1. Introduction
Beneficial properties of clay minerals and especially of thermal muds (TMs) are well known and established: their uses in both historical and modern times are thoroughly discussed in a dedicated review [
1]. Among the many curative applications, the ancient practice of pelotherapy has been carried out for centuries worldwide [
2,
3] and it gained popularity also for wellness purposes [
4]. Pelotherapy consists in the application of hot TMs (40 ± 2 °C) in a thick-layer, directly on the skin of the patient that is then covered with an insulating cloth, in order to preserve heat.
Pelotherapy has a stimulatory, antiphlogistic, analgesic action [
1,
4,
5] and it is recommended either as treatment or adjuvant therapy for rheumatic disorders and other musculoskeletal conditions, e.g., osteoarthritis [
6,
7,
8], fibromyalgia [
9,
10], rheumathoid arthritis [
9], low back pain [
11] and traumatic injuries [
12]. Pelotherapy is suggested also for other conditions, e.g., dermatological disorders [
13], neuro- and vasculopathies [
14,
15] and to improve stress resilience [
16].
The overall quality of TMs is determined by several factors, i.e., composition of raw mud, thermal water characteristics and maturation procedure. In fact, TMs gain their therapeutic properties during the maturation process, in which mud is blended with thermal water under specific conditions [
12,
17]. Complex inorganic and organic changes of the mud matrix lead to the improvement of physico-chemical, rheological and biological properties required for an effective pelotherapy [
1,
2,
12]. Most pelotherapy centers employ raw muds occurring in situ, but where exploitation of natural reserves is strictly regulated [
18], TMs are currently regenerated, i.e., after the first application on the patient they are matured and used anew [
4]. In the past, some authors discussed the need to establish standard quality criteria for TMs intended to be used in therapy [
3,
4]. Several studies evaluated chemical, mineralogical, radiological and granulometric properties of raw muds under the perspective of human health safety e.g., [
19,
20,
21,
22]. The mobility of hazardous chemical elements possibly contained in clay materials was also assessed with peculiar in vitro leaching tests [
23,
24].
So far, biological investigations mainly addressed the characterization of thermophilic microorganisms (e.g., diatoms and cyanobacteria) involved in the maturation process and release of therapeutically active biogenic compounds [
2,
3,
25,
26,
27]. On the contrary, hygienic aspects of TMs microbiology have been contemplated only by a few studies [
3,
28,
29,
30,
31,
32]. However, no exhaustive research on microbiological hygiene quality of TMs has been published so far. Studies evaluating either the effectiveness of mud cultivation in terms of sanitization or the possible transfer of microorganisms from patient to TM are also missing.
The present work aims at investigating the microbiological quality of TMs from the microbiological hygiene perspective. A dedicated laboratory method was implemented and a set of suitable indicator parameters was tested. Especially, microbial safety of every step of the TM cultivation process was evaluated to highlight critical points and peculiar contamination risks.
4. Discussion
The relevance of microbiological hygiene quality has long been neglected for TMs and literature addressing this topic is still quite limited. Sanchez-Espejo et al. [
30] evaluated the microbiological compliance of five raw clay samples used to prepare TMs with the limits proposed by the European regulations for medicinal products. Free-living pathogenic amoebas were sought in mud samples by Scaglia et al. [
29]. “Disappearance of pathogens” after maturation was reported by Galzigna et al., but no methods nor numeric results were provided [
28]. Tentatively, a study from Turkey addressed the microbial contamination of TMs, but methods and results are ambiguous and reproducibility is rather penalized [
31]. Quintela et al. [
32] published a pilot study on the microbiological quality of maturated volcanic muds: they evaluated the total microbial count at 22 and 37 °C by pour-plate method and total coliforms by membrane filtration technique. In another work they also succinctly discussed, but didn’t assess, microbial content of TMs, suggesting to take into account some indicator parameters, i.e., enterobacteria,
Streptococcus,
E. coli and total and fecal coliforms, to ensure safety of patients [
3].
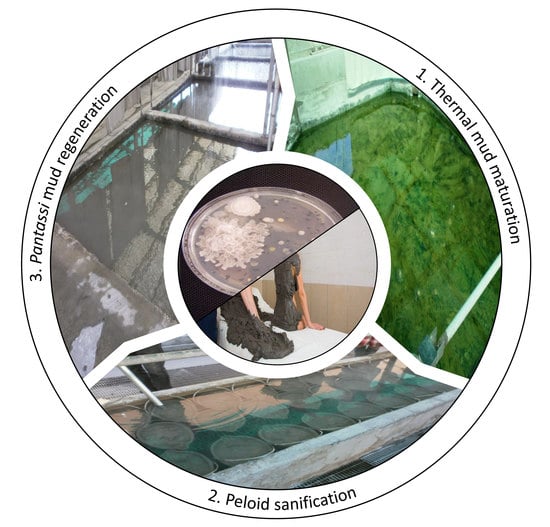
Evaluation of microbiological hygiene quality of TMs carried out in the present study encompasses a precise knowledge of both maturation process and pelotherapy protocol, so as to ensure a comprehensive assessment of the whole mud cultivation chain and its critical points. Surveyed facilities are located in the Euganean Thermal District, Veneto Region, NE Italy. This territory is one the oldest thermal areas in Europe and boasts an ancient pelotherapy tradition. In the Euganean thermal basin, virgin mud for pelotherapy is exclusively drawn from two mining concessions, i.e., the Lispida (N 45°16′41″ E 11°46′13″) and the (N 45°16′11″ E 11°44′37″) Arquà Lakes. Currently, a maximum amount of 1000 m
3 can be extracted per year, as virgin mud is a non-renewable resource [
18]. Thermal facilities are thus forced to reuse TM for multiple therapeutic applications. By law, virgin mud must be independently matured by each pelotherapy facility.
