Abstract
Despite optimal control of serum phosphate level being imperative to avoid undesirable health outcomes, hyperphosphataemia is a highly prevalent mineral abnormality among the dialysis population. This study aimed to determine factors associated with hyperphosphatemia among hemodialysis patients in Malaysia. Multiple linear regression analysis was used to ascertain the possible factors that influence serum phosphate levels. A total of 217 hemodialysis patients were recruited. Hyperphosphatemia was prevalent. Only approximately 25% of the patients were aware that optimal control of hyperphosphatemia requires the combined effort of phosphate binder medication therapy, dietary restriction, and dialysis prescription. The presence of diabetes mellitus may affect serum phosphate levels, complicating dietary phosphorus management. Patients who were less depressive portrayed higher serum phosphate levels, implying intentional non-compliance. Better compliance on phosphate binder, longer sleep duration, and higher social support was associated with a lower level of serum phosphate. Despite sleep disturbance being one of the most prevalent and intense symptom burdens identified by hemodialysis patients, relatively few studies have addressed this issue. It is time to formulate sleep therapeutic interventions besides the encouragement of strong social support, hoping which many clinical outcomes including hyperphosphatemia can be better controlled among hemodialysis patients.
1. Introduction
Kidney failure is a worldwide public health problem, with increasing incidence and prevalence, high health care costs, and poor outcomes [1,2,3]. Progressive deterioration of kidney function among patients with end-stage renal disease (ESRD) results in elevated serum phosphorus concentrations which have been associated with a number of clinical complications including abnormal bone and mineral metabolism, soft tissue and vascular calcification, cardiovascular morbidity and mortality [4], with a significant impact on health care costs [5].
Patients on hemodialysis (HD), the most common treatment modality, are required to adhere strictly to treatment regimen namely diet, fluids, medications (phosphate binder), and dialysis therapy [6]. The most common dialysis prescriptions, however, do not provide adequate phosphate removal [7], with hyperphosphataemia frequently reported among HD patients [8,9,10]. Precise reasons for this gap remain unclear and various factors have been linked to poor phosphate compliance among HD patients including socio-demographic factors [11,12], depression [13,14], social support [15,16], medication adherence [11,17,18], nutritional knowledge on phosphorus [11,19], and sleep quality [20,21] with inconsistencies existing [22,23,24]. A burgeoning body of research has greatly advanced our understanding of the manifestations and management of kidney diseases, nevertheless, hyperphosphatemia remains prevalent among HD patients in Malaysia [11,25,26]. Despite hyperphosphatemia being prevalent, there is a paucity of data pertaining to potential determinants of hyperphosphatemia among HD patients in the local setting. The current practice of phosphate management is largely emphasized on the use of phosphate binders, with the dietary management of phosphorus and dialysis as adjuncts [27]. In view of the notably low adherence of phosphate binders and the complexity of dietary management, sub-optimal control in phosphate management among HD patients is expected. Inevitably, patients’ factors such as their perceived social support and depressive symptoms have been implicated as influential drivers of compliance behavior [27], but this has not been addressed adequately in the routine phosphate management of patients. Taken together, the present study aimed to determine the factors influencing serum phosphate among HD patients, hoping the research outcomes can optimize the existing management of serum phosphate among dialysis patients.
2. Materials and Methods
This study was conducted among HD patients receiving treatment at dialysis centers. Hemodialysis involves diverting blood into an external machine, made up of a series of membranes that act as filters. During hemodialysis, the membranes will filter waste products such as phosphate, potassium, and urea. During this process, important nutrients such as amino acids will be removed as well, resulting in a high prevalence of malnutrition among hemodialysis patients. Filtered blood is passed back into the patient’s body. Most patients require dialysis three times a week on alternate days, with each session lasting for four hours. Data collection was performed while patients were on dialysis as most patients would prefer to rush home after dialysis. During dialysis sessions, patients can sit or lie on a couch, recliner, or bed depending on the setting of the dialysis centers. Some patients may feel a bit sick, dizzy, and may have muscle cramps during the procedure, hence, data collection was performed only when patients were comfortable and at ease.
2.1. Subjects
The present study is a cross-sectional study whereby 230 eligible patients were recruited from seven selected HD centers via multistage sampling. The inclusion criteria entailed all Malaysians HD patients aged 18 years and above, able to understand and speak Mandarin, English or Malay languages and had undergone HD treatment for at least 3 months. Non-Malaysians and HD patients with dementia and mental illness, acute chronic or chronic hepatitis B and C, history of parathyroidectomy as well as recent hospitalization due to complications related to hemodialysis (e.g., vascular access-related infection or bleeding, hypotension or hypertension, pulmonary edema, and or cardiac arrhythmias) were excluded from this study. There were 13 patients with incomplete data and were excluded from the analysis. This study was performed in accordance with the principles of the Declaration of Helsinki. Ethical approval for the study protocol was obtained from the university’s Ethics Committee for Research Involving Human Subjects (JKEUPM-2018-231) while approvals were gained from the respective HD centers. Eligible patients were provided with a subject information sheet and written informed consent was obtained from all eligible patients prior to data collection with the assurance of confidentiality and anonymity. Figure 1 depicts the consort diagram of the study.
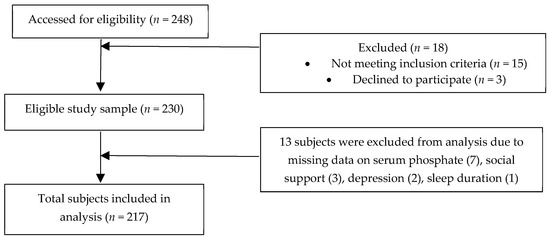
Figure 1.
Consort Flow Diagram.
2.2. Instrumentation
A pre-tested structured questionnaire was used to ascertain information on socio-demographic characteristics and clinical background of patients. Beck Depression Inventory (BDI) [28] was used to assess the severity of depressive symptoms among the patients. A universal recognized reliable tool for assessment of depression among hemodialysis patients, revealing 91% sensitivity and 86% specificity [29], BDI consists of 21 items with the first 13 questions concerning the cognitive-affective area, while other questions concern the somatic problems accompanying mood disorders. Each question is measured via a 4-point scale (0–3), which gives a total possible score of 0 to 63. Patients were classified into four levels of depression, namely none or minimal depression, mild to moderate depression, moderate to severe depression, or severe depression.
