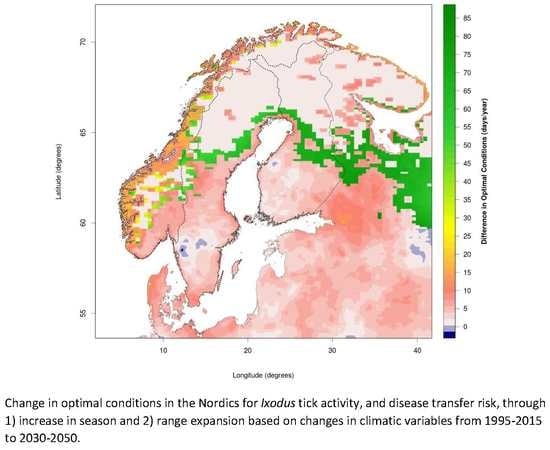
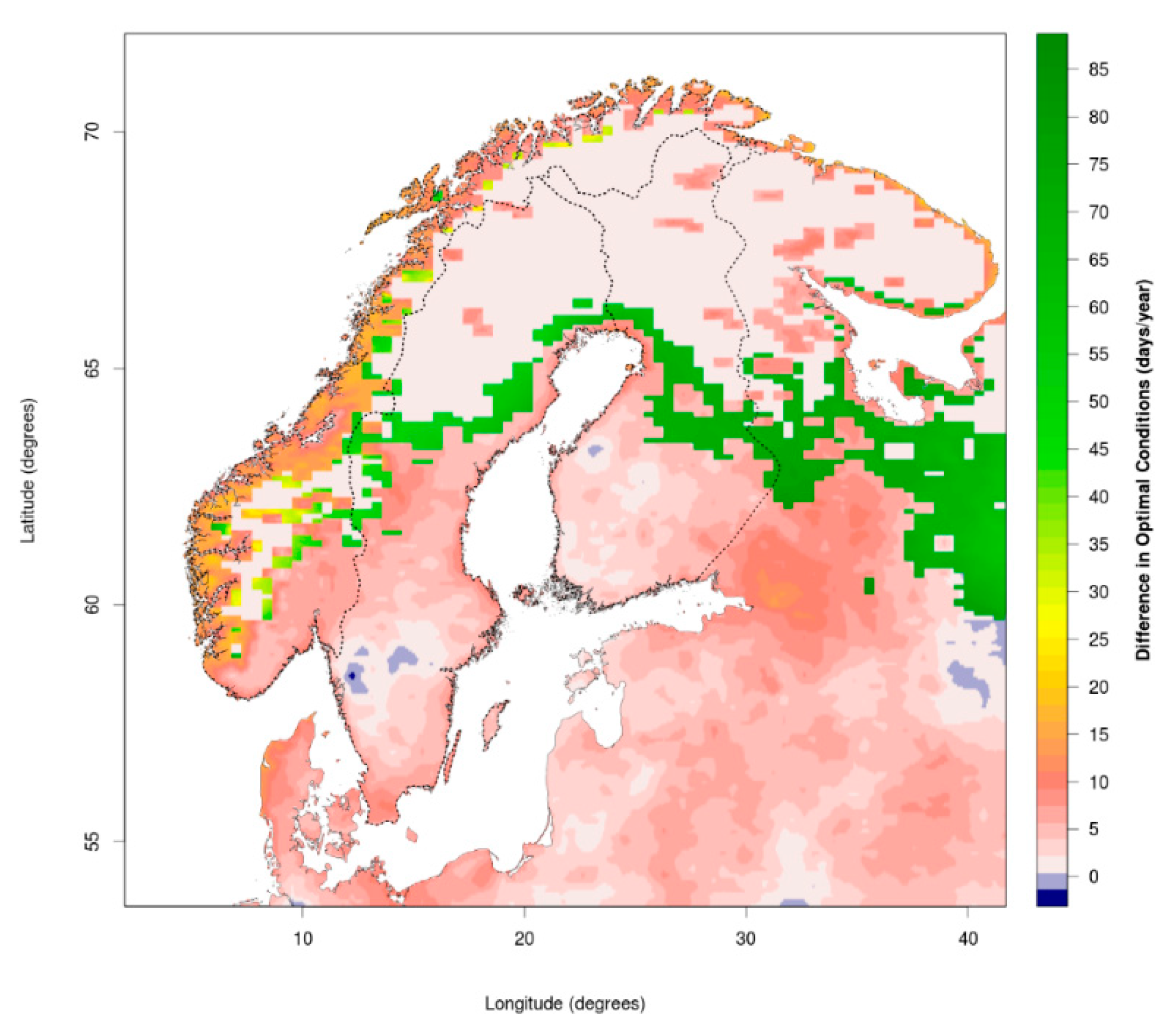
A Mini-Review of Ixodes Ticks Climate Sensitive Infection Dispersion Risk in the Nordic Region
