Effects of Ibuprofen Intake in Muscle Damage, Body Temperature and Muscle Power in Paralympic Powerlifting Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Measurements
2.4. Statistical Analysis
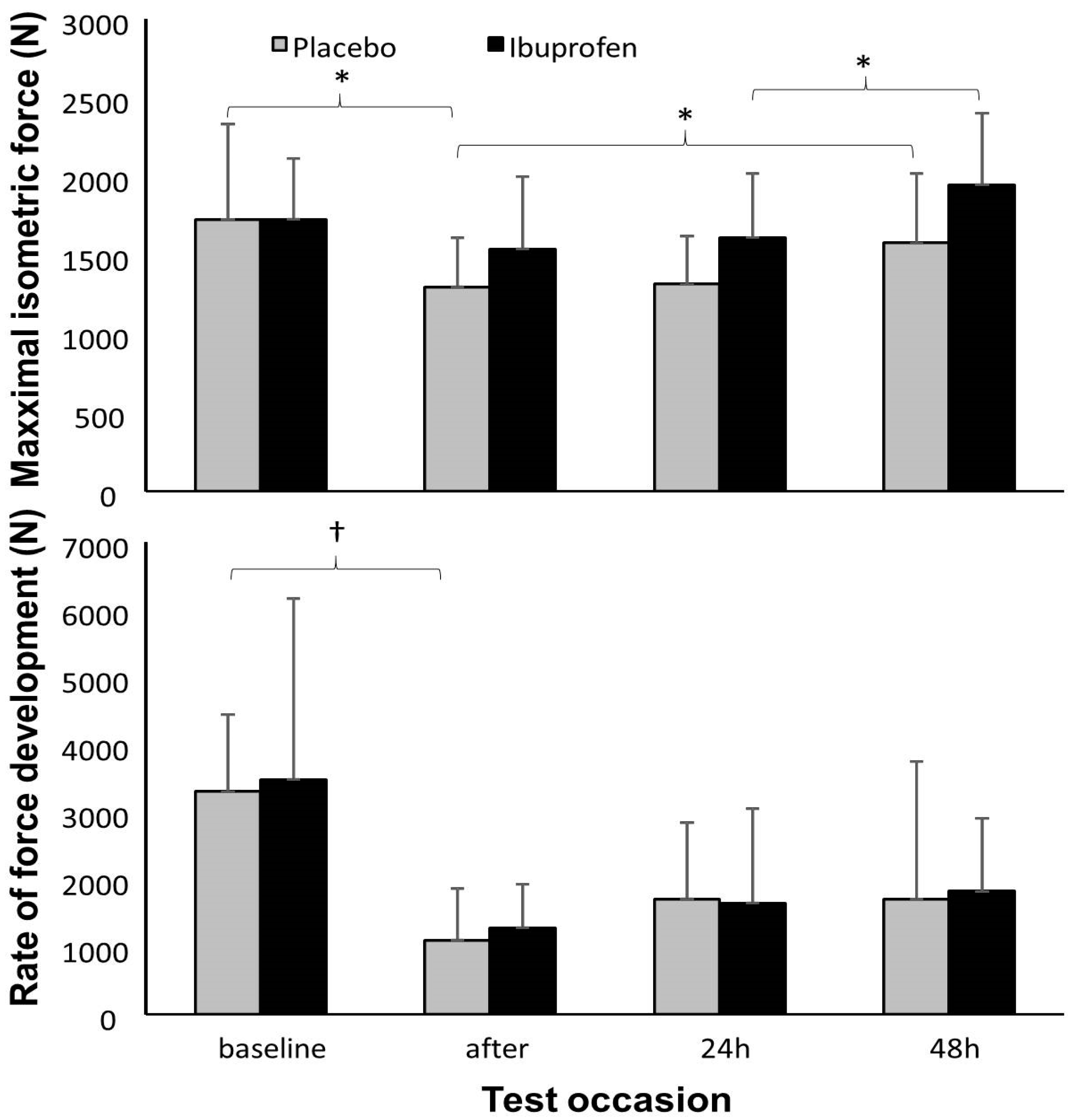
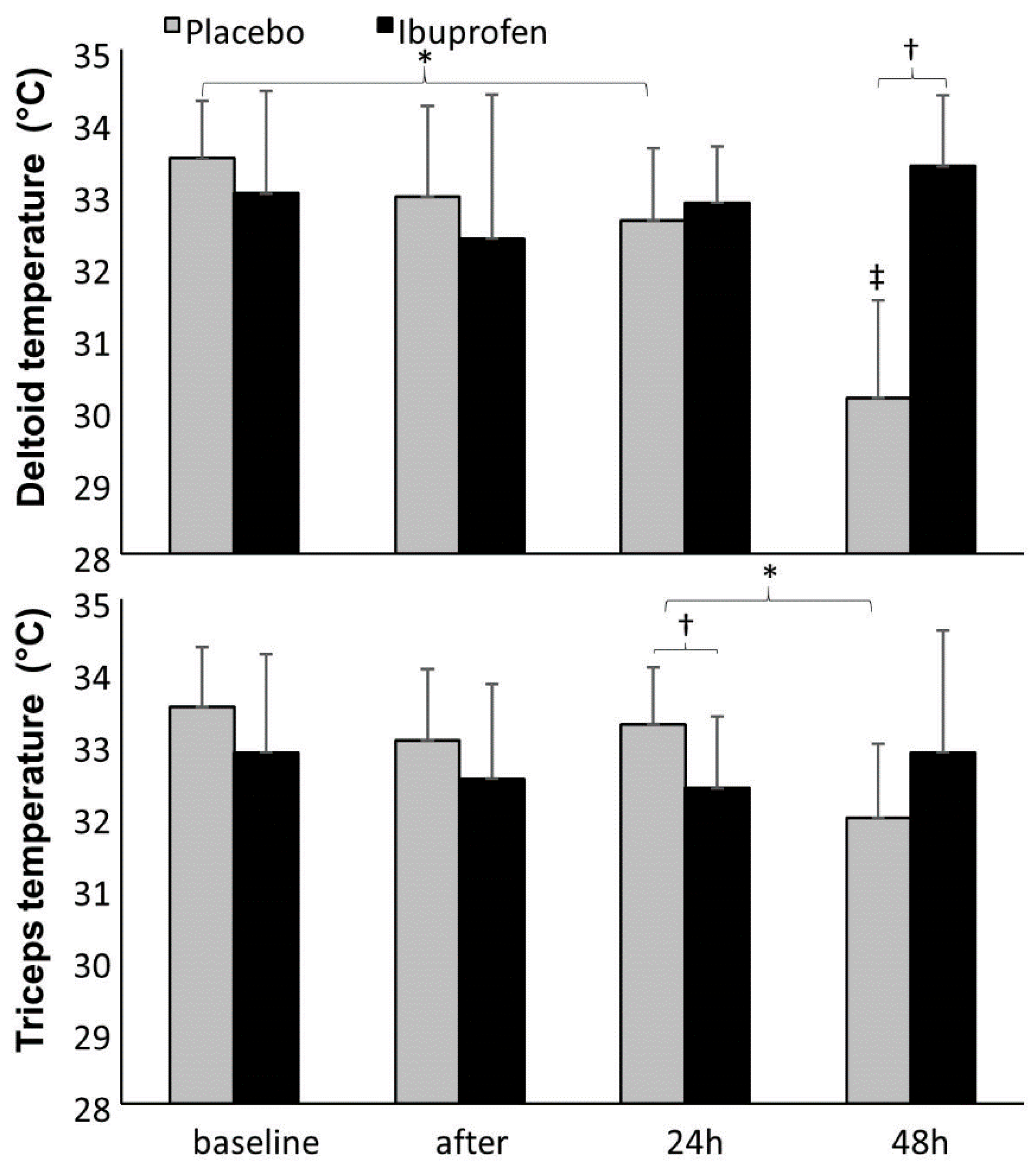
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bishop, P.A.; Jones, E.; Woods, A.K. Recovery from training: A brief review. J. Strength Cond. Res. 2008, 22, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Poppendieck, W.; Faude, O.; Wegmann, M.; Meyer, T. Cooling and performance recovery of trained athletes: A meta-analytical review. Int. J. Sports Physiol. Perform. 2013, 8, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehab. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Warden, S.J. Prophylactic use of NSAIDs by athletes: A risk/benefit assessment. Phys. Sportsmed. 2010, 38, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-body cryotherapy in athletes: From therapy to stimulation. An updated review of the literature. Front. Physiol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Vella, L.; Markworth, J.F.; Paulsen, G.; Raastad, T.; Peake, J.M.; Snow, R.J.; Cameron-Smith, D.; Russell, A.P. Ibuprofen ingestion does not affect markers of post-exercise muscle inflammation. Front. Physiol. 2016, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Trappe, T.A.; Mylona, E.; White, F.; Lambert, C.P.; Evans, W.J.; Pizza, F.X. Ibuprofen and acetaminophen: Effect on muscle inflammation after eccentric exercise. Med. Sci. Sports Exerc. 2003, 35, 892–896. [Google Scholar] [CrossRef]
- Tokmakidis, S.P.; Kokkinidis, E.; Smilios, I.; Douda, H. The effects of ibuprofen on delayed muscle soreness and muscle performance after eccentric exercise. J. Strength Cond. Res. 2003, 17, 53–59. [Google Scholar]
- Morelli, K.M.; Brown, L.B.; Warren, G.L. Effect of NSAIDs on recovery from acute skeletal muscle injury: A systematic review and meta-analysis. Am. J. Sports Med. 2018, 46, 224–233. [Google Scholar] [CrossRef]