Perceived social support of patients from three aspects (family, friends, and significant others) was assessed by using the 12-item Multidimensional Scale of Perceived Social Support (MSPSS) [30]. Response for each item was scored on a 7-point Likert scale (very strongly disagree to very strongly agree). The MSPSS was divided into 3 subscales, which were Family Subscale, Friends Subscale, and Significant Others Scale along with a Total Score Scale. The total possible score ranges from 12 to 84, with a higher score indicating greater social support perceived by an individual. The level of perceived social support for each subscale and the total score scale was classified into low, moderate, or high, accordingly.
Medication adherent (specifically referred to phosphate binder) was ascertained as missing any dosage of phosphate binders for the past one week. The 6-item Simplified Medication Adherence Questionnaire (SMAQ) [31] was adopted and adapted to further ascertain common barriers leading to non-adherence of phosphate binder. Nutritional knowledge on phosphorus (renal diet and phosphorus control) was adapted from Karavetian, Abboud, Elzein, Haydar, and Vries [32]. Questions assessing the nutritional knowledge of patients regarding phosphorus control and renal diet were adapted in the study. Multiple responses were allowed for selected questions. The total score ranged from 0–11 and was converted into a percentage and a cut-off score of 60% indicated sufficient knowledge. One score was given to each question answered correctly with all the correct answer options being selected.
Sleep quality of patients in the past month was ascertained using the validated Pittsburgh Sleep Quality Index (PSQI) [33]. The PSQI is composed of seven components, namely subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, sleep medication use, and daytime dysfunction. Each sleep component has a scale factor from 0 to 3, and the seven components collectively form a global score ranging from 0 to 21. A higher global PSQI score indicates lower sleep quality. A global PSQI score of ≤5 indicates satisfactory sleep quality, whereas a score of >6 indicates poor sleep quality. Cronbach’s alpha of this index was 0.823, indicating that the instrument has acceptable internal consistency.
Objective adherence to phosphate was obtained retrospectively from the medical records using the pre-dialysis serum phosphate levels. Patients were considered non-adherent when the average of the pre-dialysis serum phosphate levels (past nine months from the day of data collection) exceeded 1.6 mmol/L based on recommendations from Clinical Practice Guidelines on Renal Replacement Therapy [34]. Other key clinical parameters pertaining to HD such as serum calcium, parathyroid hormone, alkaline phosphatase, duration of dialysis, and adequacy of dialysis (Kt/V) were ascertained in the study.
2.3. Data Analysis
All data analyses were performed using SPSS Version 24.0 (IBM Corp., Armonk, NY, USA) [35]. Descriptive statistics were used to present socio-demographic characteristics, clinical factors, depression level, sleep duration, social support, nutrition knowledge on phosphorus, and serum phosphate level. Variables with p < 0.25 in the simple linear regression [36] were entered in the stepwise multivariate model to determine the contribution of socio-demographic characteristics (age, employment, income), duration of sleep, presence of comorbidities, medication adherence, depression, and social support to serum phosphate level. All tests were two-sided, with a significance level set at 0.05.
3. Results
A total of 217 eligible patients were recruited with their characteristics depicted in Table 1. The mean age of the patients was 57 years old, made up of a comparative proportion of men and women. The majority of our patients were married (78.3%) and possessed a secondary education level (47.9%). Most of them were retirees (47.0%) and approximately 91.0% of the patients had low and middle household incomes. Hypertension was most prevalent (81.1%) followed by diabetes mellitus (55.8%). Mean HD vintage of patients was approximately 11 years.

Table 1.
Selected sociodemographic and clinical characteristics of patients (n = 217).
With regards to depression, the mean BDI score attained by the patients was 5.76 ± 5.66 with approximately one-quarter of them presented with mild to moderate and moderate to severe depression, respectively. In addition, there were approximately three-quarters of the patients perceived a high level of social support with merely 2% of the patients perceived themselves as having a low level of social support. On the other hand, a majority of the patients perceived a high level of social support on Family (87.6%) and Significant Others (80.6%) subscales but perceived relatively less support from Friends (58.5%). The mean duration of sleep of the patients was approximately 5.6 h per day, with less than half of the patients slept more than 6 h a day, while another one-third of them slept less than 5 h per day. This could be a worrying scenario as the majority of the subjects did not achieve the recommended sleep duration of 7 h for optimal health as proposed by the American Academy of Sleep Medicine.
Means serum calcium, phosphate, iPTH, and Kt/V were comparable with national renal registry data. Serum calcium was normal in 56% of the subjects, followed by approximately a quarter of the patients with hypocalcemia and hypercalcemia, respectively. The mean serum phosphate level of patients was 1.83 ± 0.50 mmol/L, with 77.4% of them failing to achieve the recommended target range of serum phosphate. There was 84.3%, 72.8%, 53.5%, and 27.8% of the patients’ perceived optimal serum phosphate control depending on a low phosphorus diet, phosphate binder, and dialysis process, and all three aspects respectively. All the patients were on a calcium-based phosphate binder. Approximately 70% of the patients had difficulties to adhere to phosphate binders in the present study, with more than half to approximately two-thirds of them forgetful and careless when taking phosphate binder medications. There were another 40% who had issues with the high pill burden and adverse effects of the phosphate binder (gastrointestinal discomfort such as constipation and unpleasant taste). A total of 94.0% of the patients scored less than 60% on nutrition knowledge on phosphorus, reflecting insufficient knowledge on phosphorus. This was affirmed despite the majority of patients (84.3%) acknowledging the importance of avoiding phosphate-rich foods in serum phosphate controls, while only approximately 13% were able to identify foods with high phosphorus content (data not shown).