Abstract
:1. Introduction
2. Climate-Sensitive Infections: The Tick
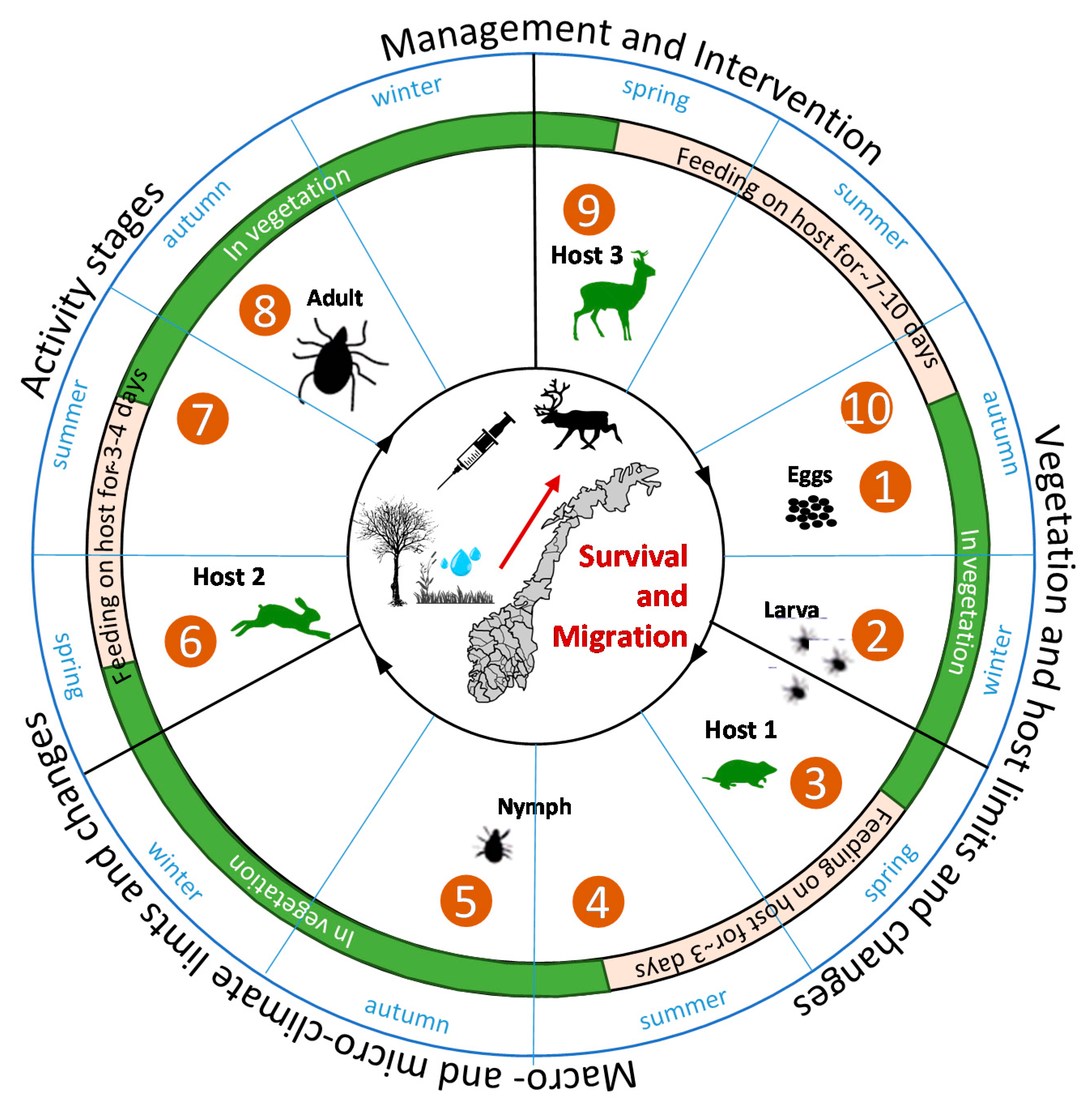
2.1. Current Distribution, Lifecycle and Spatio-Temporal Vulnerabilities for Tick-Borne Infections
2.2. Ticks and Climate Requirements: Results from Literature Review
3. What Does a Changing Climate Mean for Ticks and CSIs?
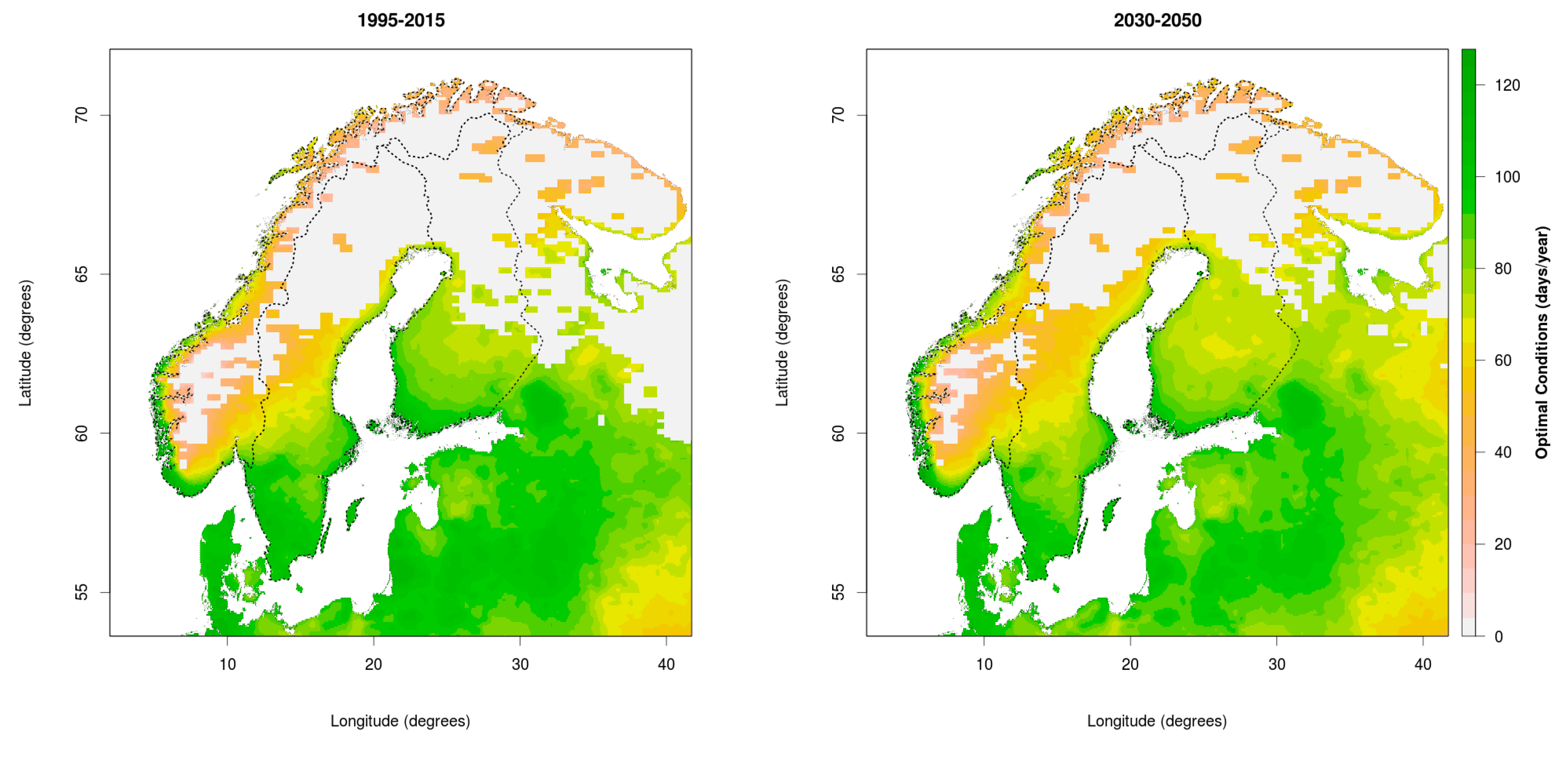
3.1. Changing Distribution Attributed to Climate Change
3.2. Comparing Current and Potential Future Distribution to Observations
4. Effects of Societal and Structural Factors on Tick Occurrences
4.1. Ticks Are a Management and Policy Issue
4.2. Potential Risks for Migrating Reindeer
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Box, J.E.; Colgan, W.T.; Christensen, T.R.; Schmidt, N.M.; Lund, M.; Parmentier, F.-J.W.; Brown, R.; Bhatt, U.S.; Euskirchen, E.S.; Romanovsky, V.E.; et al. Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Omazic, A.; Bylund, H.; Boqvist, S.; Högberg, A.; Björkman, C.; Tryland, M.; Evengård, B.; Koch, A.; Berggren, C.; Malogolovkin, A.; et al. Identifying climate-sensitive infectious diseases in animals and humans in Northern regions. Acta Vet. Scand. 2019, 61, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tryland, M.; Nymo, I.H.; Sánchez Romano, J.; Mørk, T.; Klein, J.; Rockström, U. Infectious Disease Outbreak Associated with Supplementary Feeding of Semi-Domesticated Reindeer. Front. Vet. Sci. 2019, 6, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tryland, M. “Reinpest” og andre epizootier hos rein i Fennoskandia—Et historisk tilbakeblikk. Nor. Veterinærtidsskrift 2014, 126, 154–160. [Google Scholar]
- Lundmark, L. Samernas Skatteland i Norr-Och Västerbotten under 300 år; Institutet för Rättshistorisk Forskning: Stockholm, Sweden, 2006; ISBN 978-91-85190-78-2. [Google Scholar]
- Nordkvist, M. Renens Sjukdomar: Kort Översikt; Lappväsendet. Renforskningen. Småskrift, 4: Stockholm, Sweden, 1960. [Google Scholar]
- Pettersson, J.H.-O.; Shi, M.; Bohlin, J.; Eldholm, V.; Brynildsrud, O.B.; Paulsen, K.M.; Andreassen, Å.; Holmes, E.C. Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci. Rep. 2017, 7, 10870. [Google Scholar] [CrossRef] [Green Version]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Stuen, S. Tick-Borne Fever (Anaplasma phagocytophilum Infection) in Sheep—A Review. J. Vet. Med. Res. 2016, 3, 1062. [Google Scholar]
- Soleng, A.; Kjelland, V. Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in Ixodes ricinus ticks in Brønnøysund in northern Norway. Ticks Tick Borne Dis. 2013, 4, 218–221. [Google Scholar] [CrossRef]
- Mysterud, A.; Easterday, W.; Qviller, L.; Viljugrein, H.; Ytrehus, B. Spatial and seasonal variation in the prevalence of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in questing Ixodes ricinus ticks in Norway. Parasit. Vectors 2013, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Qviller, L.; Grøva, L.; Viljugrein, H.; Klingen, I.; Mysterud, A. Temporal pattern of questing tick Ixodes ricinus density at differing elevations in the coastal region of western Norway. Parasit. Vectors 