- de Souza, R.F.; de Matos, D.G.; Ferreira, A.R.; Chilibeck, P.; de Almeida Barros, N.; de Oliveira, A.S.; Cercato, L.M.; Soares da Silva, B.; Aidar, F.J. The effect of ibuprofen on muscle, hematological and renal function, hydric balance, pain, and performance during intense long-distance running. J. Strength Cond. Res. 2018. Ahead of print. [Google Scholar] [CrossRef]
- International Paralympic Committee (IPC). Official website of IPC Powerlifting. Available online: http://www.paralympic.org/powerlifting/about (accessed on 10 January 2020).
- Fleck, S.J.; Kraemer, W.J. Designing Resistance Training Programs, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Austin, D.; Mann, B. Powerlifting: The Complete Guide to Technique, Training, and Competition; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Bento, P.C.; Pereira, G.; Ugrinowitsch, C.; Rodacki, A.L. Peak torque and rate of torque development in elderly with and without fall history. Clin. Biomech. 2010, 25, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Bonsu, B.; Terblanche, E. The training and detraining effect of high-intensity interval training on post-exercise hypotension in young overweight/obese women. Eur. J. Appl. Physiol. 2016, 116, 77–84. [Google Scholar] [CrossRef]
- Milner-Brown, H.S.; Mellenthin, M.; Miller, R.G. Quantifying human muscle strength, endurance and fatigue. Arch. Phys. Med. Rehab. 1986, 67, 530–535. [Google Scholar]
- Ammer, K.; Ring, E.F. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, 33–46. [Google Scholar]
- Cohen, J. Statistics a power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.S.; Cadore, E.L.; Baroni, B.M.; Da Silva, E.R.; Bijoldo, J.M.; Pinto, R.S.; Kruel, L.F. Effects of prophylactic anti-inflammatory non-steroidal ibuprofen on performance in a session of strength training. Braz. J. Sports Med. 2013, 19, 116–119. [Google Scholar]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Corvino, R.B.; Caputo, F.; Oliveira, A.C.; Greco, C.C.; Denadai, B.S. Rate of strength development at different speeds of muscle contractions. Braz. J. Sports Med. 2009, 15, 428–431. [Google Scholar]