Table 2 shows the single and multiple linear regression (stepwise method) analysis of sociodemographic factors (age, employment status, household income), clinical and medical factors (adequacy of dialysis, presence of co-morbidities, adherence on phosphate binder, duration of dialysis), sleep duration, depression, social support, nutritional knowledge on phosphorus and serum phosphate levels. Only variables that resulted in a p-value of less than 0.25 at the simple linear regression were selected for the multiple linear analysis. Adequate dialysis (β = −0.413, p < 0.05), adherence to phosphate binder (β = −0.368, p < 0.05), age (β = −0.305, p < 0.05), sleep duration (β = −0.288, p < 0.05), presence of diabetes mellitus (β = 0.272, p < 0.05), presence of depression (β = −0.265, p < 0.05), and social support (β = −0.141, p < 0.05)significantly contributed to serum phosphate level among hemodialysis patients after controlling for sex, as serum phosphate level was significantly different between male and their female counterparts. The prediction model was statistically significant (F = 12.4, p < 0.05) with the factors above accounting for 23.6% of the variance in serum phosphate level. There were no significant correlations between nutrition knowledge on phosphorus with serum phosphate levels.

Table 2.
Determinants of serum phosphate level among hemodialysis patients.
Table 3 depicts serum phosphate of patients according to specific variables which contributed significantly to serum phosphate in the multiple linear regression. Patients who adhered to phosphate binder prescription, older persons (defined as more than 60 years of age), diabetics, patients with adequate dialysis, depression, and higher social support had lower serum phosphate than their counterparts. While patients who slept less than 5 h per day had significantly higher serum phosphate than their counterparts, there was comparable serum phosphate between patients who slept 6 h per day and those sleeping equal to or more than 7 h.

Table 3.
Serum phosphate of patients according to specific variables.
4. Discussion
Hyperphosphataemia is a secondary complication in established renal failure patients. Approximately 80% of the patients in this study had elevated serum phosphate levels, which echoed earlier studies and reaffirmed the challenge in optimal control of serum phosphate levels. The unemployment rate of patients in this study was more than 76%, which is unexceptionally high considering only approximately 40% of the patients were aged more than 60 years old, a retirement age for a Malaysian. This is a universal scenario as HD patients often require an early retirement or sacrifice their employment or job opportunities to fit in the HD schedule [37,38]. Loss of productivity due to unemployment among HD patients had been reported to be higher compared to peritoneal dialysis [39,40], besides reduced quality of life attributed to the necessity for frequent travel.
Approximately one-quarter of the patients had depression, which was congruent with the reported prevalence rate of depression of 20% to 30% [29,41], despite being lower than the previous local study [42]. However, it should be noted that a different diagnostic tool was used in the previous study, making direct comparison not possible. Findings in our present study dictated most of the patients had none or minimal depression, which was contradicted with others [43,44,45,46] who found that most hemodialysis patients exhibited mild to moderate depression. The plausible reason to this discrepancy could be due to the overlap between symptoms of depression and uremic symptoms related to end-stage renal failure, resulting in patients that presented with uremic syndrome may be screened positive for depression, especially when self-reported measures were being utilized [47,48,49]. This present study utilized an interviewer-administered questionnaire method, which permitted the clarification of the differences to the patients. For social support, the high level of social support level perceived by the subjects were comparable to other studies [50,51]. Culture and religious beliefs that emphasized the role of family during times of sickness may contribute to the high level of family support attained [52,53]. The high level of perceived social support by patients may partly explain the low level of depression among this cohort.
Optimization of the phosphate binder used by patients with ESRD to achieve target serum phosphorus levels toward the recommended range is of utmost importance to minimize morbidity and mortality risks [54]. Nevertheless, nonadherence to phosphate binder is common with an estimation of up to 74% of ESRD patients are noncompliant to phosphate binder medication therapy [55]. Forgetfulness and carelessness were the main contributors to unintentional medication adherence among patients in this study, which was congruent with previous studies [11,56,57,58]. Adverse effects associated with phosphate binders such as gastrointestinal discomfort and high pill burden [11,59], necessity for strict adherence to the timing and dosing of the phosphate binder medication along with a busy social life and schedule may further aggravate this condition [58,60]. Considering polypharmacy is a common phenomenon among HD patients [61,62] due to the presence of various complications and underlying issues of the patients which may attribute to poor medication adherence, future studies focusing particularly on optimal clinical targets and lenient treatment strategies are warranted. On the other hand, a shared or active decision-making approach has been shown to improve patients’ medication adherence [63] and such an approach should be considered for hemodialysis patients who require long term continuum of care. Use of electronic monitoring devices to remind patients to take their medications at prescribed times may be helpful in improving medication adherence among forgetful patients [64].
Theoretically, patients with better knowledge possess higher awareness and are able to make better choices compared to lower knowledge patients. Several studies also have highlighted the lack of knowledge as a contributory factor to poor phosphate control, despite the association between phosphorus nutrition knowledge and serum phosphorus level being equivocal [65]. Similar to our findings, previous studies have reported better knowledge does not always translate to a better serum phosphate level [11,22,65,66,67]. With the growing body of evidence that educating hemodialysis patients on phosphorus improved serum phosphate levels [68,69,70,71], it is incumbent upon the healthcare professional community to acknowledge that education programs are indispensable for the optimal management of hemodialysis patients in routine care [72]. On the other hand, it would be appropriate to consider the application of more interactive approaches or technologies such as the internet and mobile telephones as education tools to extend support beyond the hospital setting, and enhancing accessibility to information aims to improve patients’ self-care management [66]. The lacking of association between better knowledge and better serum phosphorus control further suggests the use of educational empowerment techniques such as cognitive behavior interventions [73] or patient-centred approaches such as motivational interviewing [74] may be superior to the traditional approaches of information giving. Prevalence of poor nutritional knowledge on phosphorus among the patients was extremely high and worrying, which was in agreement with previous studies [11,19,22,67,75,76]. Complexity of the dietary regimen as well as the rigidity and lack of freedom that reduces the enjoyment and pleasure while dining might contribute to the failure to achieve the recommended target range of serum phosphate of the patients [22,57,77]. Emerging user-friendly educational initiatives such as motivational interviewing techniques and “traffic light” scheme to classify foods based on low, intermediate, or high phosphorus content may be useful. Empowerment of patients to tailor the phosphorus content of food to their phosphate binder use per meal will help in achieving optimal control of hyperphosphatemia while reducing the need for stringent dietary restrictions [78].
