Effect of Surface Treatment by O3 and Chemical Activation by Alkali Metal on the Performance of ACFs on Adsorption and Desorption of BTX Gases
Abstract
1. Introduction
2. Materials and Methods
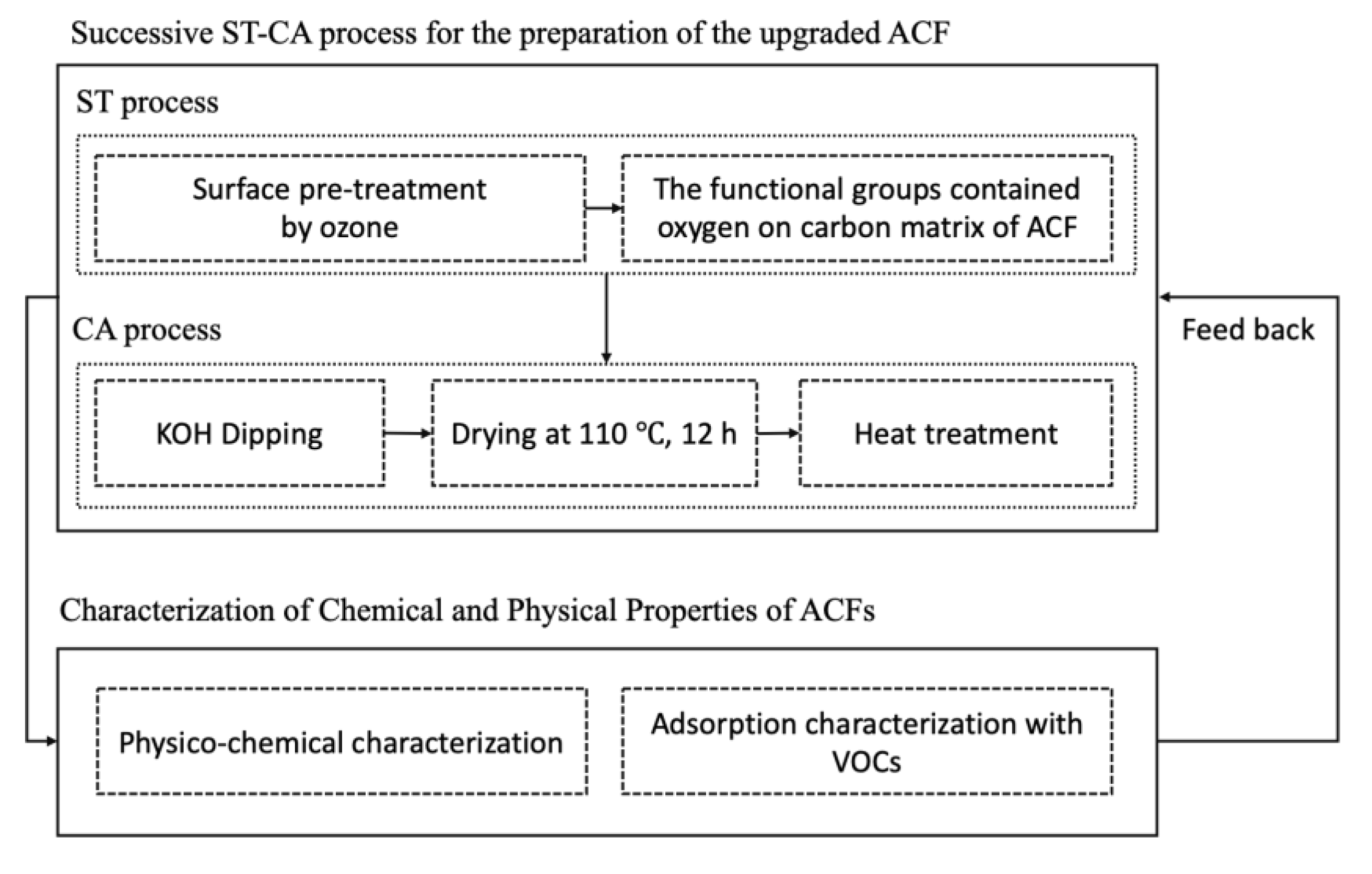
2.1. Successive ST-CA Process for the Preparation of the Upgraded ACFs
2.2. Characterization of Chemical and Physical Properties of ACFs
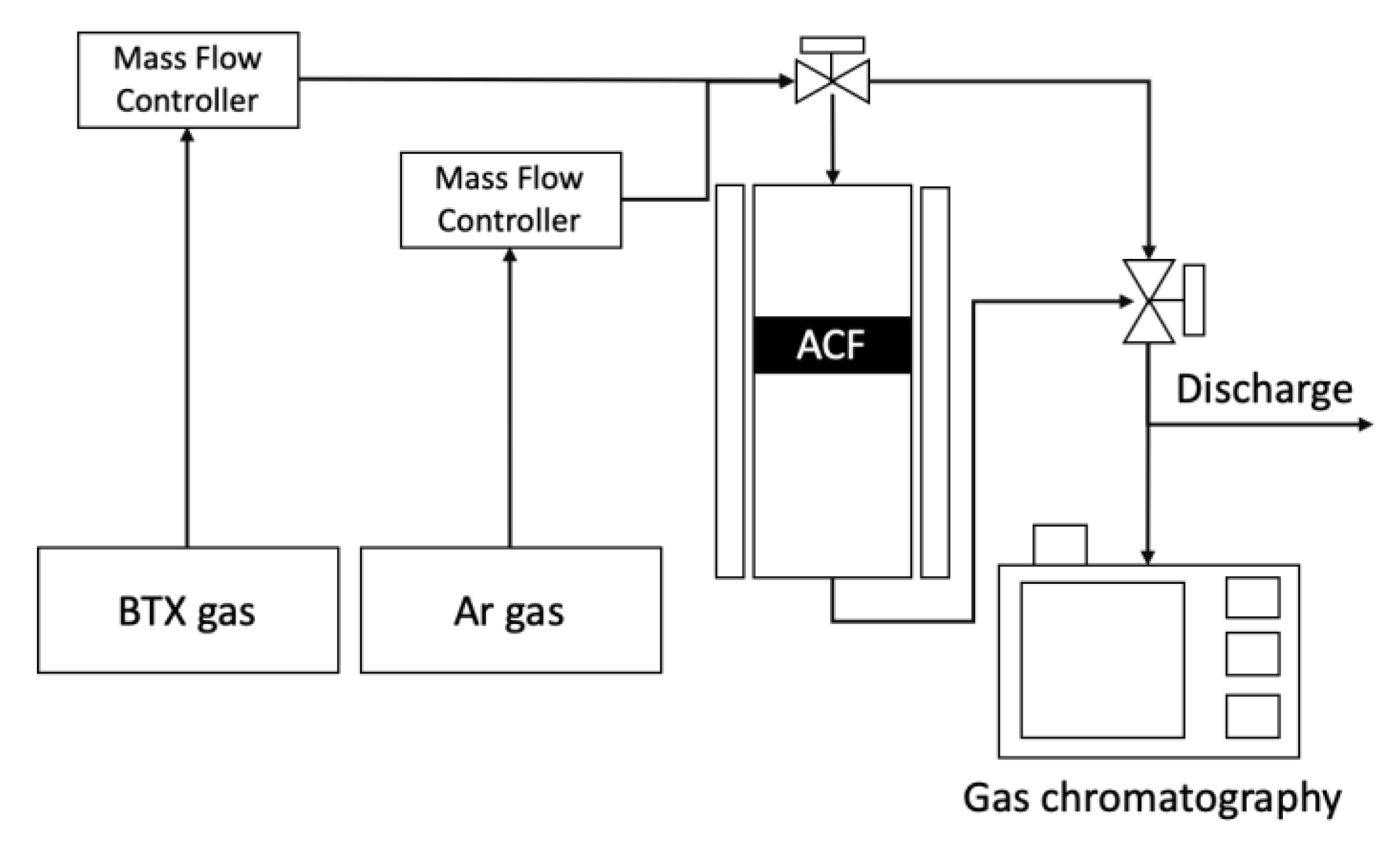
2.3. VOCs Adsorption—Desorption with ACFs before and after Surface Treatment and Chemical Activation
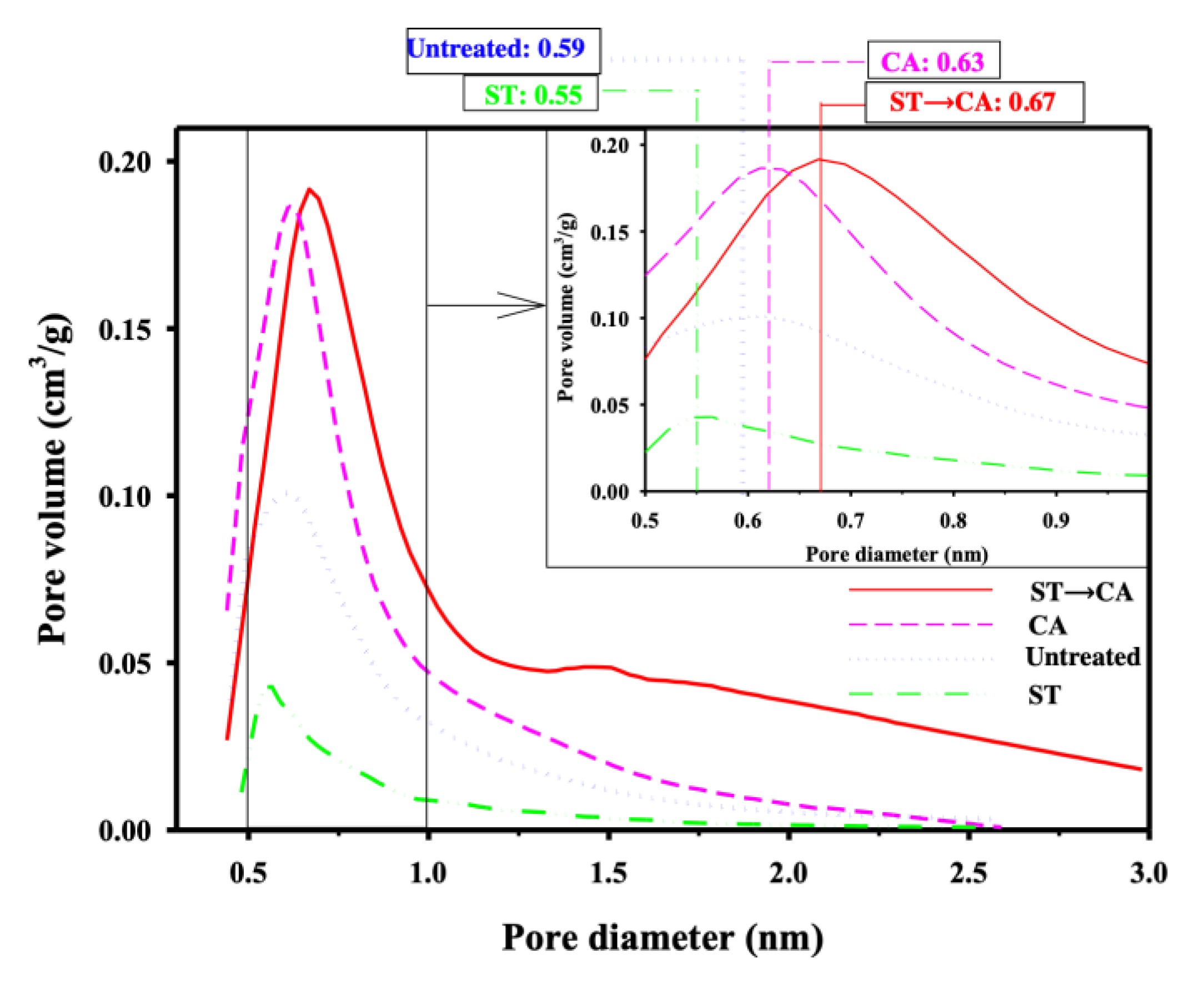
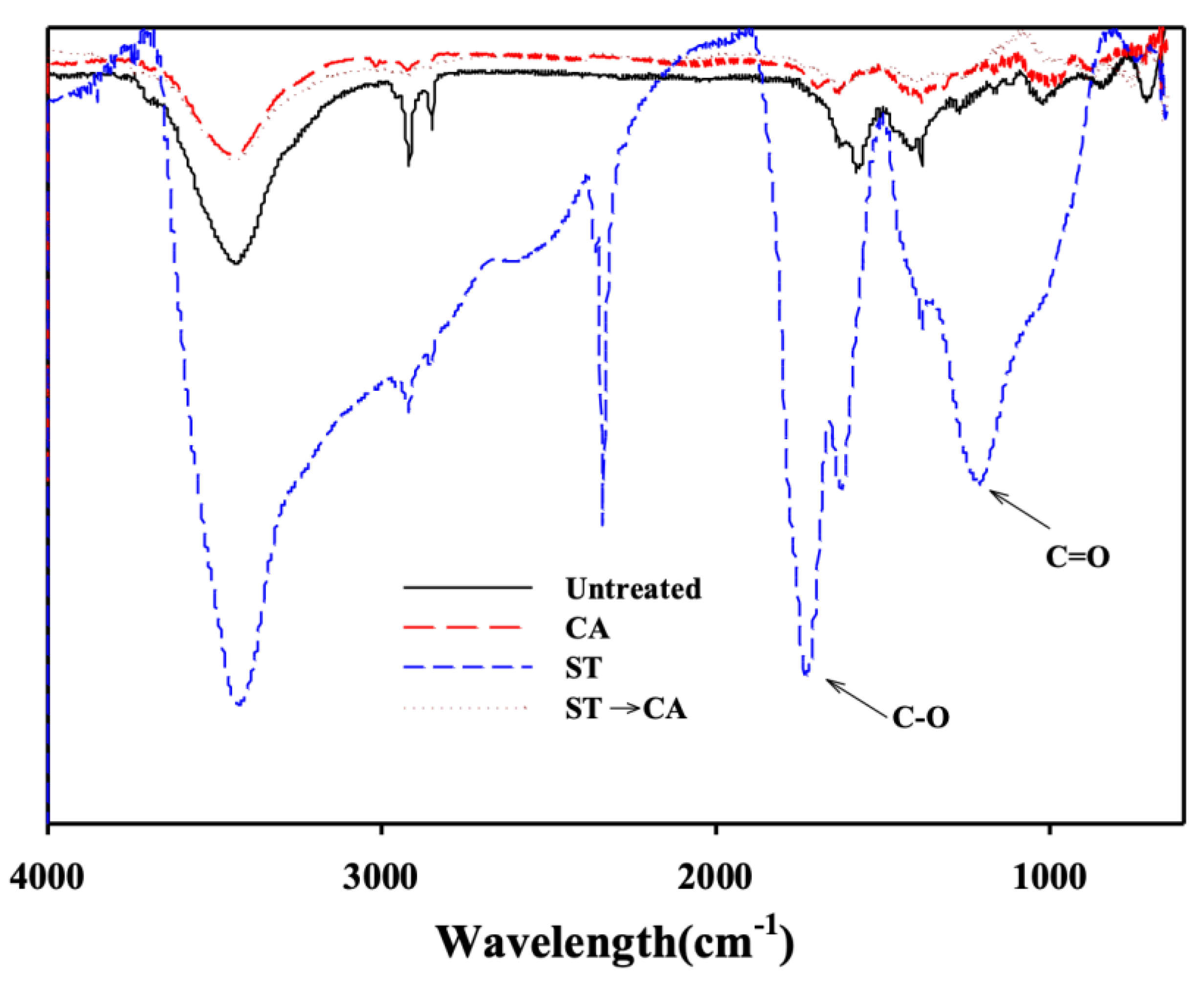
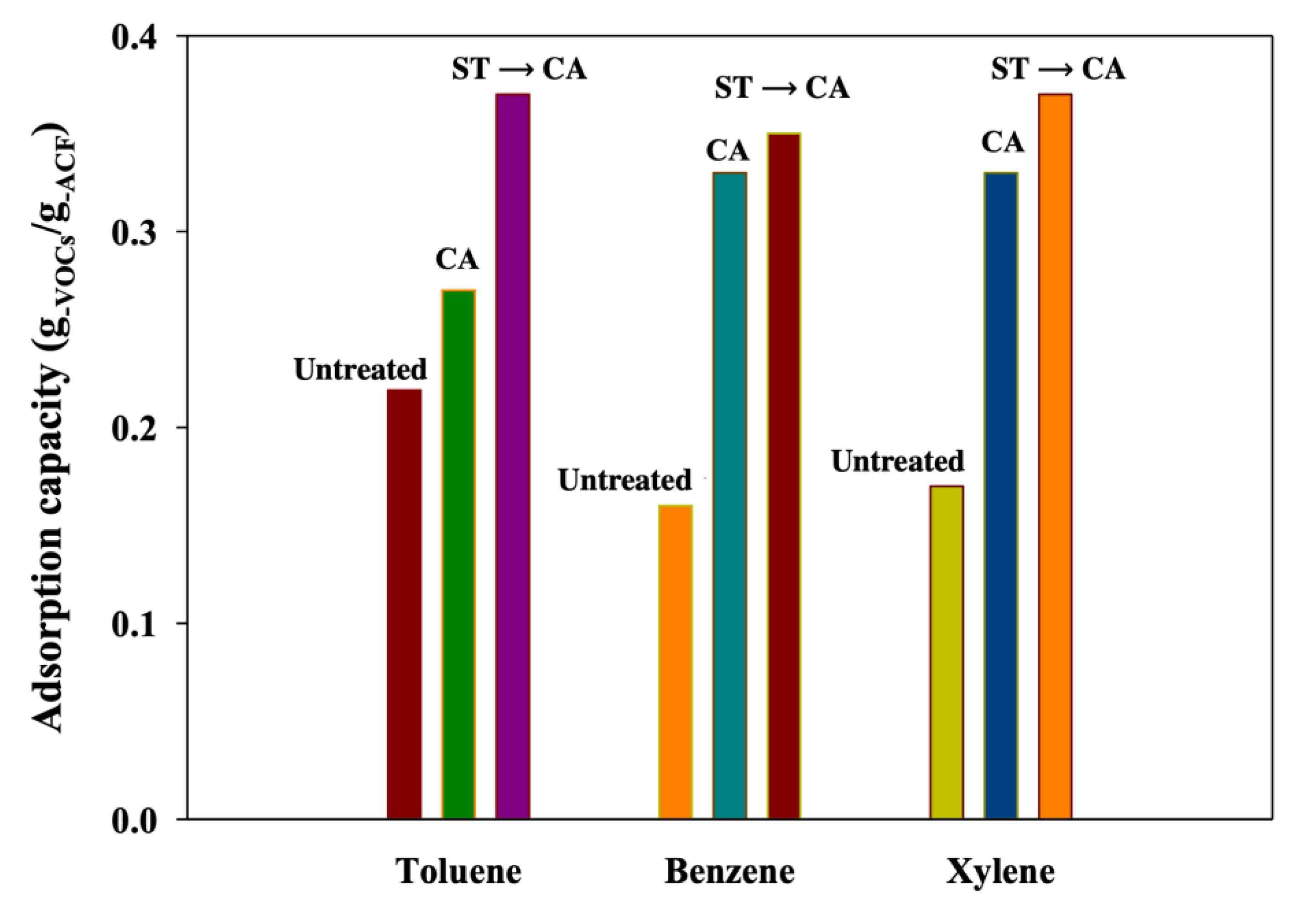
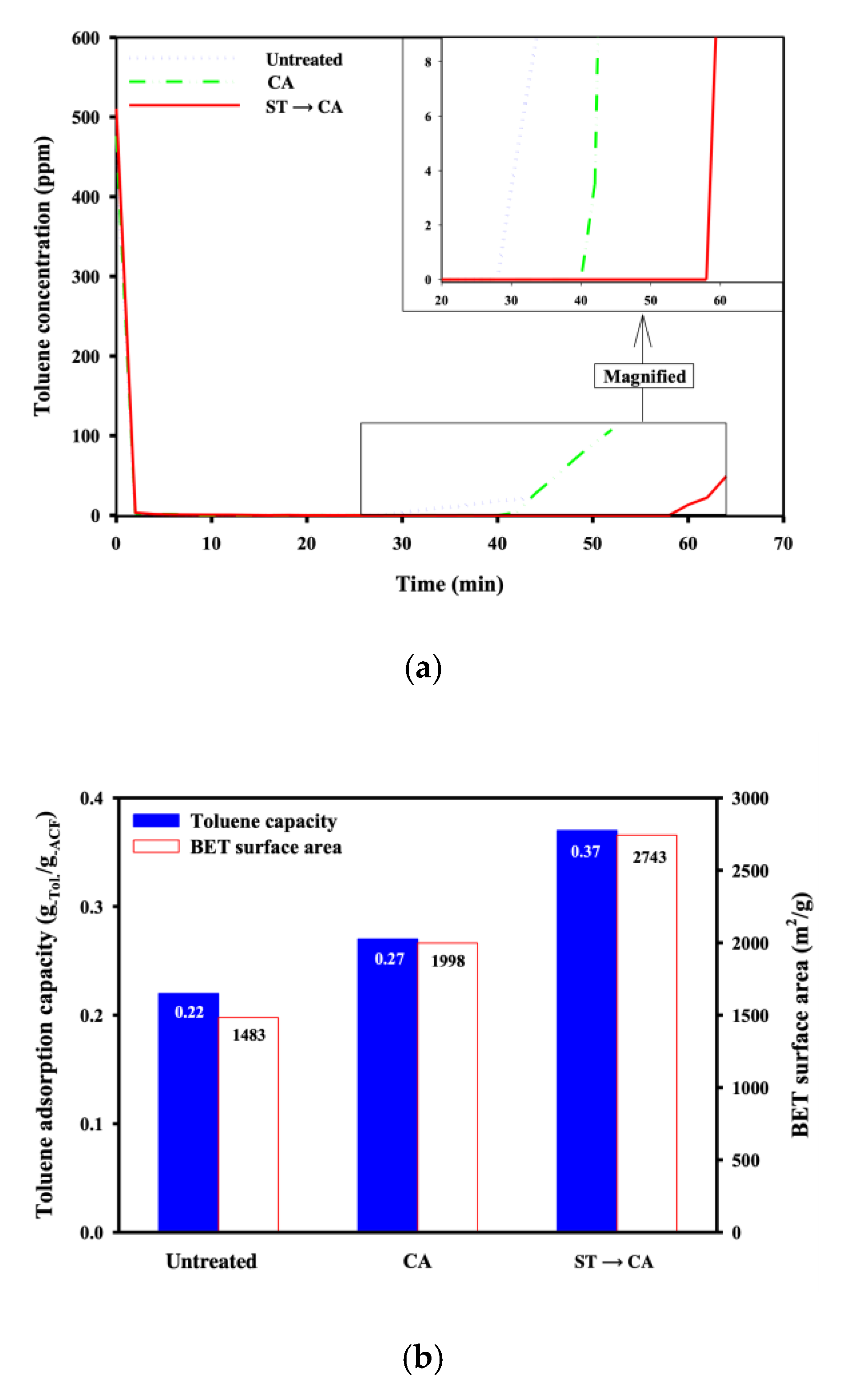
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okrasa, M.; Hitz, J.; Nowak, A.; Brochocka, A.; Thelen, C.; Walczak, Z. Adsorption Performance of Activated-Carbon-Loaded Nonwoven Filters Used in Filtering Facepiece Respirators. Int. J. Environ. Res. Public Health 2019, 16, 1973. