Use of Generalized Additive Model to Detect the Threshold of δ-Aminolevulinic Acid Dehydratase Activity Reduced by Lead Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Health Examination
2.3. DNA Extraction
2.4. ALAD Genotyping
2.5. ALAD Activity
2.6. Statistical Analysis
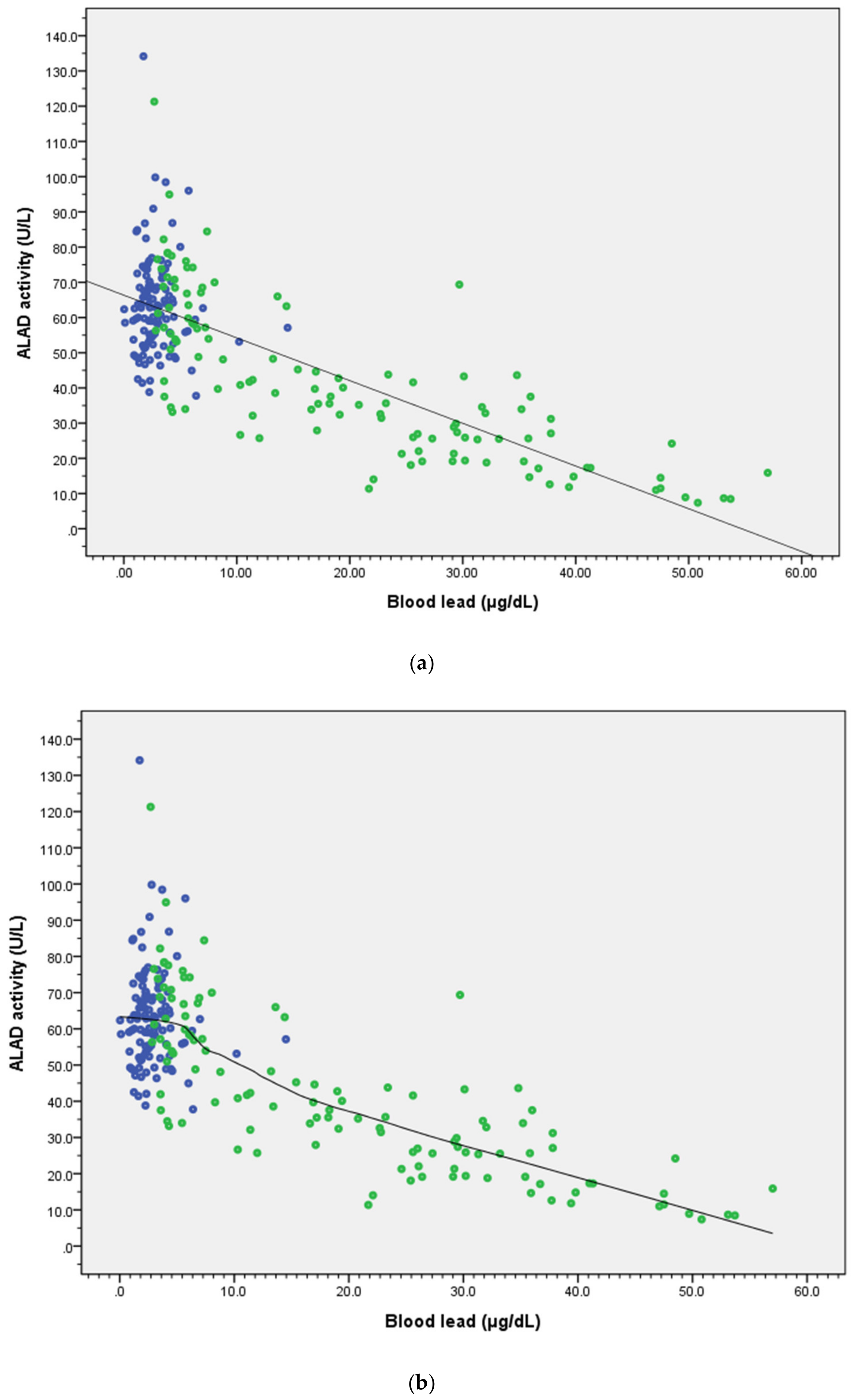
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chao, K.Y.; Shin, W.Y.; Chuang, H.Y.; Wang, J.D. The distribution of blood lead levels and job titles among lead-acid battery workers in Taiwan. Kaohsiung J. Med. Sci. 2002, 18, 347–354. [Google Scholar] [PubMed]
- Castellino, N.; Castellino, P.; Sannolo, N. Inorganic Lead Exposure: Metabolism and Intoxication; Lewis Publishers: Boca Raton, FL, USA, 1995; p. 516. [Google Scholar]
- Chisolm, J.J., Jr.; Thomas, D.J.; Hamill, T.G. Erythrocyte porphobilinogen synthase activity as an indicator of lead exposure in children. Clin. Chem. 1985, 31, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.K.; Bagla, S.; Michini, P.A. Reevaluation of a sensitive indicator of early lead exposure. Measurement of porphobilinogen synthase in blood. Biol. Trace Elem. Res. 1991, 28, 223–231. [Google Scholar] [CrossRef]
- Rogan, W.J.; Reigart, J.R.; Gladen, B.C. Association of amino levulinate dehydratase levels and ferrochelatase inhibition in childhood lead exposure. J. Pediatr. 1986, 109, 60–64. [Google Scholar] [CrossRef]
- Warren, M.J.; Cooper, J.B.; Wood, S.P.; Shoolingin-Jordan, P.M. Lead poisoning, haem synthesis and 5-aminolaevulinic acid dehydratase. Trends Biochem. Sci. 1998, 23, 217–221. [Google Scholar] [CrossRef]
- Himani; Kumar, R.; Ansari, J.A.; Mahdi, A.A.; Sharma, D.; Karunanand, B.; Datta, S.K. Blood Lead Levels in Occupationally Exposed Workers Involved in Battery Factories of Delhi-NCR Region: Effect on Vitamin D and Calcium Metabolism. Indian J. Clin. Biochem. 2020, 35, 80–87. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Chapman & Hall/CRC: New York, NY, USA; Boca Raton, FL, USA, 1990. [Google Scholar] [CrossRef]
- Chuang, H.Y.; Schwartz, J.; Tsai, S.Y.; Lee, M.L.; Wang, J.D.; Hu, H. Vibration perception thresholds in workers with long term exposure to lead. Occup. Environ. Med. 2000, 57, 588–594. [Google Scholar] [CrossRef]
- Hsieh, L.L.; Liou, S.H.; Chen, Y.H.; Tsai, L.C.; Yang, T.; Wu, T.N. Association between aminolevulinate dehydrogenase genotype and blood lead levels in Taiwan. J. Occup. Environ. Med. 2000, 42, 151–155. [Google Scholar] [CrossRef]
- Hu, H.; Wu, M.T.; Cheng, Y.; Sparrow, D.; Weiss, S.; Kelsey, K. The delta-aminolevulinic acid dehydratase (ALAD) polymorphism and bone and blood lead levels in community-exposed men: The Normative Aging Study. Environ. Health Perspect. 2001, 109, 827–832. [Google Scholar] [CrossRef][Green Version]