2014, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliasson, H.; Lindbäck, J.; Nuorti, J.P.; Arneborn, M.; Giesecke, J.; Tegnell, A. The 2000 Tularemia Outbreak: A Case-Control Study of Risk Factors in Disease-Endemic and Emergent Areas, Sweden. Emerg. Infect. Dis. 2002, 8, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Rydén, P.; Björk, R.; Schäfer, M.L.; Lundström, J.O.; Petersén, B.; Lindblom, A.; Forsman, M.; Sjöstedt, A.; Johansson, A. Outbreaks of Tularemia in a Boreal Forest Region Depends on Mosquito Prevalence. J. Infect. Dis. 2012, 205, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehringer, H.; Schacht, E.; Maylaender, N.; Zeman, E.; Kaysser, P.; Oehme, R.; Pluta, S.; Splettstoesser, W.D. Presence of an emerging subclone of Francisella tularensis holarctica in Ixodes ricinus ticks from south-western Germany. Ticks Tick-Borne Dis. 2013, 4, 93–100. [Google Scholar] [CrossRef]
- Paduraru, O.A.; Buffet, J.P.; Cote, M.; Bonnet, S.; Moutailler, S.; Paduraru, V.; Femenia, F.; Eloit, M.; Savuta, G.; Vayssier-Taussat, M. Zoonotic Transmission of Pathogens by Ixodes ricinus Ticks, Romania. Emerg. Infect. Dis. 2012, 18, 2089–2090. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J. Mosquitoes (Diptera: Culicidae), in Contrast to Ticks (Acari: Ixodidae), Do Not Carry Francisella tularensis in a Natural Focus of Tularemia in the Czech Republic. J. Med. Entomol. 1997, 34, 660–663. [Google Scholar] [CrossRef]
- De Keukeleire, M. Epidemiological and Environmental Risk Factors of Tick-Borne Diseases: Combining Hazard and Exposure. Ph.D. Thesis, Université Catholique de Louvain, Louvain-la-Neuve, Belgium, 2018. [Google Scholar]
- Jore, S.; Vanwambeke, S.O.; Viljugrein, H.; Isaksen, K.; Kristoffersen, A.B.; Woldehiwet, Z.; Johansen, B.; Brun, E.; Brun-Hansen, H.; Westermann, S.; et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasit. Vectors 2014, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Fons, F.; Fernández-de-Mera, I.G.; Acevedo, P.; Gortázar, C.; de la Fuente, J. Factors Driving the Abundance of Ixodes ricinus Ticks and the Prevalence of Zoonotic I. ricinus-Borne Pathogens in Natural Foci. Appl. Environ. Microbiol. 2012, 78, 2669–2676. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, E.; Tälleklint, L.; Polfeldt, T. Impact of Climatic Change on the Northern Latitude Limit and Population Density of the Disease-Transmitting European Tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Eisen, L.; Comstedt, P.; Mejlon, H.A.; Lindgren, E.; Bergström, S.; Olsen, B. Risk indicators for the tick Ixodes ricinus and Borrelia burgdorferi sensu lato in Sweden. Med. Vet. Entomol. 2009, 23, 226–237. [Google Scholar] [CrossRef]
- Lindgren, E.; Andersson, Y.; Suk, J.E.; Sudre, B.; Semenza, J.C. Monitoring EU Emerging Infectious Disease Risk Due to Climate Change. Science 2012, 336, 418–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tälleklint, L.; Jaenson, T.G.T. Seasonal Variations in Density of Questing Ixodes ricinus (Acari: Ixodidae) Nymphs and Prevalence of Infection with B. burgdorferi s.l. in South Central Sweden. J. Med. Entomol. 1996, 33, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S. Ixodes ricinus seasonal activity: Implications of global warming indicated by revisiting tick and weather data. Int. J. Med. Microbiol. 2008, 298 (Suppl. 1), 19–24. [Google Scholar] [CrossRef]
