A Systems Approach to Assess Transport and Diffusion of Hazardous Airborne Particles in a Large Surgical Suite: Potential Impacts on Viral Airborne Transmission
Abstract
1. Introduction
2. Materials and Methods
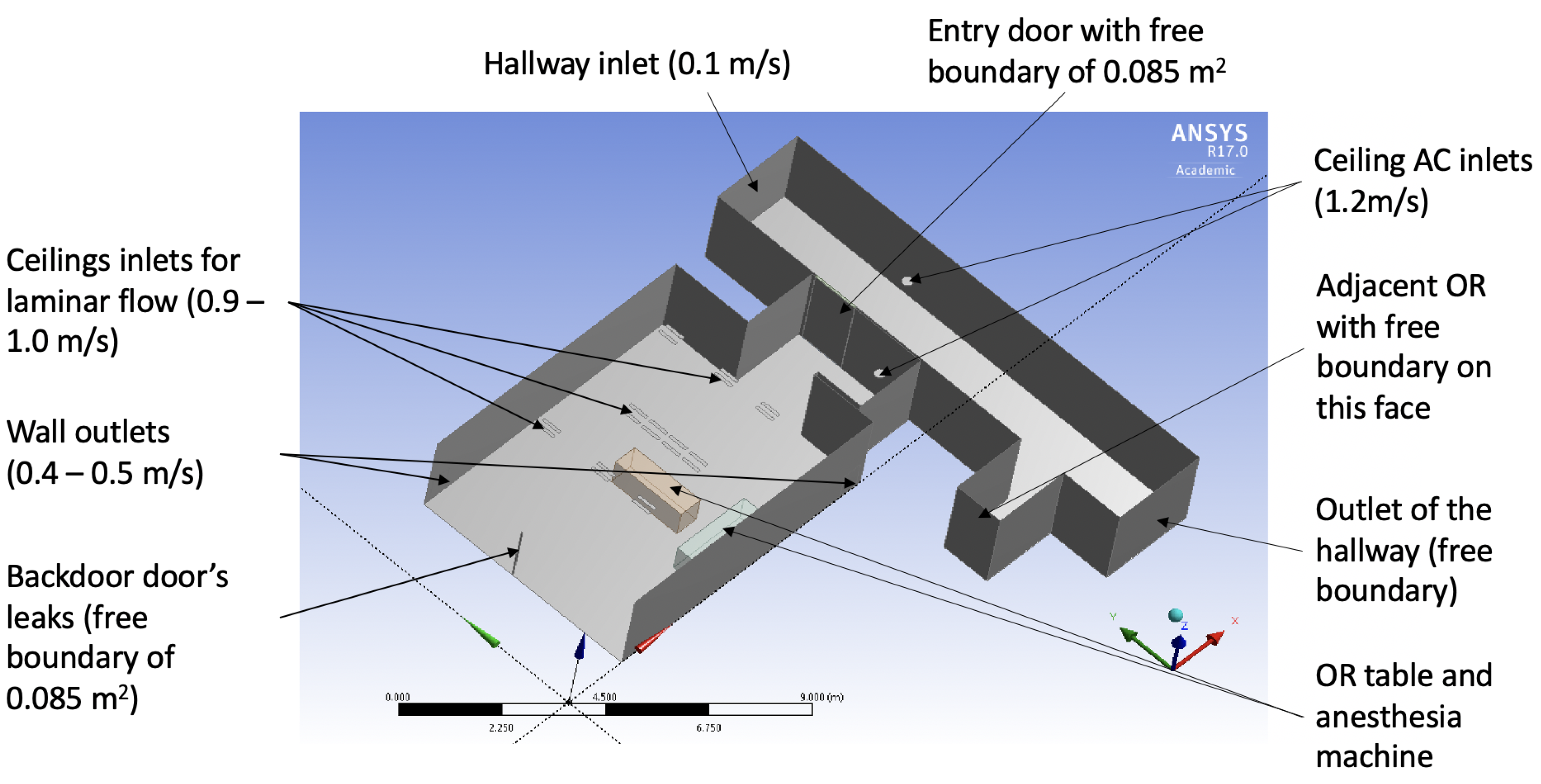
2.1. Computational Air Flow Simulation of the Operating Room
Surgical Suite Model as a System
2.2. Hybrid Box-PDE Model of Airborne Contamination in the Surgical Suite
2.2.1. Experimental Setup to Assess the Source of Particles and Smoke
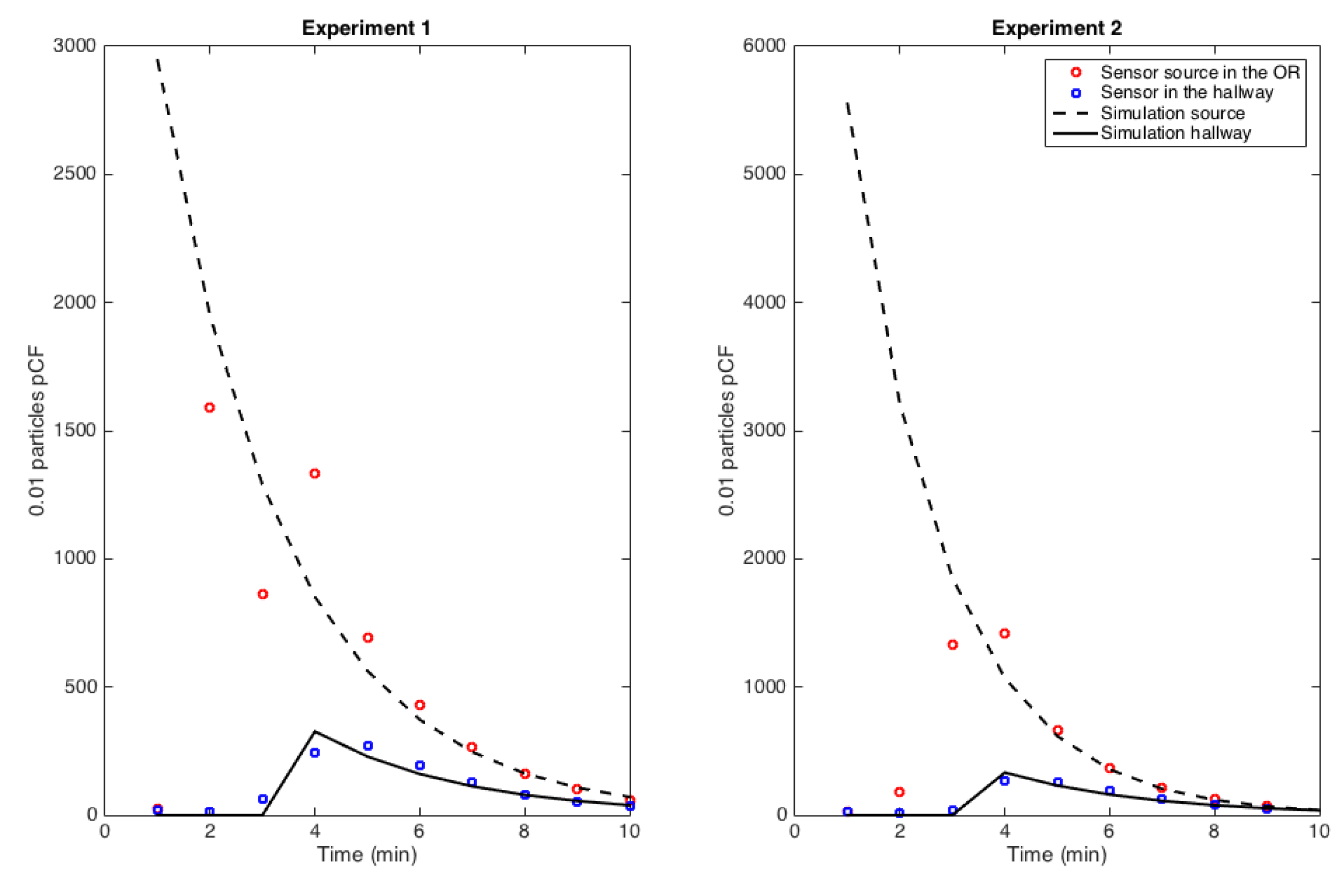
2.2.2. Assimilation of Experimental Data
- Phase 1: anesthesia preparation label as State = 1.