Maturation of virgin mud lasts several months, during which the mud is submerged with thermal water. The first hygienic issue concerns the presence of undesirable microorganisms in maturing mud, as the result of a naturally-occurring contamination of the extraction lake sediments. Optimal maturation of TMs in terms of
bioglea growth and production of therapeutic compounds should be carried out in the 30–42 °C temperature range [
33]. Registered temperatures in M sampling sites were compared to the suggested range. A low compliance was generally observed (i.e., about 30% of facilities), since maturing muds were mostly kept at >42 °C. Although affecting the maturation process, this should not penalize hygienic aspects but rather supporting the elimination of undesired environmental microorganisms.
Once maturation is achieved, TMs are traditionally “pasteurized” with thermal water for about 24–48 h prior to therapy [
33]. Microbiological analysis of ready-to-use TMs is thus indicative of the efficacy of this intended pre-therapy sanification. Pasteurization of TMs in surveyed facilities was carried out by 75% of facilities at a temperature of ≤60 °C.
As mentioned, due to the locally enforced TM extraction limit [
18], all surveyed facilities re-use TMs for multiple treatments. The hygienic concern relevant to this step, regards the possible transfer of microorganisms from the patient’s skin to the applied mud. After treatment, the used mud is immediately transferred in a dedicated collection tank filled with thermal water. The regeneration step lasts about three days. Since regeneration will be followed by a new maturation cycle, correct management of this step, in terms of thermal water temperature, is essential to avoid persistence of microbial contaminants throughout the cultivation process. By the way, only six facilities employed water ≥60 °C amidst SS and AS. Moreover, statistical comparison of thermal water temperatures between the two sampling campaigns suggests that R temperatures were significantly lower during AS (average difference = −5 °C), probably due to the enhanced cooling of thermal water during autumn environmental conditions.
Microbiological analysis of environmental samples usually employs indicator parameters to evaluate the overall quality or potential contamination of samples: contextualization of selected indicator parameters and interpretation of obtained results is hereby presented.
Total viable count is a generic indicator parameter representative for the broad mud microbial colonization, and it is essentially unrelated to pathogen species. TVCs highlighted a copious microbial growth in all samples. Significantly higher TVC counts were observed during AS, if compared to SS. Lower thermal water temperatures reported for R tanks during AS could explain this finding, but still a similar explanation can’t be invoked for M and P samples. Supposedly, environmental variables other than thermal water temperature (e.g., sunlight, air temperature, rainfall) are capable of influencing microbial growth, but an in-depth understanding of such microbial ecological dynamics falls outside the scope of the present research.
Total coliforms encompass a broad class of bacteria commonly found in the natural environment, whereas
E. coli stands for recent fecal contamination. Total coliforms in M samples should thus not come unexpected. Virgin mud most likely carries coliforms as natural colonizers that further survive in the cultivation plant thanks to the permissive thermal water temperature of maturation tanks. Two M samples (1M-SS; 5M-AS) were found positive also for
E. coli. Its presence could depend once more on pristine contamination of virgin mud or either on a faulty regeneration procedure of the used mud. Actually, it was impossible to determine whether sampled M mud was virgin mud or regenerated mud. Consistently, temperatures of both M and R sampling sites were quite low for the two facilities (
Table 2). Hygienic quality of P samples is of core importance. Whereas presence of total coliforms in P samples can still be tolerated, absence of
E. coli should be required. Among the analyzed mature mud samples, no one resulted contaminated by
E. coli. Consistently, presence of
E. coli in TMs can be prevented by pasteurization with a correct thermal water temperature and contact time.
E. coli was found in 2 R samples as well (1R-SS with 20 CFU/g; 15R-SS with 24 CFU/g. Thermal water in involved facilities once more was too cold to ensure proper sanification of used mud (
Table 2). Similarly to coliforms, the presence of enterococci in natural muds is quite predictable and acceptable. Several studies pointed out how lake sediments are significant reservoirs of enterococci [
34]. Members of the
Staphylococcus genus can be either saprophytic environmental species or microorganisms transferred from the patient’s skin, so that peculiar attention was paid to the possible presence of opportunistic pathogen species
S. aureus in P and R samples. Nevertheless, no sample resulted positive for this indicator. Among the investigated parameters, anaerobic clostridia, which are commonly found in the environment, represented the most abundant bacterial class. Mud is
per se an anoxic matrix that favors the proliferation of clostridia and their variable abundance is probably influenced by the mud cultivation and mixing procedures implemented by each facility.