- Lindström, A.; Jaenson, T.G.T. Distribution of the Common Tick, Ixodes ricinus (Acari: Ixodidae), in Different Vegetation Types in Southern Sweden. J. Med. Entomol. 2003, 40, 375–378. [Google Scholar] [CrossRef]
- ECDC—European Centre for Disease Prevention and Control and European Food Safety Authority. Ixodes ricinus—Current Known Distribution. Available online: https://www.ecdc.europa.eu/en/publications-data/ixodes-ricinus-current-known-distribution-july-2019 (accessed on 9 March 2020).
- Gilbert, L.; Brunker, K.; Lande, U.; Klingen, I.; Grøva, L. Environmental risk factors for Ixodes ricinus ticks and their infestation on lambs in a changing ecosystem: Implications for tick control and the impact of woodland encroachment on tick-borne disease in livestock. Agric. Ecosyst. Environ. 2017, 237, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Kjær, L.J.; Soleng, A.; Edgar, K.S.; Lindstedt, H.E.H.; Paulsen, K.M.; Andreassen, Å.K.; Korslund, L.; Kjelland, V.; Slettan, A.; Stuen, S.; et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway and Sweden, 2016. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Mysterud, A.; Jore, S.; Østerås, O.; Viljugrein, H. Emergence of tick-borne diseases at northern latitudes in Europe: A comparative approach. Sci. Rep. 2017, 7, 16316. [Google Scholar] [CrossRef] [Green Version]
- Jore, S.; Vanwambeke, S.O.; Slunge, D.; Boman, A.; Krogfelt, K.A.; Jepsen, M.T.; Vold, L. Spatial tick bite exposure and associated risk factors in Scandinavia. Infect. Ecol. Epidemiol. 2020, 10, 1764693. [Google Scholar] [CrossRef]
- MacLeod, J. Ixodes ricinusin Relation to its Physical Environment: II. The Factors Governing Survival and Activity. Parasitology 1935, 27, 123–144. [Google Scholar] [CrossRef]
- Gray, J. Studies on the dynamics of active populations of the sheep tick, Ixodes ricinus L. in Co. Wicklow, Ireland. Acarologia 1984, 25, 167–178. [Google Scholar]
- Perret, J.-L.; Guigoz, E.; Rais, O.; Gern, L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 2000, 86, 554–557. [Google Scholar] [CrossRef]
- Mannelli, A.; Bertolotti, L.; Gern, L.; Gray, J. Ecology of Borrelia burgdorferi sensu lato in Europe: Transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol. Rev. 2012, 36, 837–861. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of Climate Change on Ticks and Tick-Borne Diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 1–12. [Google Scholar] [CrossRef]
- Raffel, T.R.; Romansic, J.M.; Halstead, N.T.; McMahon, T.A.; Venesky, M.D.; Rohr, J.R. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 2013, 3, 146–151. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Lindgren, E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis. 2011, 2, 44–49. [Google Scholar] [CrossRef]