A younger age was associated with higher serum phosphate levels which was congruent with previous studies [79,80,81,82]. A difference in the lifestyle between older and younger patients along with self-perception of being less vulnerable to complications of non-compliance might have contributed to this [6,82,83,84]. Increased undernutrition with age, decreased renal phosphate reabsorption, and hormonal factors may also attribute to this [85]. Duration of dialysis was not associated with serum phosphate in the present study which was contradicting with evidence before this [11,86]. Earlier studies showed that as time passes, ESRD patients may easily get frustrated with the need to comply with long lists of restrictions [87], resulting in higher serum phosphate. We do not have an exact explanation but it is postulated that the heterogeneity of the patients with a wide range of duration of dialysis may have attributed to this discrepancy.
While it is well known that ESRD prevalence is increasing with the rise in the number of diabetic nephropathy patients, presence of diabetes mellitus was significantly correlated with serum phosphate levels in the present study. This finding was incongruent with previous studies [88,89] with inconsistencies existed [85,90]. Earlier studies revealed that medicines prescribed for the treatment of diabetes mellitus may incorporate highly bioavailable inorganic phosphate as an additive, which attributes to the elevation of serum phosphate levels [91]. Nevertheless, the exact mechanism concerning hyperphosphataemic episodes in diabetic hemodialysis patients remains unclear and requires more extensive and in-depth studies in the future. More studies are warranted to evaluate the precise association between diabetes and phosphatemia and its mechanism [85].
Evidences are growing that hyperphosphatemia was associated with poor sleep quality [20,21,92,93], despite inconsistencies existing [24]. While the presence of hyperphosphatemia-related pruritus could be the mediating factor for poor sleep quality [20], to the best of our knowledge, there is no clear mechanism of how poor sleep quality may influence serum phosphate level among the dialysis population. In light of the challenges of optimal control of hyperphosphatemia, despite the use of a new generation of phosphate binders and dialysis membranes, the findings of this study signify more work is needed on how sleep interventions may affect serum phosphate level as well as the possible mechanisms.
Our finding shows that less depressive hemodialysis patients may have a higher serum phosphate level, which was consistent with earlier studies [9,94,95,96,97]. It is possible that patients who were more carefree and positive may perceive themselves as less vulnerable to the risks of non-compliance, hence exercising more freedom in their diet or medication, resulting in higher serum phosphate levels. In light of the presence of psychiatric disorders such as depression, which have often been associated with a higher likelihood of adverse clinical outcomes including hospitalization and mortality in CKD patients, our findings should be interpreted carefully and more studies are needed to delineate the potential associations between depression and clinical outcomes including serum phosphate level. Besides, subjects in our study might perceive themselves as having stronger self-willpower in controlling their serum phosphate level, hence social support did not seem to exert a great impact on their phosphate compliance. Our finding was in accordance with previous findings [15,51,82,98] which demonstrated a higher level of social support was associated with better clinical outcomes. Consistent encouragement from a social support network could facilitate changes on an individual’s lifestyle [23], enhance patients’ quality of life and satisfaction from the provided care, improving treatment adherence, results in laboratory results (lower phosphate and potassium), and lead to better clinical outcomes [82,99]. As expected, patients with adequate dialysis and better adherence to phosphate binders possessed a lower serum phosphate level, emphasizing the importance of adequate dialysis and phosphate binders in optimizing control of serum phosphate.
Several limitations identified in this present study were the cross-sectional study design which limited the determination of causal relationships between the variables. We acknowledge that not all potential risk factors but rather only those which are routinely available were included in this study. Interviewer-administered questionnaires introduced bias and were highly dependent on the literacy and honesty of the subjects. We did not perform objective assessment on sleep such as polysomnography. Further studies with polysomnogram or other objective measures are needed. Despite the limitations present, this study highlighted several important findings that demand further in-depth research and investigations.
5. Conclusions
In conclusion, our findings reaffirmed poor compliance on serum phosphate levels among our hemodialysis patients. Besides, the present study also drew our attention to the role of diabetes mellitus in serum phosphate controls among HD patients, elucidating the need for healthcare professionals to monitor hyperphosphatemia closely among hemodialysis patients with diabetes mellitus. It is worth to note that 94% of the subjects in our study had an insufficient level of nutritional knowledge on phosphorus. Hence, healthcare professionals especially dietitians play an important role by providing interventions and increasing their awareness concerning this issue. Acknowledgement of barriers and factors affecting serum phosphate level compliance aids the provision of appropriate strategies and coping strategies that help to improve the clinical outcomes of HD patients. We hope by the identification of these non-conventional factors, namely social support, sleep duration and depression can assist the nephrology team in implementing a more comprehensive strategy in lessening hyperphosphatemia risk among the dialysis population.
Author Contributions
Conceptualization, E.S.Y.N., C.T.S.L. and Y.M.C.; methodology, E.S.Y.N., P.Y.W. and A.T.H.K.; Y.M.C.; formal analysis, E.S.Y.N. and Y.M.C.; resources, C.T.S.L. and Y.M.C.; data curation, E.S.Y.N. and Y.M.C.; writing–original draft preparation, E.S.Y.N.; writing–review & editing, C.T.S.L. and Y.M.C.; supervision, C.T.S.L. and Y.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank all our enthusiastic patients who participated in this study and staffs of dialysis centres for their assistance throughout the data collection process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eknoyan, G.; Lameire, N.; Barsoum, R.; Eckardt, K.U.; Levin, A.; Levin, N.; Locatelli, F.; MacLeod, A.; Vanholder, R.; Walker, R.; et al. The burden of kidney disease: Improving global outcomes. Kidney Int. 2004, 66, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. Global Facts: About Kidney Disease—The National Kidney Foundation. Available online: https://www.kidney.org/kidneydisease/global-facts-about-kidney-disease (accessed on 7 April 2020).