- Phase 2: surgical preparation to access as State = 2.
- Phase 3: surgery procedure as State = 3.
- Phase 4: surgery closing as State = 2.
- Phase 5: ending anesthesia as State = 1.
- Phase 6: room in the process of cleaning or free as State = 0.
2.3. Clinical Model of Airborne Hazard and Integration of Human Behavior in the Simulation
3. Results
3.1. Measuring Sources of Surgical Smoke
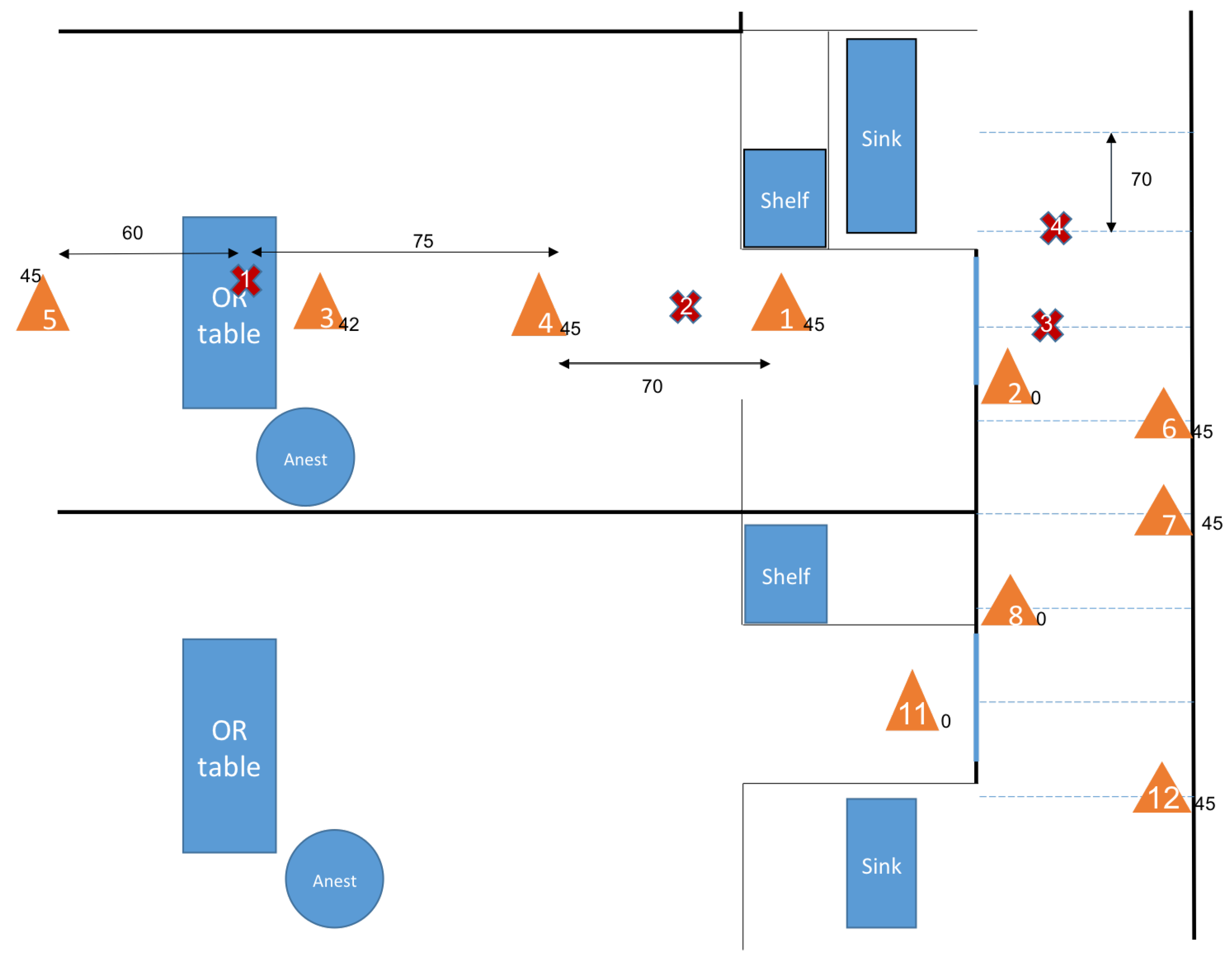
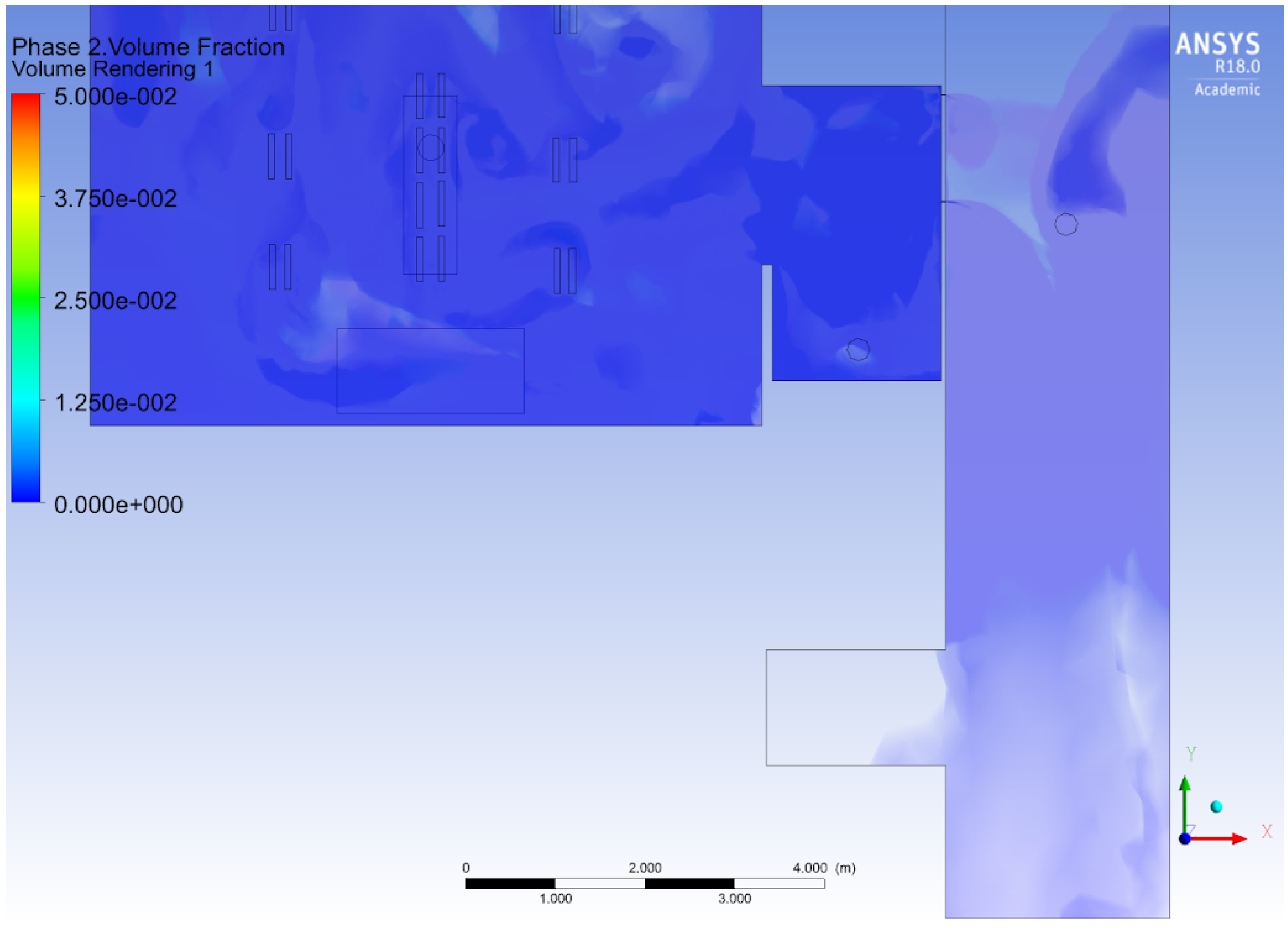
3.2. Airborne Transport-Diffusion at the Operating Room Scale
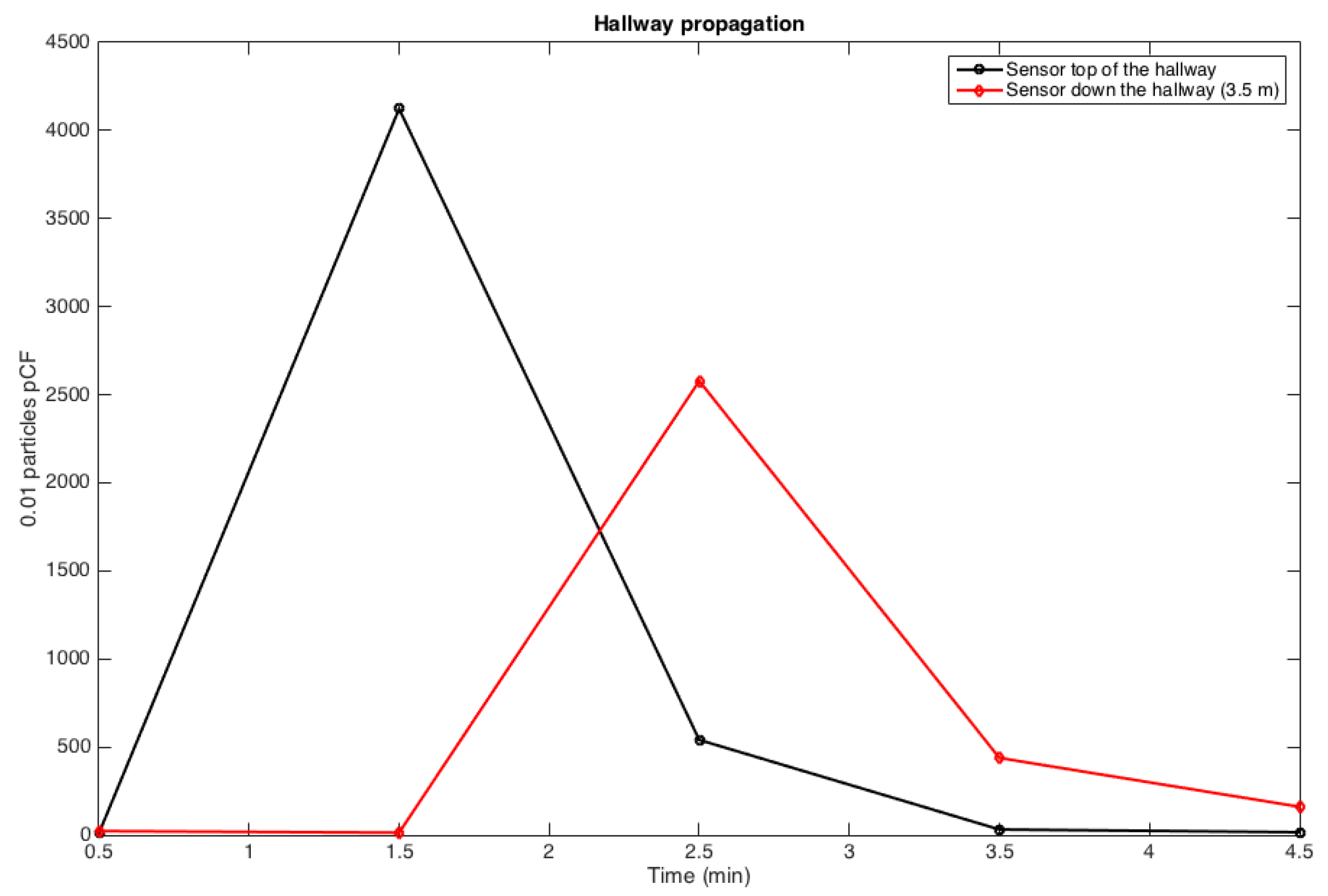
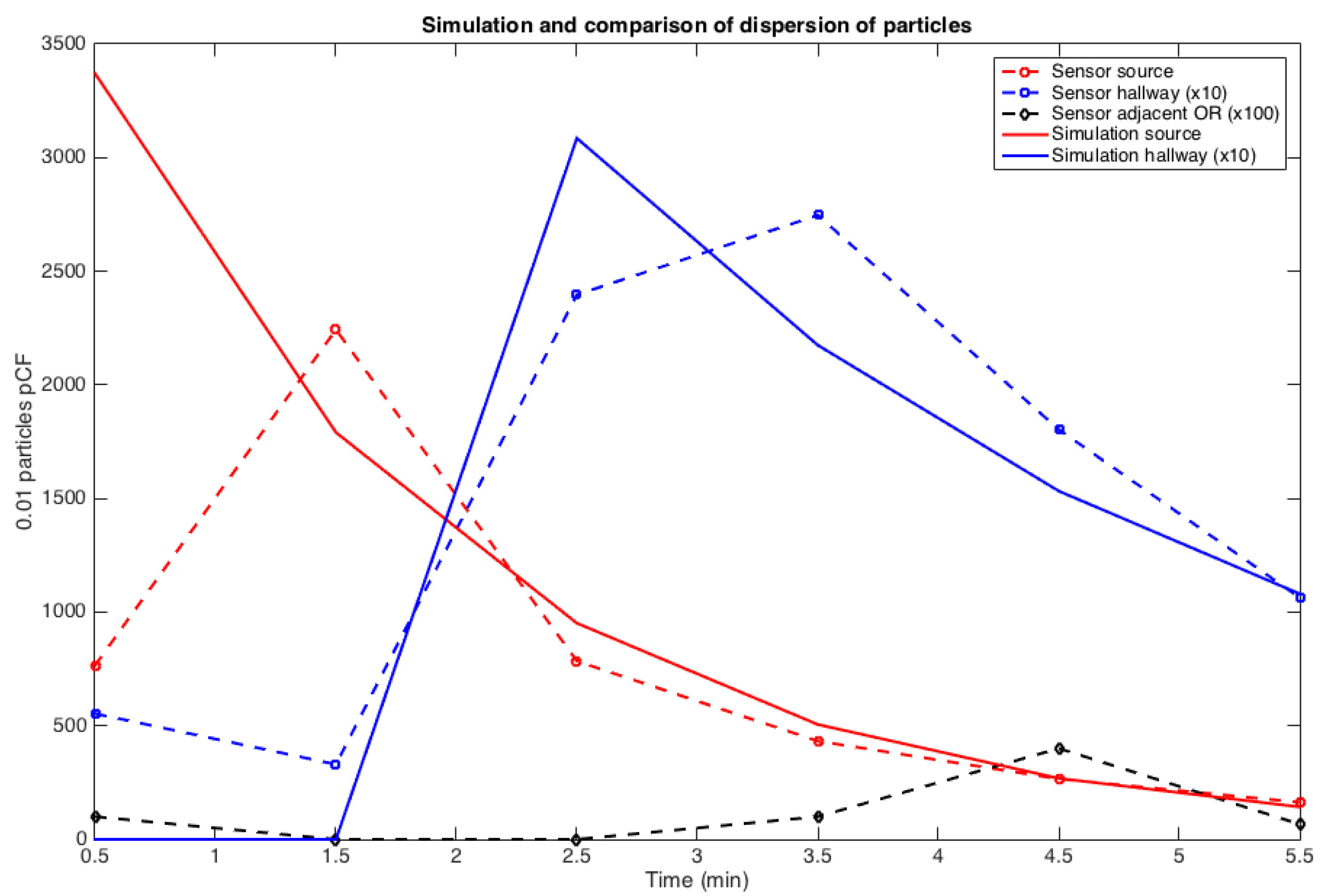
3.3. Transmission Condition and Transport-Diffusion of Particles at the Surgical Suite scale
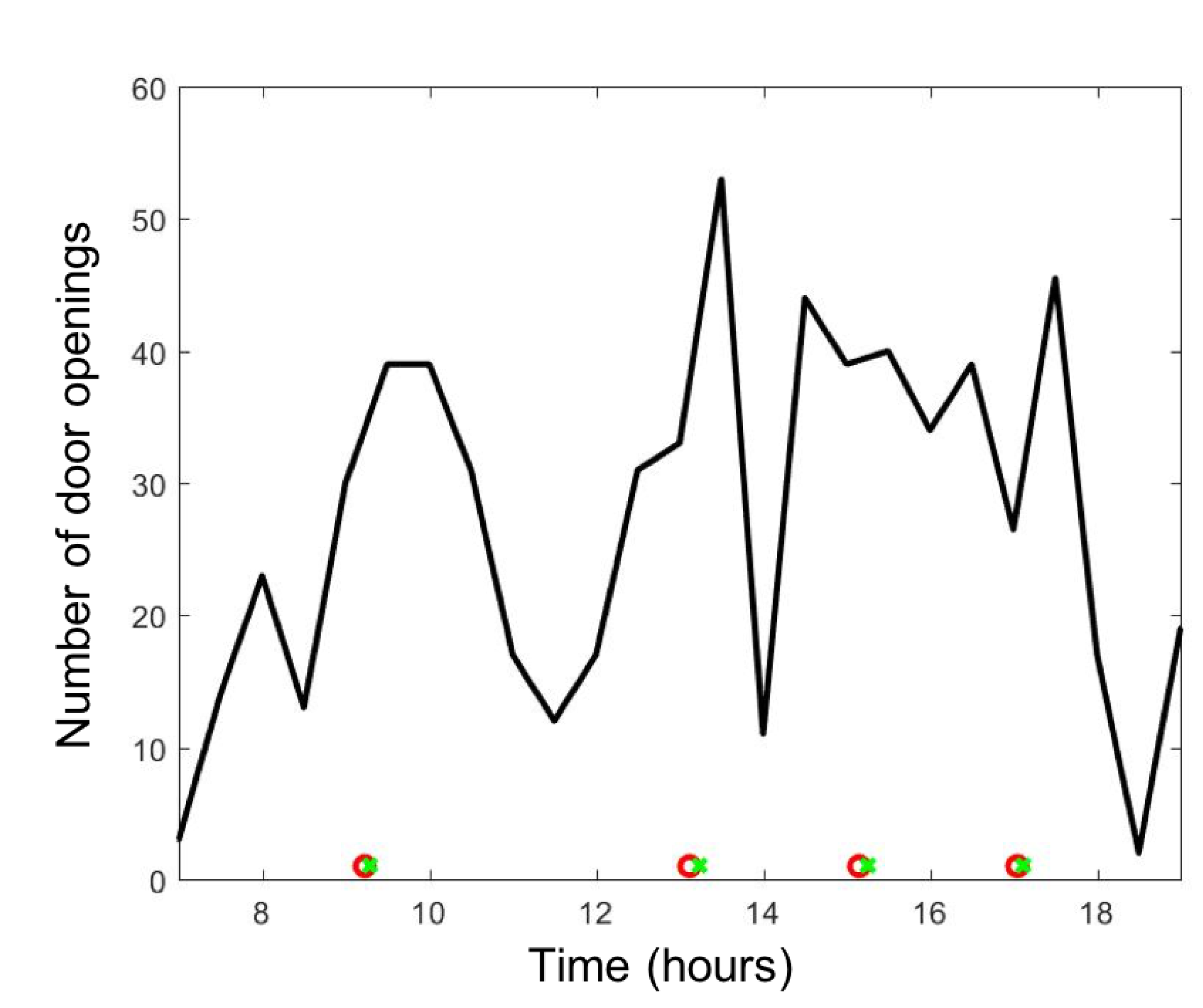
3.4. Clinical Agent-Based Model of Hazardous Airborne Particles
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef] [PubMed]
- Bourouiba, L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA 2020, 323, 1837–1838. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Passarini, F.; Gennaro, G.D.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Gilio, A.D.; Piscitelli, P.; Miani, A. Airborne Transmission Route of COVID-19: Why 2 Meters/6 Feet of Inter-Personal Distance Could Not Be Enough. Int. J. Environ. Res. Public Health 2020, 17, 2932. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Hu, X.; Yan, L.; Zhu, X. Review Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among the gynecologists. J. Cancer 2019, 10, 2788–2799. [Google Scholar] [CrossRef] [PubMed]
- Barrett, W.; Garber, S. Surgical smoke—A review of the literature: Is this just a lot of hot air? Surg. Endosc. 2003, 17, 979–987. [Google Scholar] [CrossRef]
- Hill, D.; O’Neill, J.; Powell, R.; Oliver, D. Surgical smoke—A health hazard in the operating theatre: A study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units. J. Plast Reconstr. Aesthet Surg. 2012, 65, 911–916. [Google Scholar] [CrossRef]