- Häkkinen, K.; Alen, M.; Komi, P.V. Changes in isometric force-and relaxation-time, electromyographic and muscle fiber characteristics of human skeletal muscle during strength training and detraining. Acta Physiol. 1985, 125, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rosell, D.; Pareja-Blanco, F.; Aagaard, P.; González-Badillo, J.J. Physiological and methodological aspects of rate of force development assessment in human skeletal muscle. Clin. Physiol. Funct. Imaging 2018, 38, 743–762. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Dumke, C.L.; Oley, K.; Mcanulty, S.R.; Davis, J.M.; Murphy, E.A.; Utter, A.C.; Lind, R.H.; Mcanulty, L.S.; et al. Ibuprofen use, endotoxemia, inflammation, and plasma cytokines during ultramarathon competition. Brain Behav. Immun. 2006, 20, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Alaranta, A.; Alaranta, H.; Helenius, I. Use of prescription drugs in athletes. Sports Med. 2008, 38, 449–463. [Google Scholar] [CrossRef]
- Linnane, D.M.; Bracken, R.M.; Brooks, S.; Cox, V.M.; Ball, D. Effects of hyperthermia on the metabolic responses to repeated high-intensity exercise. Eur. J. Appl. Physiol. 2004, 93, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, F.; Neves, E.B.; Moura, M.A.; Nohama, P. The thermography in support for diagnosis of muscle injury in sport. Braz. J. Sports Med. 2014, 20, 59–64. [Google Scholar]
- Neves, E.B.; Moreira, T.R.; Lemos, R.; Vilaça-Alves, J.; Rosa, C.; Reis, V.M. Using skin temperature and muscle thickness to assess muscle response to strength training. Braz. J. Sports Med. 2015, 21, 350–354. [Google Scholar] [CrossRef]

| Week | Day | Day | Day | |
|---|---|---|---|---|
| Week 1 | Day 1 1RM test | Day 2 1RM re-test | ||
