75) | 4 (1.75; 4.75) | 21 | 0.035 * | 11 (8; 14) | 16 (10; 18) | 3 (2; 5) | 45 | 0.009 * |
| Gait score | 5.5 (4.25; 7.5) | 6 (6; 8.25) | 0.5 (0; 1) | 6 | 0.174 | 3 (3; 6) | 5 (4; 7) | 1 (0; 1) | 21 | 0.026 * |
| Balance score | 9 (9; 11.25) | 13 (10.5; 15.5) | 3.5 (1.5; 4) | 21 | 0.035 * | 7 (5; 8) | 10 (6; 11) | 3 (1; 3) | 45 | 0.009 * |
| GDS 5–6 | ||||||||||
| Control (N = 17) | Experimental (N = 14) | |||||||||
| Holden scale | 14 (9; 17) | 14 (8; 16) | −1 (−2; 0) | 7.5 | 0.023 * | 16.5 (9.25; 24.5) | 13 (8; 19.25) | −3.5 (−5; −2.25) | 0 | 0.002 * |
| Tinetti scale | ||||||||||
| Total score | 16 (13; 20) | 17 (14; 21) | 1 (0; 3) | 78 | 0.002 * | 15 (11; 18) | 18 (14; 19.75) | 3 (2; 3) | 78 | 0.002 * |
| Gait score | 7 (4; 9) | 7 (4; 9) | 0 (0; 0) | 6 | 0.149 | 5.5 (3.25; 8.5) | 6 (3.5; 8) | 0 (0; 1) | 25 | 0.065 |
| Balance score | 10 (8; 11) | 11 (10; 12) | 1 (0; 3) | 78 | 0.002 * | 9 (7; 10) | 12 (9.25; 12) | 2 (1; 3) | 91 | 0.001 * |
| TOTAL | ||||||
|---|---|---|---|---|---|---|
| βEG (95% CI) | t-Value | p-Value | βEG:bl (95% CI) | t-Value | p-Value | |
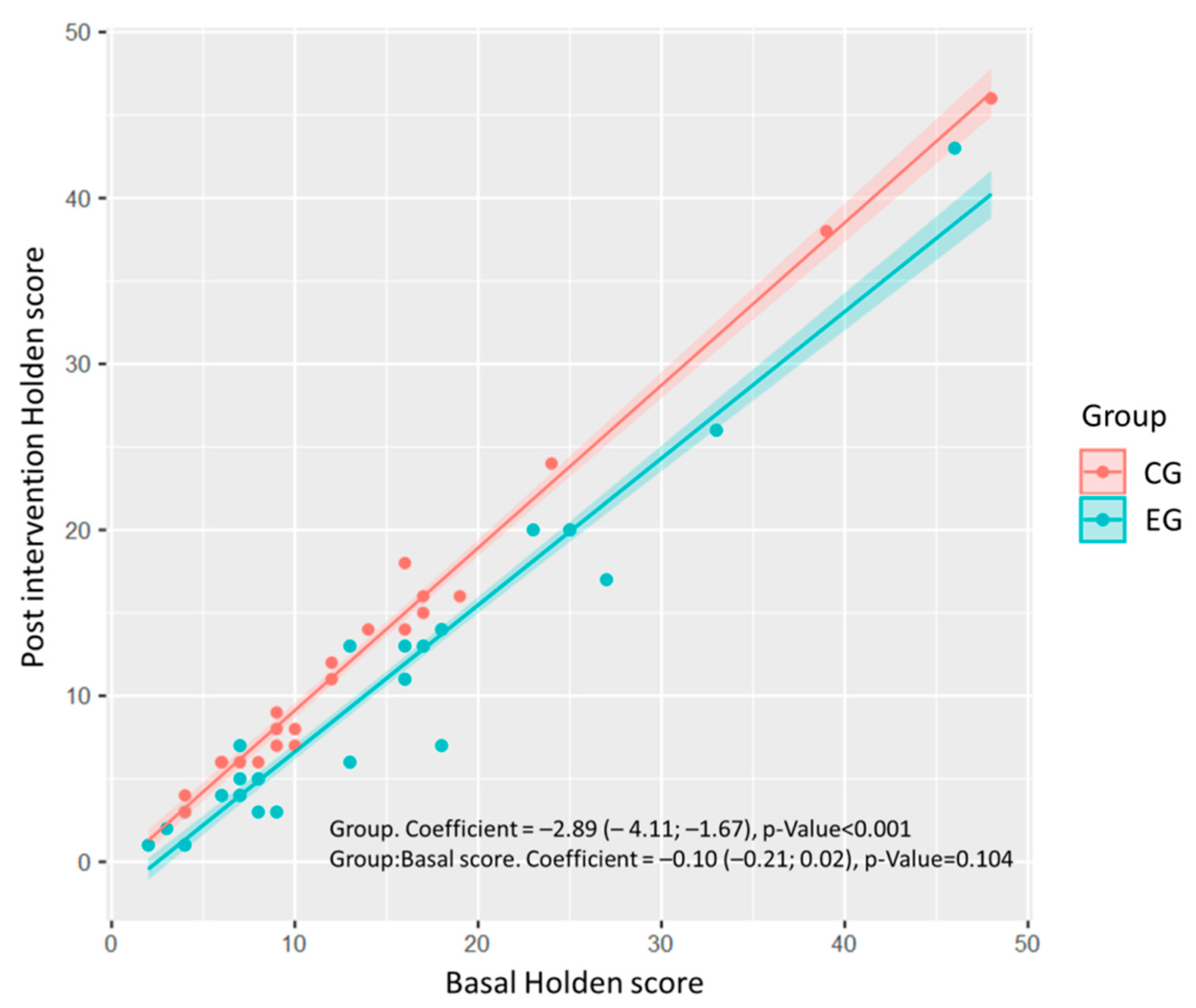
| Holden scale | −2.889 (−4.114–1.665) | −4.761 | <0.001 * | −0.096 (−0.212–0.02) | −1.663 | 0.104 |
| Tinetti scale | ||||||
| Total score | 0.199 (−0.942–1.34) | 0.352 | 0.726 | 0.08 (−0.121–0.281) | 0.802 | 0.427 |
| Gait score | 0.263 (−0.159–0.685) | 1.258 | 0.215 | −0.014 (−0.159–0.131) | −0.199 | 0.843 |
| Balance score | −0.082 (−1.04–0.877) | −0.172 | 0.864 | 0.098 (−0.204–0.4) | 0.655 | 0.516 |
| GDS 2–4 | ||||||
| Holden scale | −2.405 (−3.447–1.363) | −5.081 | <0.001* | −0.229 (−0.598–0.14) | −1.366 | 0.199 |
| Tinetti scale | ||||||
| Total score | 0.208 (−2.778–3.194) | 0.153 | 0.881 | 0.009 (−0.6–0.618) | 0.032 | 0.975 |
| Gait score | −0.018 (−1.02–0.985) | −0.039 | 0.97 | 0.18 (−0.255–0.615) | 0.91 | 0.382 |
| Balance score | 0.597 (−2.139–3.334) | 0.481 | 0.64 | −0.487 (−1.472–0.498) | −1.088 | 0.3 |