- Steege, A.; Boiano, J.; Sweeney, M. Secondhand smoke in the operating room? Precautionary practices lacking for surgical smoke. Am. J. Ind. Med. 2016, 59, 1020–1031. [Google Scholar] [CrossRef]
- Nezhat, C.; Winer, W.; Nezhat, F. Smoke from Laser Surgery: Is there a health hazard? Lasers Surg. Med. 1987, 7, 376–382. [Google Scholar] [CrossRef]
- Garden, J.; O’Banion, K.; Shelnitz, L.; Pinski, K.S.; Bakus, A.D.; Reichmann, M.E.; Sundberg, J.P. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. J. Am. Med. Assoc. 1988, 259, 1199–1202. [Google Scholar] [CrossRef]
- Romano, F.; Gusten, J.; De Antonellis, S.; Joppolo, C.M. Electrosurgical Smoke: Ultrafine Particle Measurements and Work Environment Quality in Different Operating Theatres. Int. J. Environ. Res. Public Health 2017, 14, 137. [Google Scholar] [CrossRef]
- Sisler, J.D.; Shaffer, J.; Soo, J.C.; LeBouf, R.F.L.; Harper, M.; Qian, Y.; Lee, T. In vitro toxicological evaluation of surgical smoke from human tissue. J. Occup. Med. Toxicol. 2018, 13. [Google Scholar] [CrossRef]
- Wu, X.; Nethery, R.C.; Sabath, B.M.; Braun, D.; Dominici, F. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. medRxiv 2020. [Google Scholar] [CrossRef]
- Long, Y.; Hu, T.; Liu, L.; Chen, R.; Guo, Q.; Yang, L.; Cheng, Y.; Huang, J.; Du, L. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J. Evid.-Based Med. 2020, 13, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bałazy, A.; Toivola, M.; Adhikari, A.; Sivasubramani, S.K.; Reponen, T.; Grinshpun, S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am. J. Infect. Control 2006, 34, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Stocks, G.W.; Self, S.D.; Thompson, B.; Adame, X.A.; O’Connor, D.P. Predicting bacterial populations based on airborne particulates: A study performed in nonlaminar flow operating rooms during joint arthroplasty surgery. Am. J. Infect. Control 