| Week 2 (experiment) | Day 3 Pre-exercise data | Training | Day 3 Post-exercise data | Day 4 and Follow-up 24 and 48 h |
| Week 3 (experiment) | Day 6 Pre-exercise data | Training | Day 7 Post-exercise data | Day 8 and 9 Follow-up 24 and 48 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga, G.S.; Aidar, F.J.; Matos, D.G.; Marçal, A.C.; Santos, J.L.; Souza, R.F.; Carneiro, A.L.; Vasconcelos, A.B.; Da Silva-Grigoletto, M.E.; van den Tillaar, R.; et al. Effects of Ibuprofen Intake in Muscle Damage, Body Temperature and Muscle Power in Paralympic Powerlifting Athletes. Int. J. Environ. Res. Public Health 2020, 17, 5157. https://doi.org/10.3390/ijerph17145157
Fraga GS, Aidar FJ, Matos DG, Marçal AC, Santos JL, Souza RF, Carneiro AL, Vasconcelos AB, Da Silva-Grigoletto ME, van den Tillaar R, et al. Effects of Ibuprofen Intake in Muscle Damage, Body Temperature and Muscle Power in Paralympic Powerlifting Athletes. International Journal of Environmental Research and Public Health. 2020; 17(14):5157. https://doi.org/10.3390/ijerph17145157
Chicago/Turabian StyleFraga, Guacira S., Felipe J. Aidar, Dihogo G. Matos, Anderson C. Marçal, Jymmys L. Santos, Raphael F. Souza, André L. Carneiro, Alan B. Vasconcelos, Marzo E. Da Silva-Grigoletto, Roland van den Tillaar, and et al. 2020. "Effects of Ibuprofen Intake in Muscle Damage, Body Temperature and Muscle Power in Paralympic Powerlifting Athletes" International Journal of Environmental Research and Public Health 17, no. 14: 5157. https://doi.org/10.3390/ijerph17145157
APA StyleFraga, G. S., Aidar, F. J., Matos, D. G., Marçal, A. C., Santos, J. L., Souza, R. F., Carneiro, A. L., Vasconcelos, A. B., Da Silva-Grigoletto, M. E., van den Tillaar, R., Cabral, B. T., & Reis, V. M. (2020). Effects of Ibuprofen Intake in Muscle Damage, Body Temperature and Muscle Power in Paralympic Powerlifting Athletes. International Journal of Environmental Research and Public Health, 17(14), 5157. https://doi.org/10.3390/ijerph17145157