- Wu, M.T.; Kelsey, K.; Schwartz, J.; Sparrow, D.; Weiss, S.; Hu, H. A delta-aminolevulinic acid dehydratase (ALAD) polymorphism may modify the relationship of low-level lead exposure to uricemia and renal function: The normative aging study. Environ. Health Perspect. 2003, 111, 335–341. [Google Scholar] [CrossRef]
- Chia, S.E.; Yap, E.; Chia, K.S. Delta-aminolevulinic acid dehydratase (ALAD) polymorphism and susceptibility of workers exposed to inorganic lead and its effects on neurobehavioral functions. Neurotoxicology 2004, 25, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Y.; Chang, P.W.; Wu, C.C.; Lai, J.S.; Kuo, H.W. Lack of association of delta-aminolevulinic acid dehydratase genotype with cytogenetic damage in lead workers. Int. Arch. Occup. Environ. Health 2004, 77, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.E.; Zhou, H.; Tham, M.T.; Yap, E.; Dong, N.V.; Tu, N.H.; Chia, K.S. Possible influence of delta-aminolevulinic acid dehydratase polymorphism and susceptibility to renal toxicity of lead: A study of a Vietnamese population. Environ. Health Perspect. 2005, 113, 1313–1317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mani, M.S.; Kunnathully, V.; Rao, C.; Kabekkodu, S.P.; Joshi, M.B.; D’Souza, H.S. Modifying effects of δ-Aminolevulinate dehydratase polymorphism on blood lead levels and ALAD activity. Toxicol. Lett. 2018, 295, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.Y.; Lee, M.L.; Chao, K.Y.; Wang, J.D.; Hu, H. Relationship of blood lead levels to personal hygiene habits in lead battery workers: Taiwan, 1991–1997. Am. J. Ind. Med. 1999, 35, 595–603. [Google Scholar] [CrossRef]
- Wetmur, J.G. Influence of the common human delta-aminolevulinate dehydratase polymorphism on lead body burden. Environ. Health Perspect. 1994, 102 (Suppl. 3), 215–219. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Schaller, K.H. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z. Klin. Chem. Klin. Biochem. 1974, 12, 389–390. [Google Scholar]
- Berlin, A.; Schaller, K.H.; Grimes, H.; Langevin, M.; Trotter, J. Environmental exposure to lead: Analytical and epidemiological investigations using the European standardised method for blood delta-aminolevulinic acid dehydratase activity determination. Int. Arch. Occup. Environ. Health 1977, 39, 135–141. [Google Scholar] [CrossRef]
- Venables, W.N.; Dichmont, C.M. GLMs, GAMs and GLMMs: An overview of theory for applications in fisheries research. Fish. Res. 2004, 70, 319–337. [Google Scholar] [CrossRef]
- Sakai, T.; Morita, Y. Delta-Aminolevulinic acid in plasma or whole blood as a sensitive indicator of lead effects, and its relation to the other heme-related parameters. Int. Arch. Occup. Environ. Health 1996, 68, 126–132. [Google Scholar]
- Campagna, D.; Huel, G.; Girard, F.; Sahuquillo, J.; Blot, P. Environmental lead exposure and activity of delta-aminolevulinic acid dehydratase (ALA-D) in maternal and cord blood. Toxicology 1999, 134, 143–152. [Google Scholar] [CrossRef]