2010, 38, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Cristina, M.L.; Spagnolo, A.M.; Sartini, M.; Panatto, D.; Gasparini, R.; Orlando, P.; Ottria, G.; Perdelli, F. Can particulate air sampling predict microbial load in operating theatres for arthroplasty? PLoS ONE 2012, 7, e52809. [Google Scholar] [CrossRef]
- Lu, J.; Gu, J.; Li, K.; Xu, C.; Su, W.; Lai, Z.; Zhou, D.; Yu, C.; Xu, B.; Yang, Z. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1628. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 2020, 323, 1610–1612. [Google Scholar] [CrossRef]
- Meeuwsen, F.; Guédon, A.; Arkenbout, E.; van der Elst, M.; Dankelman, J.; van den Dobbelsteen, J. The Art of Electrosurgery: Trainees and Experts. Surg. Innov. 2017, 24, 373–378. [Google Scholar] [CrossRef]
- Tang, J.; Nicolle, A.; Pantelic, J.; Klettner, C.; Su, R.; Kalliomaki, P.; Saarinen, P.; Koskela, H.; Reijula, K.; Mustakallio, P.; et al. Different types of door-opening motions as contributing factors to containment failures in hospital isolation rooms. PLoS ONE 2013, 8, e66663. [Google Scholar] [CrossRef]
- Eames, I.; Shoaib, D.; Klettner, C.; Taban, V. Movement of airborne contaminants in a hospital isolation room. J. R. Soc. Interface 2009, 6, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, E.S.; Grosskopf, K.R. Airflow patterns due to door motion and pressurization in hospital isolation rooms. Sci. Technol. Built Environ. Vol. 2016, 22, 379–384. [Google Scholar] [CrossRef]
- Cohen, E.; Bellville, J.; Brown, B.J. Anesthesia, pregnancy, and miscarriage: A study of operating room nurses and anesthetists. Anesthesiology 1971, 35, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, S.; Heinzerling, D.; Choi, J.I.; Persily, A. Multizone Modeling of Strategies to Reduce the Spread of Airborne Infectious Agents in Healthcare Facilities. Build. Environ. 2013, 60, 105–115. [Google Scholar] [CrossRef]