- Dautel, H.; Knülle, W. Cold hardiness, supercooling ability and causes of low-temperature mortality in the soft tick, Argas reflexus, and the hard tick, Ixodes ricinus (Acari: Ixodoidea) from Central Europe. J. Insect Physiol. 1997, 43, 843–854. [Google Scholar] [CrossRef]
- Campbell, J. The Life History and Development of the Sheep Tick Ixodes ricinus Linnaeus in Scotland, under Natural and Controlled Conditions. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1948. [Google Scholar]
- Kahl, O. Investigations on the Water Balance of Ticks (Acari: Ixodoidea) in the Course of Their Postembryonic Development with Special Reference to Active Water Vapour Uptake of the Engorgedphases. Ph.D. Thesis, Free University, Berlin, Germany, 1989. (In German). [Google Scholar]
- Kowalski, J. Die Seroprävalenz der Humanen Granulozytären Anaplasmose im Raum Berlin-Brandenburg und Ihre Beeinflussung Durch das Klima: Seroprevalence of Human Granulocytic Anaplasmosis in Berlin/Brandenburg, Germany: An Eight Year Survey. Ph.D. Thesis, Freie Universitat Berlin, Berlin, Germany, 2010. [Google Scholar]
- Süss, J.; Klaus, C.; Gerstengarbe, F.; Werner, P.C. What Makes Ticks Tick? Climate Change, Ticks, and Tick-Borne Diseases. J. Travel Med. 2008, 15, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Hvidsten, D.; Frafjord, K.; Gray, J.S.; Henningsson, A.J.; Jenkins, A.; Kristiansen, B.E.; Lager, M.; Rognerud, B.; Slåtsve, A.M.; Stordal, F.; et al. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis. 2020, 11, 101388. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Walter, M.; Vogelgesang, J.R.; Didyk, Y.M.; Fu, S.; Kahl, O. Geographical distribution, climate adaptation and vector competence of the Eurasian hard tick Haemaphysalis concinna. Ticks Tick Borne Dis. 2018, 9, 1080–1089. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.-C.; Golovljova, I.; Jaenson, T.G.T.; Jensen, J.-K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, L. 11. How landscapes shape Lyme borreliosis risk. In Ecology and Control of Vector-Borne Diseases; Braks, M.A.H., van Wieren, S.E., Takken, W., Sprong, H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; Volume 4, pp. 161–171. ISBN 978-90-8686-293-1. [Google Scholar]
- Rousseau, R.; McGrath, G.; McMahon, B.J.; Vanwambeke, S.O. Multi-criteria Decision Analysis to Model Ixodes ricinus Habitat Suitability. EcoHealth 2017, 14, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Burks, C.S.; Stewart, R.L.; Needham, G.R.; Lee, R.E. The role of direct chilling injury and inoculative freezing in cold tolerance of Amblyomma americanum, Dermacentor variabilis and Ixodes scapularis. Physiol. Entomol. 1996, 21, 44–50. [Google Scholar] [CrossRef]
- Hanssen-Bauer, I.; Førland, E.J.; Haddeland, I.; Hisdal, H.; Mayer, S.; Nesje, A.; Nilsen, J.E.Ø.; Sandven, S.; Sandø, A.B.; Sorteberg, A.; et al. Klima i Norge 2100. Kunnskapsgrunnlag for Klimatilpasning Oppdatert i 2015; Miljødirektoratet: Oslo, Norway, 2015; p. 204. [Google Scholar]
- Vikhamar-Schuler, D.; Beldring, S.; Førland, E.J.; Roald, L.A.; Skaugen, T.E. Snow Cover and Snow Water Equivalent in Norway: -Current Conditions (1961–1990) and Scenarios for the Future (2071–2100), Met.no report; Norwegian Meteorological Institute: Oslo, Norway, 2006; p. 35. [Google Scholar]