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Errahali, M.; Tei, L.; Mangano, E.; Brandani, S.; Cossi, M.; Marchese, L. A Porous Carbon with Excellent Gas Storage Properties from Waste Polystyrene. Nanomaterials 2019, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Sathe, M.; Sharma, P.K.; Singh, V.K.; Tripathi, N.K.; Verma, V.; Sharma, S.P.; Tomar, L.N.S.; Chaturvedi, A.; Yadav, S.S.; Thakare, V.B.; et al. Chemical Protection Studies of Activated Carbon Spheres based Permeable Protective Clothing Against Sulfur Mustard, a Chemical Warfare Agent. Def. Sci. J. 2019, 69, 577–584. [Google Scholar] [CrossRef]
- Amid, H.; Maze, B.; Flickinger, M.; Pourdeyhimi, B. Dynamic adsorption of ammonia: Apparatus, testing conditions, and adsorption capacities. Meas. Sci. Technol. 2017, 28, 1–7. [Google Scholar] [CrossRef]
- Foster, K.L.; Fuerman, R.G.; Economy, J.; Larson, S.M.; Rood, M.J. Adsorption characteristics of trace volatile organic compounds in gas streams on to activated carbon fibers. Chem. Mater. 1992, 4, 1068–1073. [Google Scholar] [CrossRef]
- Wallace, L.A.; Pellizzari, E.D.; Leaderer, B.P.; Zelon, H.; Sheldon, L.S. Emissions of volatile organic compounds from building materials and consumer products. Atmos. Environ. 1987, 21, 385–393. [Google Scholar] [CrossRef]