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Friedman, E.L.I.A. Consequences and management of hyperphosphatemia in patients with renal insufficiency. Kidney Int. 2005, 67, S1–S7. [Google Scholar] [CrossRef]
- Chiroli, S.; Mattin, C.; Belozeroff, V.; Perrault, L.; Mitchell, D.; Gioni, I. Impact of mineral and bone disorder on healthcare resource use and associated costs in the European Fresenius medical care dialysis population: A retrospective cohort study. BMC Nephrol. 2012, 13, 140. [Google Scholar] [CrossRef]
- Kutner, N.G. Improving compliance in dialysis patients: Does anything work? Semin. Dial. 2001, 14, 324–327. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “Phosphorous Pyramid”: A Visual Tool for Dietary Phosphate Management in Dialysis and CKD Patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef]
- Port, F.K.; Pisoni, R.L.; Bommer, J.; Locatelli, F.; Jadoul, M.; Eknoyan, G.; Kurokawa, K.; Canaud, B.J.; Finley, M.P.; Young, E.W. Improving outcomes for dialysis patients in the international dialysis outcomes and practice patterns study. Clin. J. Am. Soc. Nephrol. 2006, 1, 246–255. [Google Scholar] [CrossRef]
- Ibrahim, S.; Hossam, M.; Belal, D. Study of Non-Compliance among Chronic Hemodialysis Patients and its Impact on Patients’ Outcomes. Saudi J. Kidney Dis. Transpl. 2015, 26, 243–249. [Google Scholar] [CrossRef]
- Correa, D.; Kruger, T.S.; Sczip, A.C.; Vieira, M.A.; Morais, J.G. Perceptions of hemodialysis patients about dietary and fluid restrictions. J. Bras. Nefrol. 2017, 39, 154–161. [Google Scholar]
- Chan, Y.M.; Zalilah, M.S.; Hii, S.Z. Determinants of Compliance Behaviours among Patients Undergoing Hemodialysis in Malaysia. PLoS ONE 2012, 7, e41362. [Google Scholar] [CrossRef] [PubMed]
- Victoria, A.; Evangelos, F.; Sofia, Z. Family support, social and demographic correlations of non-adherence among haemodialysis patients. Am. J. Nurs. Sci. 2015, 4, 60–65. [Google Scholar] [CrossRef][Green Version]
- Martin, L.R.; Williams, S.L.; Haskard, K.B.; Dimatteo, M.R. The challenge of patient adherence. Ther. Clin. Risk Manag. 2005, 1, 189–199. [Google Scholar] [PubMed]
- Rosenthal Asher, D.; Ver Halen, N.; Cukor, D. Depression and nonadherence predict mortality in hemodialysis treated end-stage renal disease patients. Hemodial. Int. 2012, 6, 387–393. [Google Scholar]
- Cohen, S.D.; Sharma, T.; Acquaviva, K.; Peterson, R.A.; Patel, S.S.; Kimmel, P.L. Social Support and Chronic Kidney Disease: An Update. Adv. Chronic Kidney Dis. 2007, 14, 335–344. [Google Scholar] [CrossRef]
- Untas, A.; Thumma, J.; Rascle, N.; Rayner, H.; Mapes, D.; Lopes, A.A.; Fukuhara, S.; Akizawa, T.; Morgenstern, H.; Robinson, B.M.; et al. The associations of social support and other psychosocial factors with mortality and quality of life in the dialysis outcomes and practice patterns study. Clin. J. Am. Soc. Nephrol. 2011, 6, 142–152. [Google Scholar] [CrossRef]
- Waheed, A.A.; Pedraza, F.; Lenz, O.; Isakova, T. Phosphate control in end-stage renal disease: Barriers and opportunities. Nephrol. Dial. Transplant. 2013, 28, 2961–2968. [Google Scholar] [CrossRef]
- Van Camp, Y.P.M.; Vrijens, B.; Abraham, I.; Van Rompaey, B.; Elseviers, M.M. Adherence to phosphate binders in hemodialysis patients: Prevalence and determinants. J. Nephrol. 2014, 27, 673–679. [Google Scholar] [CrossRef]
- Pollock, J.B.; Jaffery, J.B. Knowledge of Phosphorus Compared with Other Nutrients in Maintenance Dialysis Patients. J. Ren. Nutr. 2007, 17, 323–328. [Google Scholar] [CrossRef]
- Elder, S.J.; Pisoni, R.L.; Akizawa, T.; Fissell, R.; Andreucci, V.E.; Fukuhara, S.; Kurokawa, K.; Rayner, H.C.; Furniss, A.L.; Port, F.K.; et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2008, 23, 998–1004. [Google Scholar] [CrossRef]
- Ezzat, H.; Mohab, A. Prevalence of sleep disorders among ESRD patients. Ren. Fail. 2015, 37, 1013–1019. [Google Scholar] [CrossRef]
- Durose, C.L.; Holdsworth, M.; Watson, V.; Przygrodzka, F. Knowledge of Dietary Restrictions and the Medical Consequences of Noncompliance by Patients on Hemodialysis Are Not Predictive of Dietary Compliance. J. Am. Diet. Assoc. 2004, 104, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Thong, M.S.Y.; Kaptein, A.A.; Krediet, R.T.; Boeschoten, E.W.; Dekker, F.W. Social support predicts survival in dialysis patients. Nephrol. Dial. Transplant. 2007, 22, 845–850. [Google Scholar] [CrossRef]
- Unruh, M.L.; Hartunian, M.G.; Chapman, M.M.; Jaber, B.L. Sleep quality and clinical correlates in patients on maintenance dialysis. Clin. Nephrol. 2003, 59, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.M.; Zalilah, M.S.; Lim, C.T.S.; Goh, B.L. Factors associated with poor nutritional status Among hemodialysis patients in Malaysia. Mal. J. Med. Health Sci. 2019, 15, 77–83. [Google Scholar]
- Nor Baizura, M.Y.; Chan, Y.M.; Zalilah, M.S.; Choo, B.H. Factors associated with quality of life among hemodialysis patients in Malaysia. PLoS ONE 2013, 8, e84152. [Google Scholar] [CrossRef]