- Kölling, C. Forests under the influence of climate change—Chances and limitations of adaptation in forestry. In Warning Signal Climate. Health Risks for Plants, Animals and Human Beings; Loz’an, J., Graßl, H., Jendritzky, G., Karbe, L., Reise, K., Eds.; Wissenschaftliche Auswertungen: Hamburg, Germany, 2008. [Google Scholar]
- Høgda, K.A.; Tømmervik, H.; Karlsen, S.R. Trends in the Start of the Growing Season in Fennoscandia 1982–2011. Remote Sens. 2013, 5, 4304–4318. [Google Scholar] [CrossRef] [Green Version]
- Park, T.; Ganguly, S.; Tømmervik, H.; Euskirchen, E.S.; Høgda, K.-A.; Karlsen, S.R.; Brovkin, V.; Nemani, R.R.; Myneni, R.B. Changes in growing season duration and productivity of northern vegetation inferred from long-term remote sensing data. Environ. Res. Lett. 2016, 11, 084001. [Google Scholar] [CrossRef]
- Elmberg, J.; Berg, C.; Lerner, H.; Waldenström, J.; Hessel, R. Potential disease transmission from wild geese and swans to livestock, poultry and humans: A review of the scientific literature from a One Health perspective. Infect. Ecol. Epidemiol. 2017, 7, 1300450. [Google Scholar] [CrossRef]
- Tälleklint, L.; Jaenson, T.G.T. Increasing Geographical Distribution and Density of Ixodes ricinus (Acari: Ixodidae) in Central and Northern Sweden. J. Med. Entomol. 1998, 35, 521–526. [Google Scholar] [CrossRef]
- Jore, S.; Viljugrein, H.; Hofshagen, M.; Brun-Hansen, H.; Kristoffersen, A.B.; Nygård, K.; Brun, E.; Ottesen, P.; Sævik, B.K.; Ytrehus, B. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasit. Vectors 2011, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Handeland, K.; Qviller, L.; Vikøren, T.; Viljugrein, H.; Lillehaug, A.; Davidson, R.K. Ixodes ricinus infestation in free-ranging cervids in Norway—A study based upon ear examinations of hunted animals. Vet. Parasitol. 2013, 195, 142–149. [Google Scholar] [CrossRef]
- Soleng, A.; Edgar, K.S.; Paulsen, K.M.; Pedersen, B.N.; Okbaldet, Y.B.; Skjetne, I.E.B.; Gurung, D.; Vikse, R.; Andreassen, Å.K. Distribution of Ixodes ricinus ticks and prevalence of tick-borne encephalitis virus among questing ticks in the Arctic Circle region of northern Norway. Ticks Tick Borne Dis. 2018, 9, 97–103. [Google Scholar] [CrossRef]
- Hvidsten, D.; Stuen, S.; Jenkins, A.; Dienus, O.; Olsen, R.S.; Kristiansen, B.-E.; Mehl, R.; Matussek, A. Ixodes ricinus and Borrelia prevalence at the Arctic Circle in Norway. Ticks Tick Borne Dis. 2014, 5, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Mysterud, A.; Easterday, W.R.; Stigum, V.M.; Aas, A.B.; Meisingset, E.L.; Viljugrein, H. Contrasting emergence of Lyme disease across ecosystems. Nat. Commun. 2016, 7, 11882. [Google Scholar] [CrossRef]
- Rosà, R.; Andreo, V.; Tagliapietra, V.; Baráková, I.; Arnoldi, D.; Hauffe, H.; Manica, M.; Rosso, F.; Blaňarová, L.; Bona, M.; et al. Effect of Climate and Land Use on the Spatio-Temporal Variability of Tick-Borne Bacteria in Europe. Int. J. Environ. Res. Public Health 2018, 15, 732. [Google Scholar] [CrossRef] [Green Version]