- Awbi, H.B. Ventilation of Buildingsy, 2nd ed.; Spon Press: London, UK, 2003. [Google Scholar]
- Romano, F.; Aroccoa, L.; Gustenb, J.; Joppoloa, C.M. Numerical and experimental analysis of airborne particles control in an operating theater. Build. Environ. 2015, 89, 369–379. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T. Editorial: Indoor Air Quality and Health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef]
- Joerger, G.; Rambourg, J.; Gaspard-Boulinc, H.; Conversy, S.; Bass, B.L.; Dunkin, B.J.; Garbey, M. A Cyber-Physical System to Improve the Management of a Large Suite of Operating Rooms. ACM Trans. Cyber-Phys. Syst. 2018, 2, 34. [Google Scholar] [CrossRef]
- Huang, A.Y.; Joerger, G.; Fikfak, V.; Salmon, R.; Dunkin, B.J.; Bass, B.L.; Garbey, M. The SmartOR: A distributed sensor network to improve operating room efficiency. Surg. Endosc. 2017, 31, 3590–3595. [Google Scholar] [CrossRef]
- Huang, A.Y.; Joerger, G.; Salmon, R.; Dunkin, B.; Sherman, V.; Bass, B.L.; Garbey, M. A robust and non-obtrusive automatic event tracking system for operating room management to improve patient care. Surg. Endosc. 2016, 30, 3638–3645. [Google Scholar] [CrossRef]
- Garbey, M.; Joerger, G.; Rambourg, J.; Dunkin, B.; Bass, B. Multiscale Modeling of Surgical Flow in a Large Operating Room Suite: Understanding the Mechanism of Accumulation of Delays in Clinical Practice. Procedia Comput. Sci. 2017, 108, 1863–1872. [Google Scholar] [CrossRef]
- National Research Council. Indoor Pollutants; National Academies: Washington, DC, USA, 1981. [Google Scholar]
- Semple, S.; Ibrahim, A.; Apsley, A.; Steiner, M.; Turner, S. Using a new, low-cost air quality sensor to quantify second-hand smoke (SHS) levels in homes. Tob. Control 2015, 2, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Eames, I.; Li, Y.; Taha, Y.; Wilson, P.; Bellingan, G.; Ward, K.; Breuer, J. Door-opening motion can potentially lead to a