- Umeukeje, E.M.; Mixon, A.S.; Cavanaugh, K.L. Phosphate-control adherence in hemodialysis patients: Current perspectives. Patient Prefer. Adherence 2018, 4, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Garbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Watnick, S.; Wang, P.L.; Demadura, T.; Ganzini, L. Validation of 2 depression screening tools in dialysis patients. Am. J. Kidney Dis. 2005, 46, 919–924. [Google Scholar] [CrossRef]
- Zimet, G.D.; Dahlem, N.W.; Zimet, S.G.; Farley, F.G. The Multidimensional Scale of Perceived Social Support. J. Pers. Assess. 1988, 52, 30–41. [Google Scholar] [CrossRef]
- Knobel, H.; Alonso, J.; Casado, J.L.; Collazos, J.; González, J.; Ruiz, I.; Kindelan, J.M.; Carmona, A.; Juega, J.; Ocampo, A.; et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: The GEEMA Study. AIDS 2002, 16, 605–613. [Google Scholar] [CrossRef]
- Karavetian, M.; Abboud, S.; Elzein, H.; Haydar, S.; de Vries, N. Nutritional education for management of osteodystrophy (NEMO) trial: Design and patient characteristics, Lebanon. Nutr. Res. Pract. 2014, 8, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Ministry of Health Malaysia. Clinical Practice Guidelines: Renal Replacement Therapy, 4th ed.; Post Graduate Renal Society of Malaysia: Kuala Lumpur, Malaysia, 2017. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0; IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Morton, R.L.; Da Silva-Gane, M.; Cass, A.; Patterson, K.; Yip, A.C.W.; Handke, W.A.; Webster, A.C. Interventions to aid employment for people on dialysis and their families. Cochrane Database Syst. Rev. 2017, 2017, CD012702. [Google Scholar] [CrossRef]
- Jones, D.J.W.; Harvey, K.; Harris, J.P.; Butler, L.T.; Vaux, E.C. Understanding the impact of haemodialysis on UK National Health Service patients’ well-being: A qualitative investigation. J. Clin. Nurs. 2018, 27, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Chen, H.H.; Wu, M.J.; Hsu, B.G.; Tsai, J.C.; Kuo, C.C.; Lin, S.P.; Chen, T.H.; Sue, Y.M. Out-of-pocket costs and productivity losses in haemodialysis and peritoneal dialysis from a patient interview survey in Taiwan. BMJ Open 2019, 9, e023062. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lau, T.; Luo, N. Cost-effectiveness of haemodialysis and peritoneal dialysis for patients with end-stage renal disease in Singapore. Nephrology 2016, 21, 669–677. [Google Scholar] [CrossRef]
- Cukor, D.; Coplan, J.; Brown, C.; Friedman, S.; Cromwell-Smith, A.; Peterson, R.A.; Kimmel, P.L. Depression and anxiety in urban hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2007, 2, 484–490. [Google Scholar] [CrossRef]
- Bujang, M.A.; Musa, R.; Liu, W.J.; Chew, T.F.; Lim, C.T.S.; Morad, Z. Depression, anxiety and stress among patients with dialysis and the association with quality of life. Asian J. Psychiatr 2015, 18, 49–52. [Google Scholar] [CrossRef]
- Khalil, A.A.; Darawad, M.; Al Gamal, E.; Hamdan-Mansour, A.M.; Abed, M.A. Predictors of dietary and fluid non-adherence in Jordanian patients with end-stage renal disease receiving haemodialysis: A cross-sectional study. J. Clin. Nurs. 2013, 22, 127–136. [Google Scholar] [CrossRef]
- Al Dukhayel, A. Prevalence of Depressive Symptoms among Hemodialysis and Peritoneal Dialysis Patients. Int. J. Health Sci. (Qassim) 2015, 9, 9–16. [Google Scholar] [CrossRef]
- Nelson, V.; Gopalakrishnan, S.; Rakesh, P.S.; Simon, S.; Babu, V.; Vikraman, V.; Abraham, S.; Mohammaed, Y. Depression Among Dialysis Patients. J. Nephrol. Soc. Work. 2008, 40, 34–37. [Google Scholar]
- Čengić, B.; Resić, H. Depression in Hemodialysis Patients. Bosn. J. Basic Med. Sci. 2017, 10, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.P. Psychosocial factors in adult end-stage renal disease patients treated with hemodialysis: Correlates and outcomes. Am. J. Kidney Dis. 2000, 35, 132–140. [Google Scholar] [CrossRef]
- Cukor, D.; Peterson, R.A.; Cohen, S.D.; Kimmel, P.L. Depression in end-stage renal disease hemodialysis patients. Nat. Clin. Pract. Nephrol. 2006, 2, 678–687. [Google Scholar] [CrossRef]
- Shirazian, S.; Grant, C.D.; Aina, O.; Mattana, J.; Khorassani, F.; Ricardo, A.C. Depression in Chronic Kidney Disease and End-Stage Renal Disease: Similarities and Differences in Diagnosis, Epidemiology, and Management. Kidney Int. Rep. 2017, 2, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Rafii, F. Perceived Social Support in Hemodialysis Patients. Iran. J. Nurs. 2009, 22, 99–110. [Google Scholar]
- Theodoritsi, A.; Aravantinou, M.E.; Gravani, V.; Bourtsi, E.; Vasilopoulou, C.; Theofilou, P.; Polikandrioti, M. Factors Associated with the Social Support of Hemodialysis Patients. Iran. J. Public Health 2016, 45, 1261–1269. [Google Scholar] [PubMed]
- Bayat, A.; Kazemi, R.; Toghiani, A.; Mohebi, B.; Tabatabaee, M.N.; Adibi, N. Psychological evaluation in hemodialysis patients. J. Pak. Med. Assoc. 2012, 62, S1–S5. [Google Scholar]
- Lilympaki, I.; Makri, A.; Vlantousi, K.; Koutelekos, I.; Babatsikou, F.; Polikandrioti, M. Effect of perceived social support on the levels of anxiety and depression of hemodialysis patients. Mater. Sociomed. 2016, 28, 361–365. [Google Scholar] [CrossRef]