- Gupta, K.V.; Verma, N. Removal of volatile organic compounds by cryogenic condensation followed by adsorption. Chem. Eng. Sci. 2002, 57, 2679–2696. [Google Scholar] [CrossRef]
- Rudy, E.N.; Carroll, L.A. Select the best VOC control strategy. Chem. Eng. Prog. 1993, 89, 28–39. [Google Scholar]
- Benkhedda, J.; Jaubert, J.N.; Barth, D.; Perrin, L. Experimental and modeled results describing the adsorption of toluene onto activated carbons. J. Chem. Eng. Data 2000, 45, 650–653. [Google Scholar] [CrossRef]
- Cal, M.P.; Larson, S.M.; Rood, M.J. Experimental and modelled results describing the adsorption of acetone and benzene onto activated carbon fibres. Environ. Prog. 1994, 13, 26–30. [Google Scholar] [CrossRef]
- Dolidovich, A.F.; Akhremkova, G.S.; Effremtsev, V.S. Novel technologies of VOC decontamination in fixed, moving and fluidized catalyst-adsorbent beds. Can. J. Chem. Eng. 1999, 77, 342–355. [Google Scholar]
- Vorontsov, A.V.; Savinov, E.N.; Smirniotis, P.G. Vibro fluideized and fixed-bed photocatalytic reactors: Case of gaseous actone photooxidation. Chem. Eng. Sci. 2000, 55, 5089–5098. [Google Scholar] [CrossRef]
- Navarri, P.; Marchal, D.; Ginestet, A. Activated carbon fiber material for VOC removal. Filtr. Separat. 2001, 2, 33–40. [Google Scholar] [CrossRef]
- Suzuki, M. Activated carbon fiber: Fundamentals and applications. Carbon 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Thwaites, M.; Stencel, J.; McEnaney, B.; Derbyshire, F. Adsorbent carbon synthesis from coals by phosphoric acid activation. Carbon 1992, 30, 1089–1096. [Google Scholar] [CrossRef]
- Teng, H.; Yeh, T.S.; Hsu, L.Y. Preparation of activated carbon from bituminous coal with phosphoric acid activation. Carbon 1998, 36, 1387–1395. [Google Scholar] [CrossRef]
- Illán-Gómez, M.J.; García-García, A.; Salinas-Martínez de Lecea, C.; Linares-Solano, A. Activated carbons from Spanish coals. 2. Chemical activation. Energy Fuel 1996, 10, 1108–1119. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite: I. Activation by KOH. Carbon 2001, 39, 741–749. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite: II. Activation by NaOH. Carbon 2001, 39, 751–759. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrolysis 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Lee, K.J.; Shiratori, N.; Lee, G.H.; Miyawaki, J.; Mochida, I.; Yoon, S.-H.; Jang, J. Activated carbon nanofiber produced from electrospun polyacrylonitrile nanofiber as a highly efficient formaldehyde adsorbent. Carbon 2010, 48, 4248–4255. [Google Scholar] [CrossRef]
- Oh, G.-Y.; Ju, Y.-W.; Jung, H.-R.; Lee, W.-J. Preparation of the novel manganese-embedded PAN-based activated carbon nanofibers by electrospinning and their toluene adsorption. J. Anal. Appl. Pyrolysis 2008, 81, 211–217. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, R.; Li, S.-M. Preparation and application of efficient TiO2/ACFs photocatalyst. J. Environ. Sci. 2006, 18, 979–982. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Li, M.; Boggs, M.; Beebe, T.P.; Huang, C.P. Oxidation of single-walled carbon nanotubes in dilute aqueous solutions by ozone as affected by ultrasound. Carbon 2008, 46, 466–475. [Google Scholar] [CrossRef]
- Park, S.-J.; Park, S.-J. Effect of ozone-treated single-walled carbon nanotubes on interfacial properties and fracture toughness of carbon fiber-reinforced epoxy composites. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105937. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Ma, J.; Cui, Y.-H.; Zhang, B.-P. Effect of ozonation pretreatment on the surface properties and catalytic activity of multi-walled carbon nanotube. Appl. Catal. B Environ. 2009, 92, 301–306. [Google Scholar] [CrossRef]
- Jiménez, V.; Ramírez-Lucas, A.; Sánchez, P.; Valverde, J.L.; Romero, A. Hydrogen storage in different carbon materials: Influence of the porosity development by chemical activation. Appl. Surf. Sci. 2012, 258, 2498–2509. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Azaïs, P.; Cacciaguerra, T.; Cazorla-Amorós, D.; Linares-Solano, A.; Béguin, F. KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 2005, 43, 786–795. [Google Scholar] [CrossRef]


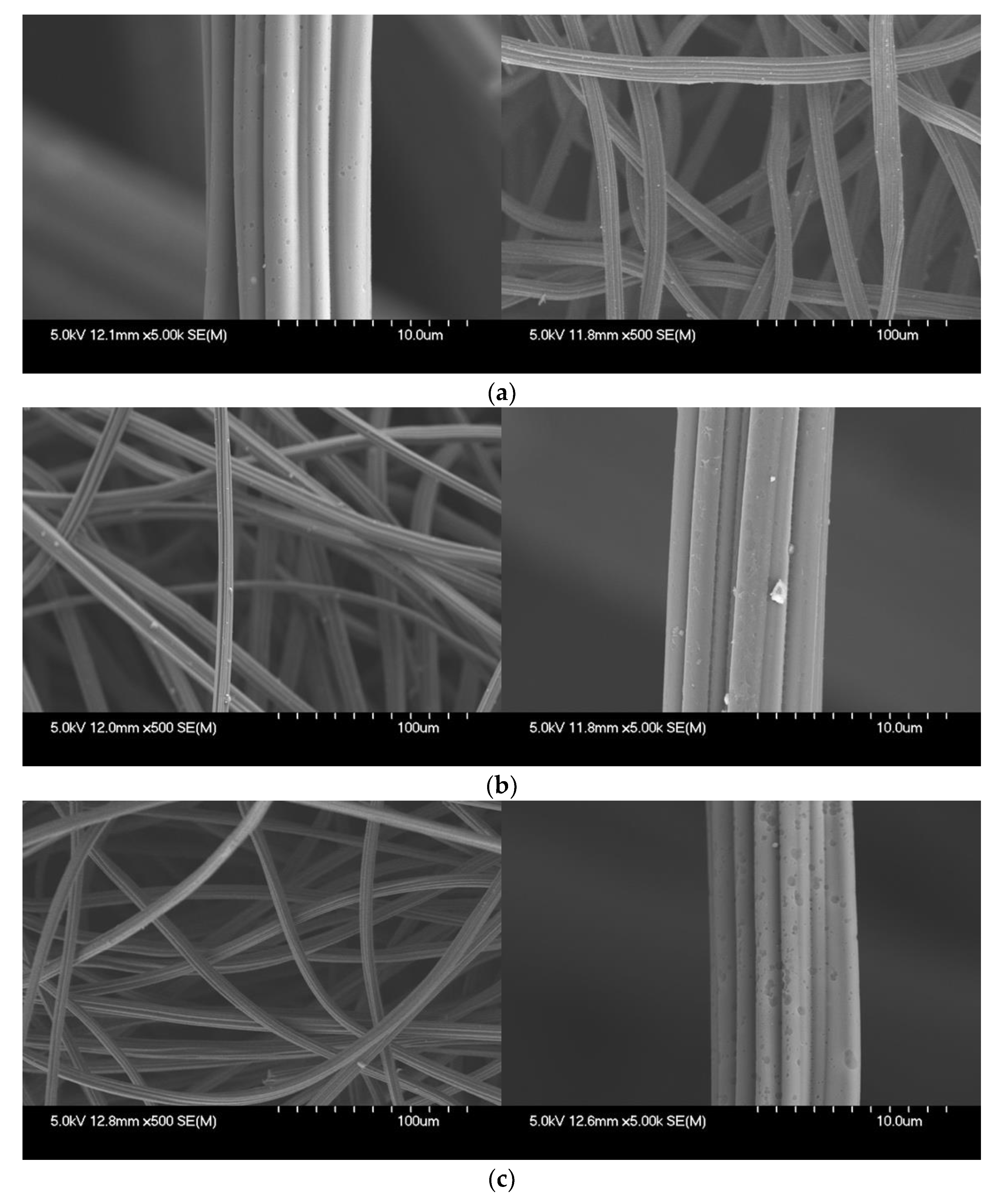
| Textural Properties of ACFs | Untreated | ST | CA | ST→CA | |