- Setten, G.; Austrheim, G. Changes in land use and landscape dynamics in mountains of northern Europe: Challenges for science, management and conservation. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2012, 8, 287–291. [Google Scholar] [CrossRef]
- Steigedal, H.; Loe, L.; Grøva, L.; Mysterud, A. The effect of sheep Ovis aries presence on the abundance of ticks Ixodes ricinus. Acta Agric. Scand. Sect. Anim. Sci. 2013, 63, 111–120. [Google Scholar] [CrossRef]
- Millins, C.; Gilbert, L.; Medlock, J.; Hansford, K.; Thompson, D.B.; Biek, R. Effects of conservation management of landscapes and vertebrate communities on Lyme borreliosis risk in the United Kingdom. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160123. [Google Scholar] [CrossRef] [Green Version]
- Bjørkhaug, H.; Rønningen, K. Crisis? What Crisis? Marginal Farming, Rural Communities and Climate Robustness: The Case of Northern Norway. Int. Jrnl. Soc. Agr. Food 2014, 21, 51–69. [Google Scholar]
- Risvoll, C. Adaptive Capacity and Adaptation Processes in Norwegian pastoral Communities in the Face of Environmental and Societal Change. Ph.D. Thesis, Nord University, Bodø, Norway, 2015. [Google Scholar]
- Hovelsrud, G.K.; Risvoll, C.; Riseth, J.Å.; Tømmervik, H.; Omazic, A.; Albihn, A. Chapter 6. Reindeer herding and coastal pastures: Climate Change Multiple Stressors. In Nordic Perspectives on the Responsible Development of the Arctic: Pathways for Action; Nord, D., Ed.; Springer: Berlin, Germany. (in review)
- Stuen, S.; Grøva, L.; Granquist, E.G.; Sandstedt, K.; Olesen, I.; Steinshamn, H. A comparative study of clinical manifestations, haematological and serological responses after experimental infection with Anaplasma phagocytophilum in two Norwegian sheep breeds. Acta Vet. Scand. 2011, 53, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Risvoll, C.; Hovelsrud, G.K. Pasture access and adaptive capacity in reindeer herding districts in Nordland, Northern Norway. Polar J. 2016, 6, 87–111. [Google Scholar] [CrossRef]
- Næss, M.W. Climate change, risk management and the end of Nomadic pastoralism. Int. J. Sustain. Dev. World Ecol. 2013, 20, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Vikhamar-Schuler, D.; Isaksen, K.; Haugen, J.E.; Tømmervik, H.; Luks, B.; Schuler, T.V.; Bjerke, J.W. Changes in Winter Warming Events in the Nordic Arctic Region. J. Clim. 2016, 29, 6223–6244. [Google Scholar] [CrossRef]
- Stuen, S. Experimental tick-borne fever infection in reindeer (Rangifer tarandus tarandus). Vet. Rec. 1996, 138, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romano, J.; Grund, L.; Obiegala, A.; Nymo, I.H.; Ancin-Murguzur, F.J.; Li, H.; Król, N.; Pfeffer, M.; Tryland, M. A Multi-Pathogen Screening of Captive Reindeer (Rangifer tarandus) in Germany Based on Serological and Molecular Assays. Front. Vet. Sci. 2019, 6, 461. [Google Scholar] [CrossRef]