- Isakova, T.; Nickolas, T.L.; Denburg, M.; Yarlagadda, S.; Weiner, D.E. KDOQI Commentary KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease—Mineral and Bone Disorder (CKD-MBD). Am. J. Kidney Dis. 2017, 70, 737–751. [Google Scholar] [CrossRef]
- Karamanidou, C.; Clatworthy, J.; Weinman, J.; Horne, R. A systematic review of the prevalence and determinants of end-stage renal disease. BMC Nephrol. 2008, 10, 1–10. [Google Scholar]
- Ossareh, S.; Tabrizian, S.; Zebarjadi, M. Prevalence of Depression in Maintenance Hemodialysis Patients and Its Correlation with Adherence to Medications. Iran. J. Kidney Dis. 2014, 8, 467–475. [Google Scholar] [PubMed]
- Covic, A.; Rastogi, A. Hyperphosphatemia in patients with ESRD: Assessing the current evidence linking outcomes with treatment adherence. BMC Nephrol. 2013, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Castelino, R.L.; Lioufas, N.M.; Peterson, G.M. Nonadherence to Medication Therapy in Haemodialysis Patients: A Systematic Review. PLoS ONE 2015, 10, e0144119. [Google Scholar] [CrossRef]
- Chan, S.; Au, K.; Francis, R.S.; Mudge, D.W.; Johnson, D.W.; Pillans, P.I. Phosphate binders in patients with chronic kidney disease. Aust. Prescr. 2017, 40, 10–14. [Google Scholar] [CrossRef]
- Arenas, M.D.; Malek, T.; Gil, M.T.; Moledous, A.; Alvarez-Ude, F.; Reig-Ferrer, A. Challenge of phosphorus control in hemodialysis patients: A problem of adherence? J. Nephrol. 2010, 23, 525–534. [Google Scholar]
- Alshamrani, M.; Almalki, A.; Qureshi, M.; Yusuf, O.; Ismail, S. Polypharmacy and Medication-Related Problems in Hemodialysis Patients: A Call for Deprescribing. Pharmacy 2018, 6, 76. [Google Scholar] [CrossRef]
- Schmidt, I.M.; Hübner, S.; Nadal, J.; Titze, S.; Schmid, M.; Bärthlein, B.; Schlieper, G.; Dienemann, T.; Schultheiss, U.T.; Meiselbach, H.; et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: The German Chronic Kidney Disease study. Clin. Kidney J. 2019, 12, 663–672. [Google Scholar] [CrossRef]
- Schoenthaler, M.A.; Lancaster, K.J.; Chaplin, W.; Butler, M.; Forsyth, J.; Ogedegbe, G. Cluster Randomized Clinical Trial of FAITH (Faith-Based Approaches in the Treatment of Hypertension) in Blacks. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004691. [Google Scholar] [CrossRef]
- Churillo, T.O. Medication Reminder Systems: An Adjunct Technique in Improving Phosphate Binder. J. Ren. Nutr. 2012, 22, e3–e9. [Google Scholar] [CrossRef]
- Pafili, Z.; Maridaki, M.; Giannaki, C.; Karatzaferi, C.; Liakopoulos, V.; Eleftheriadis, T.; Stefanidis, I.; Sakkas, G.K. Phosphorus nutritional knowledge among dialysis health care providers and patients: A multicenter observational study. Clin. Nutr. ESPEN 2019, 31, 33–37. [Google Scholar] [CrossRef]
- Collinson, A.; McMullan, M.; Tse, W.Y.; Sadler, H. Managing serum phosphate in haemodialysis patients: Time for an innovative approach? Eur. J. Clin. Nutr. 2014, 68, 392–396. [Google Scholar] [CrossRef]
- Cupisti, A.; Ferretti, V.; D’Alessandro, C.; Petrone, I.; Di Giorgio, A.; Meola, M.; Panichi, V.; Conti, P.; Lippi, A.; Caprioli, R.; et al. Nutritional Knowledge in Hemodialysis Patients and Nurses: Focus on Phosphorus. J. Ren. Nutr. 2012, 22, 541–546. [Google Scholar] [CrossRef]
- Ford, J.C.; Pope, J.F.; Hunt, A.E.; Gerald, B. The effect of diet education on the laboratory values and knowledge of hemodialysis patients with hyperphosphatemia. J. Ren. Nutr. 2004, 14, 36–44. [Google Scholar] [CrossRef]
- Reddy, V.; Symes, F.; Sethi, N.; Scally, A.J.; Scott, J.; Mumtaz, R.; Stove, R. Dietitian-Led Education Program to Improve Phosphate Control in a Single-Center Hemodialysis Population. J. Ren. Nutr. 2009, 19, 314–320. [Google Scholar] [CrossRef]
- Shaw-Stuart, N.J.; Stuart, A. The effect of an educational patient compliance program on serum phosphate levels in patients receiving hemodialysis. J. Ren. Nutr. 2000, 10, 80–84. [Google Scholar] [CrossRef]
- Lim, E.; Hyun, S.; Lee, J.M.; Kim, S.; Lee, M.J.; Lee, S.M.; Lim, E.; Hyun, S.; Lee, J.M.; Kim, S.; et al. Effects of education on low-phosphate diet and phosphate binder intake to control serum phosphate among maintenance hemodialysis patients: A randomized controlled trial. Kidney Res. Clin. Pract. 2018, 37, 69–76. [Google Scholar] [CrossRef]
- Karavetian, M.; Rizk, R. Patient education for hyperphosphatemia management: Improving outcomes while decreasing costs? Kidney Res. Clin. Pract. 2018, 37, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Matteson, M.L.; Russell, C. Interventions to improve hemodialysis adherence: A systematic review of randomized-controlled trials. Hemodial. Int. 2010, 14, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.L.; Cronk, N.J.; Herron, M.; Knowles, N.; Matteson, M.L.; Peace, L.; Ponferrada, L. Motivational Interviewing in Dialysis Adherence Study (MIDAS). Nephrol. Nurs. J. 2011, 38, 229–236. [Google Scholar] [PubMed]