|---|---|---|---|---|---|
| Before Heat Treatment | After Heat Treatment | ||||
| BET surface area (m2/g) | 1483 ± 128.2 | 796 ± 76.3 | 1390 ± 108.2 | 1998 ± 140.2 | 2743 ± 203 |
| Total pore volume (cm3/g, P/Po = 0.99) | 0.7512 ± 0.063 | 0.4313 ± 0.045 | 0.5498 ± 0.044 | 1.414 ± 0.141 | 1.5053 ± 0.193 |
| Average pore diameter (nm) | 1.931 ± 0.082 | 1.841 ± 0.077 | 1.959 ± 0.074 | 1.912 ± 0.072 | 2.512 ± 0.12 |
| Average micropore diameter (nm) | 0.744 ± 0.065 | 0.715 ± 0.080 | 0.759 ± 0.044 | 0.735 ± 0.054 | 1.014 ± 0.015 |
| Micropore volume (cm3/g, P/Po = 0.1) | 0.5477 ± 0.034 | 0.2929 ± 0.017 | 0.4992 ± 0.023 | 0.7346 ± 0.045 | 0.9621 ± 0.075 |
| Micropore volume percent (%) | 72.9 ± 5.66 | 67.9 ± 4.31 | 88.3 ± 2.4 | 51.9 ± 10.2 | 63.9 ± 3.14 |
| Micropore area (m2/g, P/Po = 0.1) | 1199 ± 102.3 | 398 ± 20.9 | 1227 ± 10.3 | 2099 ± 180.2 | 2331 ± 222.1 |
| External area (m2/g, P/Po = 0.1) | 165 ± 15.6 | 30 ± 3.2 | 163 ± 10.6 | 136 ± 20.3 | 310 ± 29.