- Nordman, C.H.; Hernberg, S. Blood lead levels and erythrocyte delta-amino-levulinic acid dehydratase activity of selected population groups in Helsinki. Scand. J. Work Environ. Health 1975, 1, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Wada, O.; Takeo, K.; Yano, Y.; Ono, T.; Nagahashi, M. delta-aminolevulinic acid dehydratase in low level lead exposure. Arch. Environ. Health 1976, 31, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Sakai, T.; Morita, Y.; Iwata, T.; Dakeishi, M. Critical dose of lead affecting delta-aminolevulinic acid levels. J. Occup. Health 2003, 45, 209–214. [Google Scholar] [CrossRef] [PubMed]
- La-Llave-Leon, O.; Mendez-Hernandez, E.M.; Castellanos-Juarez, F.X.; Esquivel-Rodriguez, E.; Vazquez-Alaniz, F.; Sandoval-Carrillo, A.; Garcia-Vargas, G.; Duarte-Sustaita, J.; Candelas-Rangel, J.L.; Salas-Pacheco, J.M. Association between Blood Lead Levels and Delta-Aminolevulinic Acid Dehydratase in Pregnant Women. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, S.; Blake, D.; Shevenan, P. Enzyme glycation may decrease activity of erythrocytic delta-aminolevulinate dehrdratase in diabetes mellitus. Clin. Chem. 1987, 33, 1807–1810. [Google Scholar] [CrossRef]
- Zorana, K.G.; Alica, P.; Jasna, J. Influence of abatement of lead exposure in Croatia on blood lead and ALAD activity. Environ. Sci. Pollut. Res. Int. 2016, 23, 898–907. [Google Scholar] [CrossRef]
- Jangid, A.P.; John, P.J.; Yadav, D.; Mishra, S.; Sharma, P. Impact of chronic lead exposure on selected biological markers. Indian J. Clin. Biochem. 2012, 27, 83–89. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Sun, P. Effects of delta-aminolevulinic acid dehydratase polymorphisms on susceptibility to lead in Han subjects from southwestern China. Int. J. Environ. Res. Public Health 2012, 9, 2326–2338. [Google Scholar] [CrossRef]
| Characteristics | Control | Lead-Exposed | p-Value |
|---|---|---|---|
| n = 117 | n = 121 | ||
| Age (years) | 41.7 ± 11.8 | 41.5 ± 8.3 | 0.916 a |
| Time on the job (years) | – | 11.7 ± 5.3 | – |
| Gender (male %) | 71 (61.1%) | 98 (81.0%) | 0.01 b |
| BMI (kg/m2) | 24.9 ± 4.1 | 24.4 ± 3.5 | 0.310 a |
| Blood lead (μg/dL) | 2.9 ± 1.8 | 19.7 ± 14.7 | <0.001 a |
| Hemoglobin (mg/dL) | 14.7 ± 1.5 | 14.9 ± 1.4 | 0.168 |
| Hematocrit (Hct) (%) | 43.5 ± 3.8 | 44.4 ± 3.5 | 0.060 a |
| ALAD activity (U/L) | 63.32 ± 14.01 | 41.58 ± 22.07 | <0.001 a |
| ALAD 1-1 type (%) | 113 (96.6%) | 116 (95.9%) | 0.773 b |
| ALAD 1-2 type (%) † | 4 (3.4%) | 5 (4.1%) | |
| Fasting glucose (mg/dL) | 106.7 ± 38.6 | 101.8 ± 32.3 | 0.309 a |
| Alcohol use (%) | 9 (7.7%) | 32 (26.4%) | <0.001 b |
| Smoking (%) | 16 (13.7%) | 49 (40.5%) | <0.001 b |
| All (n = 238) | ALAD 1-1 Type (n = 229) | |||||
|---|---|---|---|---|---|---|
| Variables | Regression Coefficient | (SE) | p-Value | Regression Coefficient | (SE) | p-Value |
| Blood lead (μg/dL) | −1.04 | (0.08) | <0.001 ** | −1.05 | (0.08) | <0.001 ** |