transient breakdown in negative-pressure isolation conditions: The importance of vorticity and buoyancy airflows. J. Hosp. Infection 2005, 61, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Garbey, M.; Joerger, G.; Huang, A.; Salmon, R.; Kim, J.; Sherman, V.; Dunkin, B.; Bass, B. An intelligent hospital operating room to improve patient health care. J. Comput. Surg. 2015, 2, 1–10. [Google Scholar] [CrossRef][Green Version]
- Weld, K.J.; Dryer, S.; Ames, C.D.; Cho, K.; Hogan, C.; Lee, M.; Biswas, P.; Landman, J. Analysis of Surgical Smoke Produced by Various Energy-Based Instruments and Effect on Laparoscopic Visibility. J. Endourol. 2007, 21, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Flaniken, B. Engineer’s HVAC Handbook; Price: Houston, TX, USA, 2011. [Google Scholar]
- American Society of Anesthesiologists Ad Hoc Committee. Occupational disease among operating room personnel: National study. Anesthesiology 1974, 41, 321–340. [Google Scholar]
- Spence, A.; Cohen, E.; Brown, B.J.; Knill-Jones, R.; Himmelberger, D. Occupational hazards for operating room–based physicians. Analysis of data from the United States and the United Kingdom. JAMA 1977, 238, 955–959. [Google Scholar] [CrossRef]
- Hemminki, K.; Kyyrönen, P.; Lindbohm, M. Spontaneous abortions and malformations in the offspring of nurses exposed to anaesthetic gases, cytostatic drugs, and other potential hazards in hospitals, based on registered information of outcome. J. Epidemiol. Community Health 1985, 39, 141–147. [Google Scholar] [CrossRef]
- Rosenberg, P.; Kirves, A. Miscarriages among operating theatre staff. Acta Anaesthesiol. Scand. Suppl. 1973, 53, 37–42. [Google Scholar]