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.H.; Han, G.B. Effect of Surface Treatment by O3 and Chemical Activation by Alkali Metal on the Performance of ACFs on Adsorption and Desorption of BTX Gases. Int. J. Environ. Res. Public Health 2020, 17, 5422. https://doi.org/10.3390/ijerph17155422
Jang JH, Han GB. Effect of Surface Treatment by O3 and Chemical Activation by Alkali Metal on the Performance of ACFs on Adsorption and Desorption of BTX Gases. International Journal of Environmental Research and Public Health. 2020; 17(15):5422. https://doi.org/10.3390/ijerph17155422
Chicago/Turabian StyleJang, Jung Hee, and Gi Bo Han. 2020. "Effect of Surface Treatment by O3 and Chemical Activation by Alkali Metal on the Performance of ACFs on Adsorption and Desorption of BTX Gases" International Journal of Environmental Research and Public Health 17, no. 15: 5422. https://doi.org/10.3390/ijerph17155422
APA StyleJang, J. H., & Han, G. B. (2020). Effect of Surface Treatment by O3 and Chemical Activation by Alkali Metal on the Performance of ACFs on Adsorption and Desorption of BTX Gases. International Journal of Environmental Research and Public Health, 17(15), 5422. https://doi.org/10.3390/ijerph17155422