- Ekramzadeh, M.; Mazloom, Z.; Jafari, P.; Ayatollahi, M.; Sagheb, M.M. Major Barriers Responsible for Malnutrition in Hemodialysis Patients: Challenges to Optimal Nutrition. Nephrourol. Mon. 2014, 6, e23158. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, R.S.; Sharifi, N. Evaluation of Nutritional Knowledge in Terms of Dietary Sources of Protein, Phosphorous, Potassium and Fluids Restriction in Hemodialysis Patients. Jentashapir J. Heal. Res. 2014, 5. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer. Adherence 2013, 7, 379–390. [Google Scholar] [CrossRef]
- Kugler, C.; Vlaminck, H.; Haverich, A.; Maes, B. Nonadherence with Diet and Fluid Restrictions Receiving Hemodialysis. J. Nurs. Scholarsh. 2005, 37, 25–29. [Google Scholar] [CrossRef]
- Baraz, S.; Parvardeh, S.; Mohammadi, E.; Broumand, B. Dietary and fluid compliance: An educational intervention for patients having haemodialysis. J. Adv. Nurs. 2010, 66, 60–68. [Google Scholar] [CrossRef]
- Youngmee, K.; Evangelista, L.S. Relationship between Illness Perceptions, Treatment Adherence, and Clinical Outcomes in Patients on Maintenance Hemodialysis. Nephrol. Nurs. J. 2010, 37, 271–281. [Google Scholar]
- Ahrari, S.; Moshki, M.; Bahrami, M. The Relationship Between Social Support and Adherence of Dietary and Fluids Restrictions among Hemodialysis Patients in Iran. J. Caring Sci. 2014, 3, 11–19. [Google Scholar]
- Park, K.A.; Choi-Kwon, S.; Sim, Y.M.; Kim, S.B. Comparison of Dietary Compliance and Dietary Knowledge Between Older and Younger Korean Hemodialysis Patients. J. Ren. Nutr. 2008, 18, 415–423. [Google Scholar] [CrossRef]
- Clark-Cutaia, M.N.; Dianxu, R. Adherence to Hemodialysis Dietary Sodium Recommendations: Influence of Patien Characteristics, Self-Efficacy and Perceived Barriers. J. Ren. Nutr. 2012, 76, 211–220. [Google Scholar] [CrossRef]
- Laurain, E.; Thilly, N.; Boini, S.; Kessler, M.; Briancon, S.; Frimat, L. Hyperphosphatemia in chronic kidney disease: Patient characteristics and dialysis mortality during the first year of dialysis. J. Nephrol. Ther. 2012, S3, 009. [Google Scholar] [CrossRef]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.W.; Twinn, S.F.; Chan, S.W. Self-reported adherence to therapeutic regimen among patients undergoing continuous ambulatory peritoneal dialysis. J. Adv. Nurs. 2010, 66, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; De Boer, I.H.; Peralta, C.A.; Adeney, K.L.; Duprez, D.A.; Jenny, N.S.; Siscovick, D.S.; Kestenbaum, B.R. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. CJASN 2009, 4, 609–615. [Google Scholar] [CrossRef]
- Van Kuijk, J.P.; Flu, W.J.; Chonchol, M.; Valentijn, T.M.; Verhagen, H.J.; Bax, J.J.; Poldermans, D. Elevated preoperative phosphorus levels are an independent risk factor for cardiovascular mortality. Am. J. Nephrol. 2010, 32, 163–168. [Google Scholar] [CrossRef]
- Naves-Díaz, M.; Passlick-Deetjen, J.; Guinsburg, A.; Marelli, C.; Fernández-Martín, J.L.; Rodríguez-Puyol, D.; Cannata-Andía, J.B. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol. Dial. Transplant. 2011, 26, 1938–1947. [Google Scholar] [CrossRef]
- Imtiaz, R.; Hawken, S.; McCormick, B.B.; Leung, S.; Hiremath, S.; Zimmerman, D.L. Diabetes Mellitus and Younger Age Are Risk Factors for Hyperphosphatemia in Peritoneal Dialysis Patients. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Han, B.; Zhu, F.X.; Shi, C.; Wu, H.L.; Gu, X.H. Association between Serum Vitamin D Levels and Sleep Disturbance in Hemodialysis Patients. Nutrients 2017, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, S.; Sarikhani, S.; Roozbeh, J. Sleep Quality in Patients Undergoing Long-term Hemodialysis Using the Pittsburgh Sleep Quality Index. Nephrourol. Mon. 2017, 9, e13137. [Google Scholar] [CrossRef]
- Acar, D.; Güneş, Z. Factors affecting therapeutic compliance in patients with chronic renal failure: Anxiety, Depression, İllness Perception. Health Prim. Care 2018, 2. [Google Scholar] [CrossRef]
- Bezerra, C.I.L.; Silva, B.C.; Elias, R.M. Decision-making process in the pre-dialysis CKD patients: Do anxiety, stress and depression matter? BMC Nephrol. 2018, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.; de Azevedo, V.; Miranda, C.; Miranda, M.; Teixeira, M.; Elias, R. Depression in hemodialysis patients: The role of dialysis shift. Clinics 2014, 69, 198–202. [Google Scholar] [CrossRef]
- Collister, D.; Rodrigues, J.C.; Mazzetti, A.; Salisbury, K.; Morosin, L.; Rabbat, C.; Brimble, K.S.; Walsh, M. Single Questions for the Screening of Anxiety and Depression in Hemodialysis. CJKHD 2019. [Google Scholar] [CrossRef]
- Alexopoulou, M.; Giannakopoulou, N.; Komna, E.; Alikari, V.; Toulia, G.; Polikandrioti, M. The Effect of Perceived Social Support on Hemodialysis Patients’ Quality of Life. Mater. Sociomed. 2016, 28, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, L.C.; Fink, N.E.; Harrington-Levey, R.; Finkelstein, F.O.; Hebah, N.; Powe, N.; Jaar, B.G. Association of social support with outcomes in incident dialysis patients. CJASN 2010, 5, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).