| ALAD type (1-2 vs. 1-1) | −4.12 | (4.75) | 0.39 | – | – | |
| Gender (M vs. F) | −8.08 | (2.27) | <0.001 ** | −8.28 | (2.31) | <0.001 ** |
| Age (years) | −0.16 | (0.09) | 0.08 | −0.19 | (0.09) | 0.05 |
| BMI (kg/m2) | 0.20 | (0.26) | 0.45 | 0.33 | (0.27) | 0.23 |
| Fasting glucose (mg/dL) | −0.11 | (0.03) | <0.001 ** | −0.12 | (0.03) | <0.001 ** |
| Smoking (yes/no) | 0.96 | (2.37) | 0.68 | 1.22 | (2.40) | 0.61 |
| Drinking (yes/no) | −5.47 | (2.69) | 0.04 * | −5.51 | (2.70) | 0.04 * |
| Constant | 83.84 | (6.76) | <0.001 ** | 82.86 | (6.91) | <0.001 ** |
| (adj R2 = 0.639) | (adj R2 = 0.644) | |||||
| Group | Range of Blood Lead (μg/dL) | No. of Subjects | ALAD Activity (U/L) | Blood Lead (μg/dL) | β | SE | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | ≦5.00 | 128 | 64.21 ± 15.04 | 2.74 ± 1.11 | – | – | – |
| 2 | 5.01–10.00 | 26 | 60.49 ± 14.15 | 6.48 ± 0.93 | −1.099 | 2.925 | 0.708 |
| 3 | 10.01–15.00 | 12 | 44.64 ± 13.30 | 12.15 ± 1.60 | −16.328 | 4.004 | <0.001 |
| 4 | 15.01–20.00 | 11 | 37.76 ± 5.36 | 17.65 ± 1.24 | −23.710 | 4.237 | <0.001 |
| 5 | 20.01–25.00 | 8 | 28.18 ± 11.40 | 22.66 ± 1.15 | −33.514 | 5.041 | <0.001 |
| 6 | 25.01–30.00 | 13 | 28.89 ± 13.65 | 27.58 ± 1.77 | −31.159 | 3.996 | <0.001 |
| 7 | 30.01–35.00 | 9 | 29.92 ± 9.27 | 31.73 ± 1.55 | −34.900 | 4.569 | <0.001 |
| 8 | 35.01–40.00 | 11 | 22.33 ± 9.14 | 37.05 ± 1.58 | −38.096 | 4.271 | <0.001 |
| 9 | 40.01 | 11 | 13.20 ± 5.17 | 48.84 ± 4.88 | −45.991 | 4.594 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Yang, C.-C.; Liu, T.-Y.; Dai, C.-Y.; Wang, C.-L.; Chuang, H.-Y. Use of Generalized Additive Model to Detect the Threshold of δ-Aminolevulinic Acid Dehydratase Activity Reduced by Lead Exposure. Int. J. Environ. Res. Public Health 2020, 17, 5712. https://doi.org/10.3390/ijerph17165712
Huang C-C, Yang C-C, Liu T-Y, Dai C-Y, Wang C-L, Chuang H-Y. Use of Generalized Additive Model to Detect the Threshold of δ-Aminolevulinic Acid Dehydratase Activity Reduced by Lead Exposure. International Journal of Environmental Research and Public Health. 2020; 17(16):5712. https://doi.org/10.3390/ijerph17165712
Chicago/Turabian StyleHuang, Chan-Ching, Chen-Cheng Yang, Te-Yu Liu, Chia-Yen Dai, Chao-Ling Wang, and Hung-Yi Chuang. 2020. "Use of Generalized Additive Model to Detect the Threshold of δ-Aminolevulinic Acid Dehydratase Activity Reduced by Lead Exposure" International Journal of Environmental Research and Public Health 17, no. 16: 5712. https://doi.org/10.3390/ijerph17165712
APA StyleHuang, C.-C., Yang, C.-C., Liu, T.-Y., Dai, C.-Y., Wang, C.-L., & Chuang, H.-Y. (2020). Use of Generalized Additive Model to Detect the Threshold of δ-Aminolevulinic Acid Dehydratase Activity Reduced by Lead Exposure. International Journal of Environmental Research and Public Health, 17(16), 5712. https://doi.org/10.3390/ijerph17165712