- Shuhaiber, S.; Koren, G. Occupational exposure to inhaled anesthetic Is it a concern for pregnant women? Can. Fam. Phys. 2000, 46, 2391–2392. [Google Scholar]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Bae, S.; Kim, M.C.; Kim, J.Y.; Cha, H.H.; Lim, J.S.; Jung, J.; Kim, M.J.; Oh, D.K.; Lee, M.K.; Choi, S.H.; et al. Effectiveness of Surgical and Cotton Masks in Blocking SARS-CoV-2: A Controlled Comparison in 4 Patients. Ann. Internal Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, X.; Garg, H.; Qi, M. Decision Support Algorithm for Selecting an Antivirus Mask over COVID-19 Pandemic under Spherical Normal Fuzzy Environment. Int. J. Environ. Res. Public Health 2020, 17, 3407. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Pallavicini, A.; Ruscio, M.; Piscitelli, P.; Colao, A.; Miani, A. Searching for SARS-COV-2 on Particulate Matter: A Possible Early Indicator of COVID-19 Epidemic Recurrence. Int. J. Environ. Res. Public Health 2020, 17, 2986. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.T.; Moschella, A.; Bruna, A.L.; Romano, G.L.; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef] [PubMed]

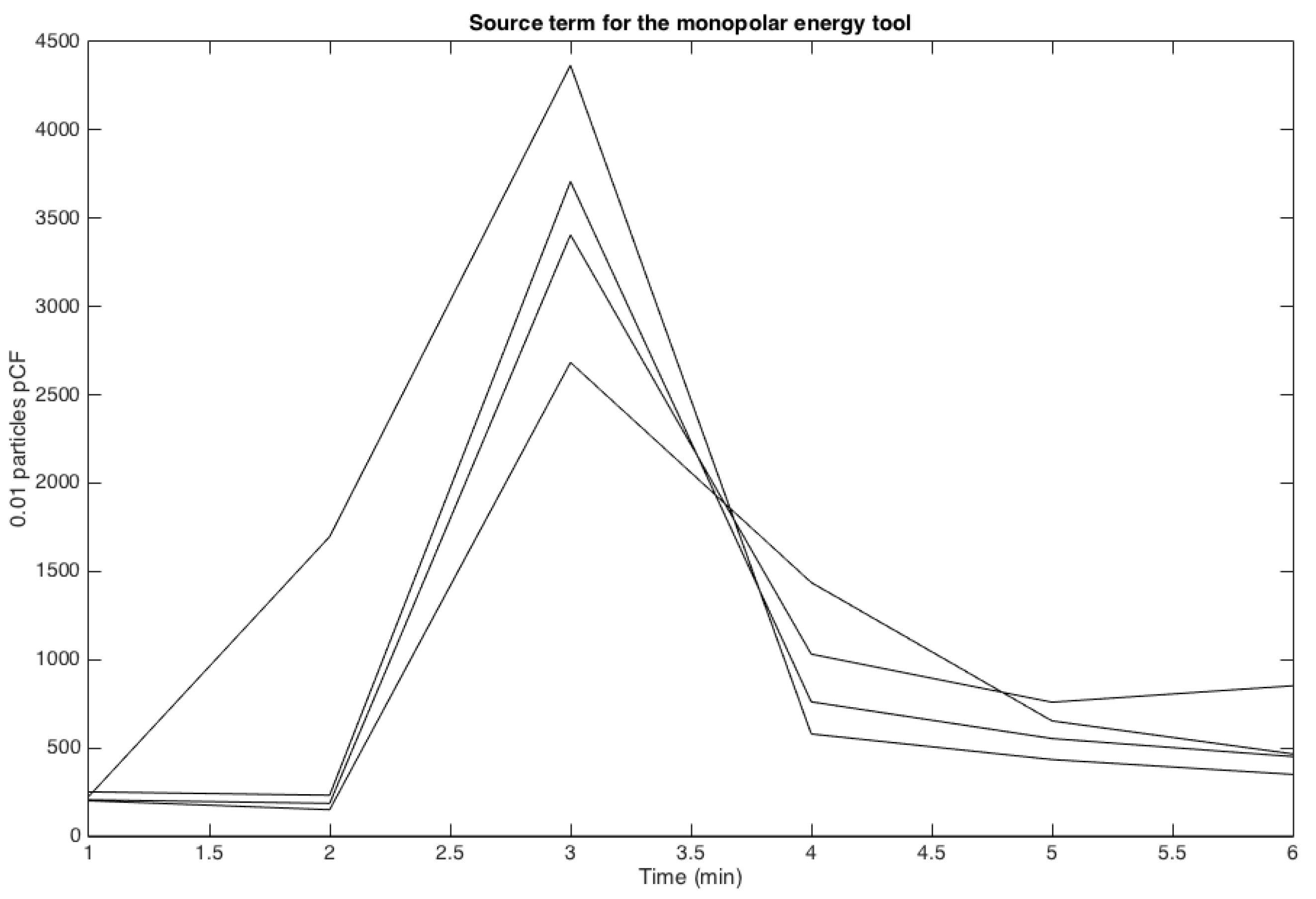
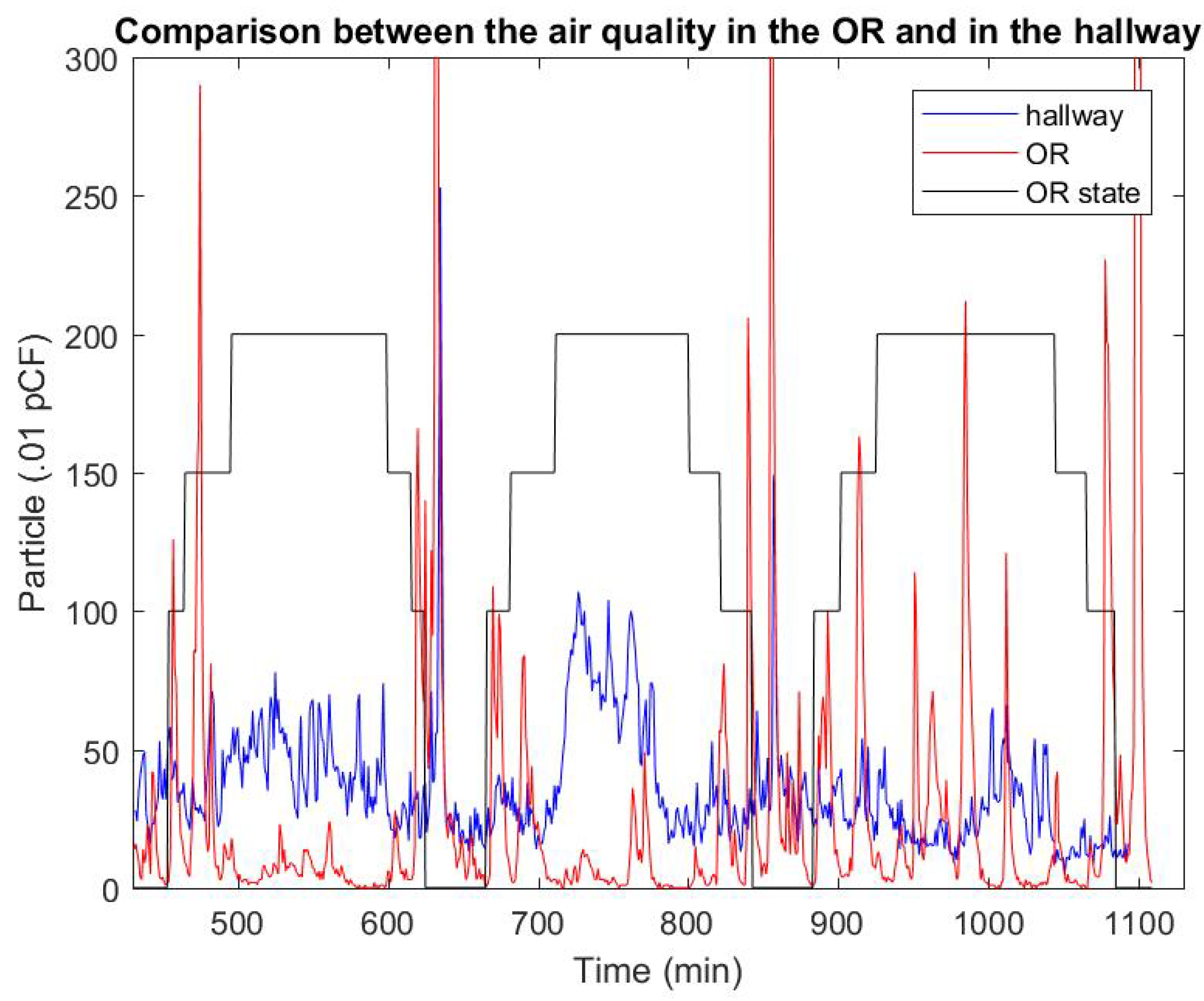
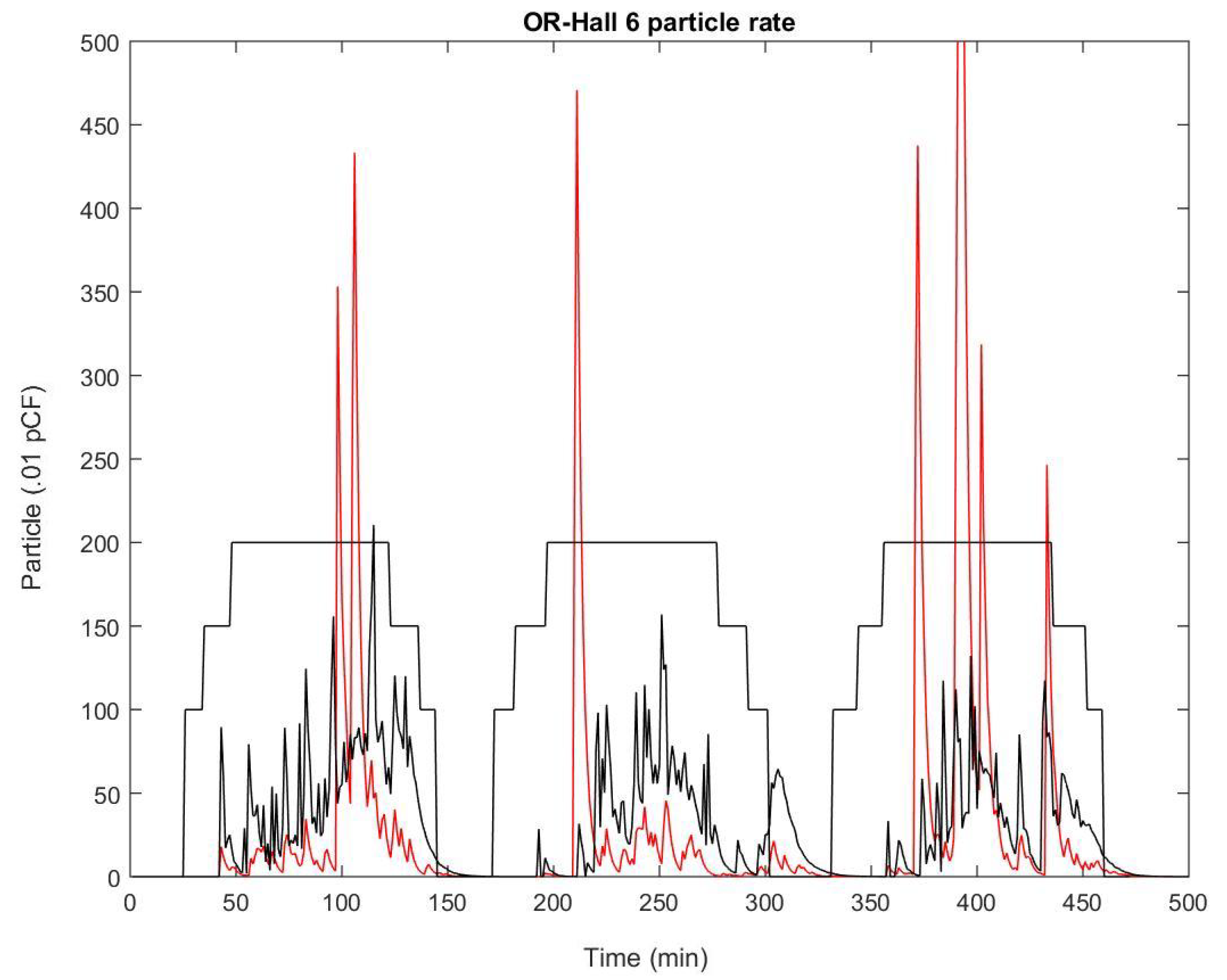
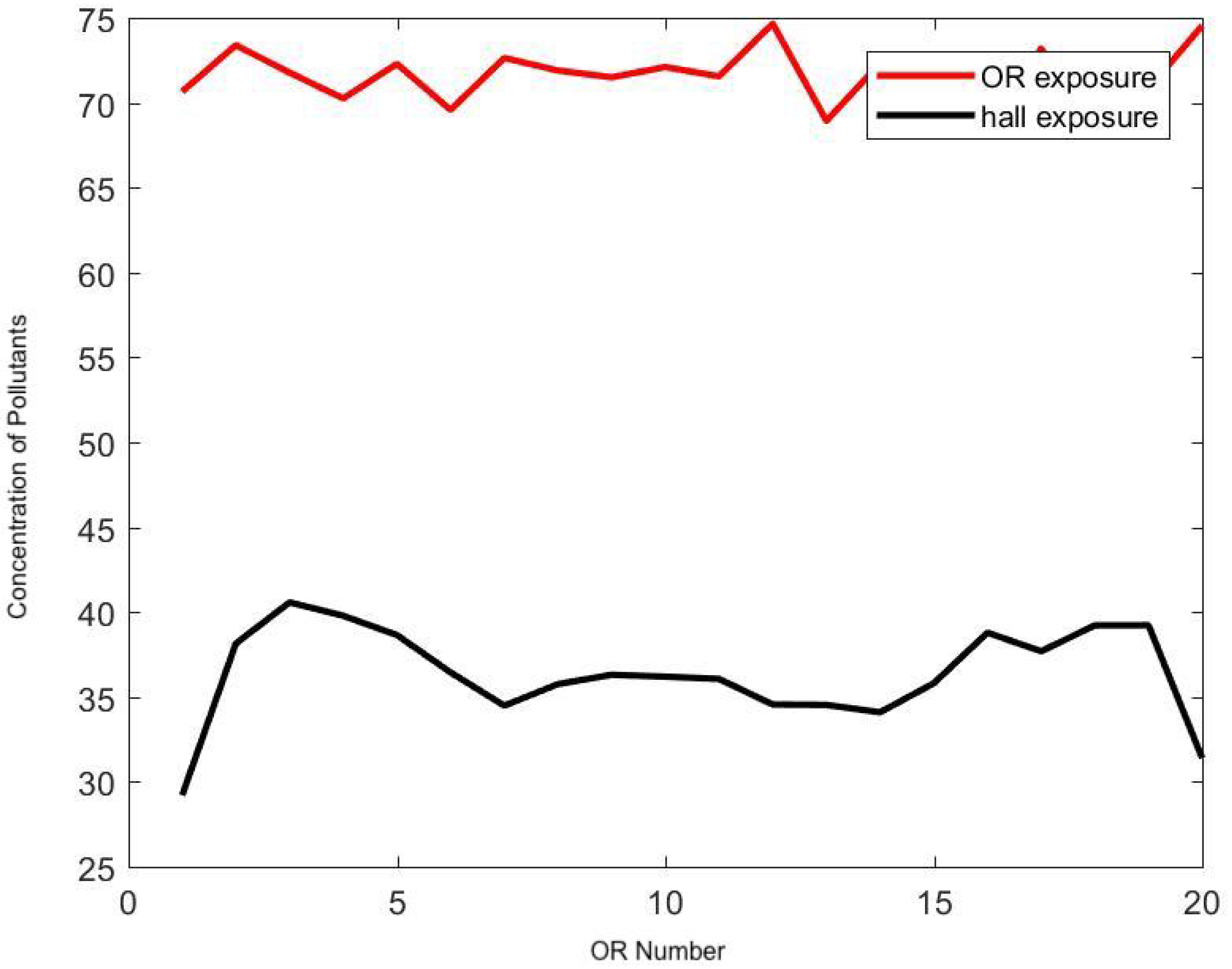

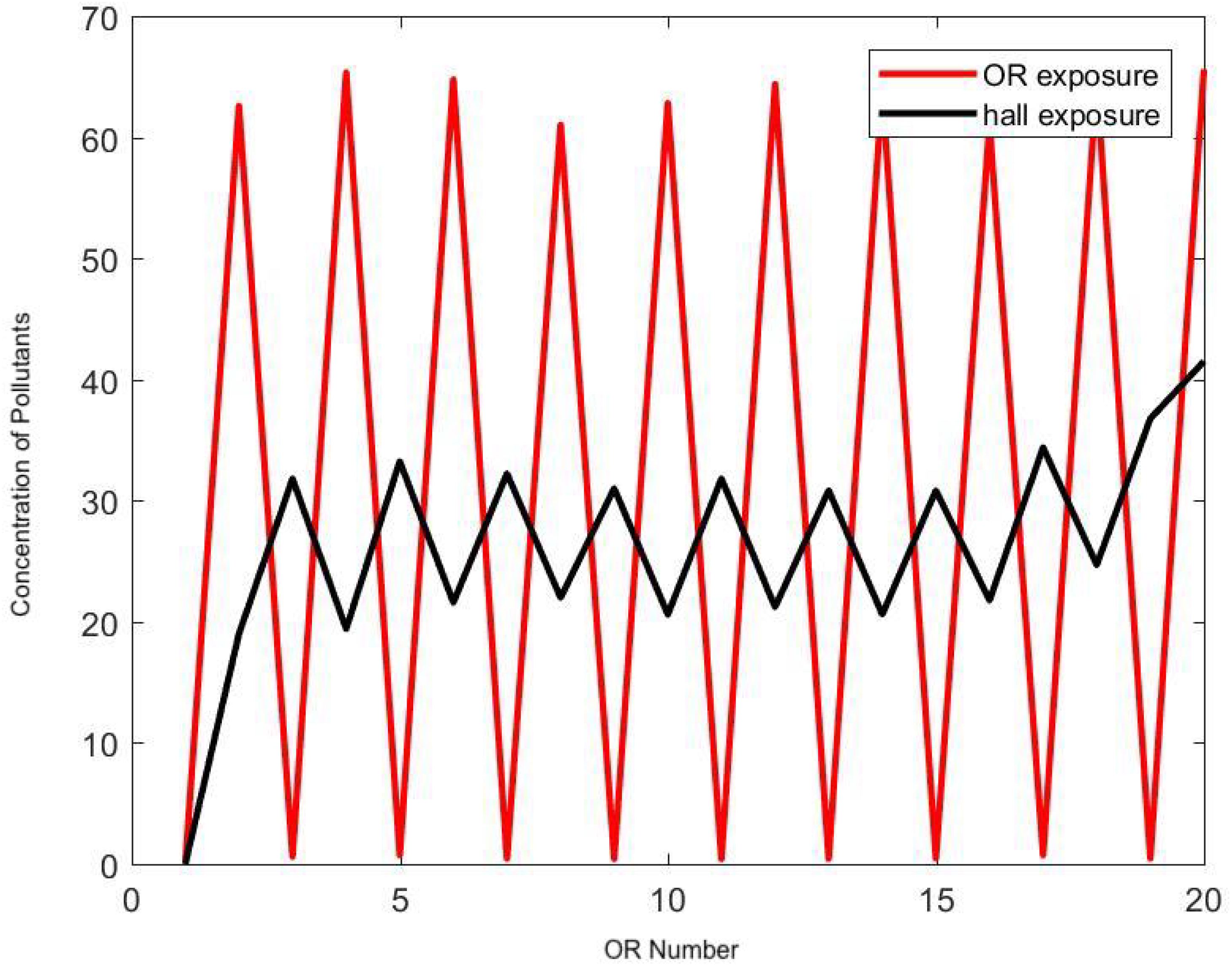
| Amplitude (.01 PpCF) | Monopolar | APC | Harmonic |
|---|---|---|---|
| Mean | 4800 | 1600 | 1050 |
| Standard Deviation | 5100 | 1100 | 870 |
| Diffusion Coefficient | Monopolar | APC | Harmonic |
| Mean | 0.65 | 0.56 | 0.36 |
| Standard Deviation | 0.13 | 0.19 | 0.3 |
| Number of Experiments | 5 | 5 | 4 |
| Boundary | Type | Velocity (m/s) | Temperature (K) |
|---|---|---|---|
| AC inside OR | Velocity inlet | 1.2 | 286.15 |
| A/C hallway | Velocity inlet | 1.2 | 292.15 |
| Top hallway | Velocity inlet | 0.1 | 293.15 |
| Inlet middle OR | Velocity inlet | 4 × 1.0 | 286.15 |
| Inlet right OR | Velocity inlet | 3 × 1.1 | 286.15 |
| Inlet left OR | Velocity inlet | 3 × 0.9 | 286.15 |
| Outlet right OR | Velocity outlet | 0.4 | 290.15 |
| Outlet left OR | Velocity outlet | 0.5 | 290.15 |