- Haigh, J.C.; Gerwing, V.; Erdenebaatar, J.; Hill, J.E. A novel clinical syndrome and detection of anaplasma ovis in mongolian reindeer (rangifer tarandus). J. Wildl. Dis. 2008, 44, 569–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuen, S.; Oppegaard, A.S.; Bergström, K.; Moum, T. Anaplasma phagocytophilum Infection in North Norway. The First Laboratory Confirmed Case. Acta Vet. Scand. 2005, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Løwe, K. Norge Kan få Flere og Farligere Flått. Available online: https://forskning.no/insekter-klima-nmbu-norges-miljo-og-biovitenskapelige-universitet/norge-kan-fa-flere-og-farligere-flatt/1333977 (accessed on 20 January 2020).
- Parkinson, A.J.; Evengard, B.; Semenza, J.C.; Ogden, N.; Børresen, M.L.; Jim, B.; Michael, B.; Anders, S.; Magnus, E.; David, M.; et al. Climate change and infectious diseases in the Arctic: Establishment of a circumpolar working group. Int. J. Circumpolar Health 2014, 73. [Google Scholar] [CrossRef] [PubMed]
| CSI | Identified Climatic Limits | Sources |
|---|---|---|
| Anaplasmosis (NO: Sjodogg) and Babesiosis (NO: Blodpiss) | Temperature below 10 °C no development or eggs −15 °C, −18.9 °C for 24 h and −10 °C for 30 days lethal 5 °C: limit for activity Tavg <5 °C for >170 days 7 °C host-seeking activity (questing) for nymphs and adults 10 °C host-seeking activity (questing) for larvae 6.7 °C growing season mean Relative humidity 80–85% 24 °C = limit for occurrence and activity. 15–17 °C = reduced activity >30 °C induces diapause 35 °C = questing limit Precipitation >90 mm per month in April–May reduces egg deposition. | [12,20,36,39,40,41,42,43,44] |
| Growing season duration: 175–180 days; 160 days; 170 days | [39,45] | |
| Snow cover duration: Below 125 days > present; Above 175 days > absent; Above 150 days > absent | [13,20,23] | |
| Tularemia (NO: Harepest) | Temperature: number of days below −12 °C Growing season duration: 160–180 days Snow cover duration: absent above 175 days of snow cover | [46,47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Oort, B.E.H.; Hovelsrud, G.K.; Risvoll, C.; Mohr, C.W.; Jore, S. A Mini-Review of Ixodes Ticks Climate Sensitive Infection Dispersion Risk in the Nordic Region. Int. J. Environ. Res. Public Health 2020, 17, 5387. https://doi.org/10.3390/ijerph17155387
van Oort BEH, Hovelsrud GK, Risvoll C, Mohr CW, Jore S. A Mini-Review of Ixodes Ticks Climate Sensitive Infection Dispersion Risk in the Nordic Region. International Journal of Environmental Research and Public Health. 2020; 17(15):5387. https://doi.org/10.3390/ijerph17155387
Chicago/Turabian Stylevan Oort, Bob E. H., Grete K. Hovelsrud, Camilla Risvoll, Christian W. Mohr, and Solveig Jore. 2020. "A Mini-Review of Ixodes Ticks Climate Sensitive Infection Dispersion Risk in the Nordic Region" International Journal of Environmental Research and Public Health 17, no. 15: 5387. https://doi.org/10.3390/ijerph17155387
APA Stylevan Oort, B. E. H., Hovelsrud, G. K., Risvoll, C., Mohr, C. W., & Jore, S. (2020). A Mini-Review of Ixodes Ticks Climate Sensitive Infection Dispersion Risk in the Nordic Region. International Journal of Environmental Research and Public Health, 17(15), 5387. https://doi.org/10.3390/ijerph17155387