P. aeruginosa was isolated in two samples (4P-SS and 9M-SS). Consistently, temperature registered for sample 4P and 9M was unsuitable for sanitization purposes (
Table 2). Skin-disease causing dermatophyte fungi were sporadically isolated in 5 R samples. Their most plausible origin is direct transfer from patient’s skin to the used mud.
Statistical analysis supports the relevance of thermal water temperature on microbial growth of indicator parameters. Presence or absence of total coliforms resulted significantly linked to different temperature clusters, as shown in
Table 11. Similarly, M samples with
E. coli and R samples with enterococci significantly correlate to lower average temperatures. Although statistical significance was achieved only by the above discussed samples, a similar trend can be observed for all considered indicators and in all mud typologies, confirming the critical role of temperature on the hygienic profiling of TMs.
Overall, some of the selected indicator parameters proved suitable for the assessment of microbiological hygienic quality of TMs. Nevertheless, the need of a precise thermal water temperature guideline value for each mud cultivation phase emerged as a critical issue. A reference protocol addressing both the optimization of cultivation temperatures and setting microbiological quality requirements can now be ventured. During maturation, priority should be given to the correct growth of the bioglea, so that TMs can achieve the best therapeutic quality. A temperature range of 30–42 °C is therefore recommended. Temperatures >42 °C are strongly discouraged since, although enhancing precocious mud sanitization, they can seriously compromise the correct maturation of TMs. Hygienic implications effectively come into play in the pre-therapy pasteurization step. It should be desirable for thermal water in P tanks to be at 60–65 °C. Of future interest, the multidisciplinary evaluation of a temperature range with an upper limit, so as to grant sanification without irretrievably denaturing therapeutic compounds. Furthermore, it should be compulsory to thoroughly sanitize the used mud before they start a new maturation cycle. Thermal water in regeneration tanks should therefore be kept at a temperature of ≥65 °C, for at least 72 h. Disruption of therapeutic compounds and of bio-active microorganisms should not be feared in this case, since the use of a “starter” bioglea can efficiently promote a new maturation process [
33]. Of course, since only cultivation techniques of the Euganean basin were considered in the present study, a limitation of the above proposal is that it should be calibrated for other pelotherapy districts. However, the general assessment of hygiene-related critical points of the mud cultivation chain is transferable to other pelotherapy operating facilities.
To the author’s knowledge, there is no national nor international legislation specifically addressing microbial requirements for thermal muds and peloids. Cosmetics regulations can possibly share some similarity, if considering the clayey nature of peloids. The European Regulation (EC) No. 1223/2009 recommends peculiar attention to microbiological purity of topical products to be used on mucous membranes in general, on damaged skin. It also stresses the importance of microbiological quality of products, if used by immunocompromised persons or by elderly people, in reason of their physiological immunosenescence. Safety issues resulting from microbiologically contaminated topical products are sporadic (e.g., infections caused by Gram-negative organisms), yet not negligible [
35,
36]. Similar recommendations could also suit peloids, especially if taking into account the typical pelotherapy applications and the elderly patients. Nevertheless, hygienic quality of thermal muds should not only focus on raw materials (i.e., virgin mud) and the “finished product” (i.e., the ready-for-therapy peloid). Quality of regenerated muds should definitely be granted by all facilities that reuse them for multiple pelotherapy applications on different patients. Of course thermal muds and peloids need not to be sterile, but they certainly should not be contaminated with undesired or potentially pathogenic microorganisms.
Among the assessed indicator parameters, some resulted not informative in respect of the hygienic quality characterization of TMs. In detail, TVCs and clostridia resulted too numerically variable among different samples to provide a reliable reference index; total coliforms and enterococci were commonly found as part of the environmental microflora. Recommended indicator parameters and guideline values thus are: absence (i.e., 0 CFU/g) of E. coli, Pseudomonas aeruginosa, Staphylococcus aureus and dermatophytes. Moreover, microbiological hygiene requirements can be reasonably demanded only for ready-for-therapy TMs, after a proper pasteurization step is carried out.
In the end, some considerations about the methodological choices are shared. The present study adopted a classic bacteriology approach after considering some pragmatic and scope-determined aspects. In the first place, a large number of mud samples (i.e., 180) had to be processed, so that the affordability of culture methods certainly played a role. Moreover, culture methods provide precious quantitative details and evaluate the microorganism vitality, a data crucially relevant for a complete risk assessment. Molecular methods such as microarrays or next generation sequencing (e.g., [
27,
37]) were considered to provide analytical details even beyond the core aim of this pilot research.