| GDS 5–6 | ||||||
| Holden scale | −2.688 (−4.089–1.288) | −3.939 | <0.001 * | −0.087 (−0.213–0.039) | −1.412 | 0.169 |
| Tinetti scale | ||||||
| Total score | 0.475 (−0.752–1.701) | 0.795 | 0.434 | 0.056 (−0.159–0.27) | 0.532 | 0.599 |
| Gait score | 0.299 (−0.191–0.789) | 1.252 | 0.221 | −0.05 (−0.209–0.109) | −0.647 | 0.523 |
| Balance score | 0.207 (−0.894–1.307) | 0.386 | 0.703 | 0.106 (−0.266–0.478) | 0.585 | 0.564 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo-Claverol, M.; Malla-Clua, B.; Marquilles-Bonet, C.; Sol, J.; Jové-Naval, J.; Sole-Pujol, M.; Ortega-Bravo, M. Animal-Assisted Therapy Improves Communication and Mobility among Institutionalized People with Cognitive Impairment. Int. J. Environ. Res. Public Health 2020, 17, 5899. https://doi.org/10.3390/ijerph17165899
Rodrigo-Claverol M, Malla-Clua B, Marquilles-Bonet C, Sol J, Jové-Naval J, Sole-Pujol M, Ortega-Bravo M. Animal-Assisted Therapy Improves Communication and Mobility among Institutionalized People with Cognitive Impairment. International Journal of Environmental Research and Public Health. 2020; 17(16):5899. https://doi.org/10.3390/ijerph17165899
Chicago/Turabian StyleRodrigo-Claverol, Maylos, Belén Malla-Clua, Carme Marquilles-Bonet, Joaquim Sol, Júlia Jové-Naval, Meritxell Sole-Pujol, and Marta Ortega-Bravo. 2020. "Animal-Assisted Therapy Improves Communication and Mobility among Institutionalized People with Cognitive Impairment" International Journal of Environmental Research and Public Health 17, no. 16: 5899. https://doi.org/10.3390/ijerph17165899
APA StyleRodrigo-Claverol, M., Malla-Clua, B., Marquilles-Bonet, C., Sol, J., Jové-Naval, J., Sole-Pujol, M., & Ortega-Bravo, M. (2020). Animal-Assisted Therapy Improves Communication and Mobility among Institutionalized People with Cognitive Impairment. International Journal of Environmental Research and Public Health, 17(16), 5899. https://doi.org/10.3390/ijerph17165899