| Backdoor | Free | N/A | 290.15 |
| Bottom hallway | Free | N/A | 295.15 |
| OR 2 | Free | N/A | 288.15 |
| Parameter | Measurement | Simulation |
|---|---|---|
| Velocities (inside leaks in m/s) | 3.55 [37] | 3.6 |
| Velocities (in front of the leaks in m/s) | 1.5 | 1.2 |
| Velocities (in front of the door in m/s) | 0.8 | 0.8 |
| Pressure (Pa) | 7.5 | 7.3 |
| 0.56 | 0.6 | |
| Delay sensor 3 (sec) | 10.33 | 10 |
| Delay sensor 1 (sec) | 31 | 29 |
| Parameter | ||||
|---|---|---|---|---|
| Mean | 0.49 | 0.25 | 0.053 | 0.2 |
| Standard Deviation | 0.098 | 0.12 | 0.026 | 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbey, M.; Joerger, G.; Furr, S. A Systems Approach to Assess Transport and Diffusion of Hazardous Airborne Particles in a Large Surgical Suite: Potential Impacts on Viral Airborne Transmission. Int. J. Environ. Res. Public Health 2020, 17, 5404. https://doi.org/10.3390/ijerph17155404
Garbey M, Joerger G, Furr S. A Systems Approach to Assess Transport and Diffusion of Hazardous Airborne Particles in a Large Surgical Suite: Potential Impacts on Viral Airborne Transmission. International Journal of Environmental Research and Public Health. 2020; 17(15):5404. https://doi.org/10.3390/ijerph17155404
Chicago/Turabian StyleGarbey, Marc, Guillaume Joerger, and Shannon Furr. 2020. "A Systems Approach to Assess Transport and Diffusion of Hazardous Airborne Particles in a Large Surgical Suite: Potential Impacts on Viral Airborne Transmission" International Journal of Environmental Research and Public Health 17, no. 15: 5404. https://doi.org/10.3390/ijerph17155404
APA StyleGarbey, M., Joerger, G., & Furr, S. (2020). A Systems Approach to Assess Transport and Diffusion of Hazardous Airborne Particles in a Large Surgical Suite: Potential Impacts on Viral Airborne Transmission. International Journal of Environmental Research and Public Health, 17(15), 5404. https://doi.org/10.3390/ijerph17155404




