Modelling Malaria Incidence in the Limpopo Province, South Africa: Comparison of Classical and Bayesian Methods of Estimation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Frame and Data Collection
2.1.1. Classical Methods
Poisson Regression Model
Negative Binomial (NB) Model
Maximum Likelihood Estimation
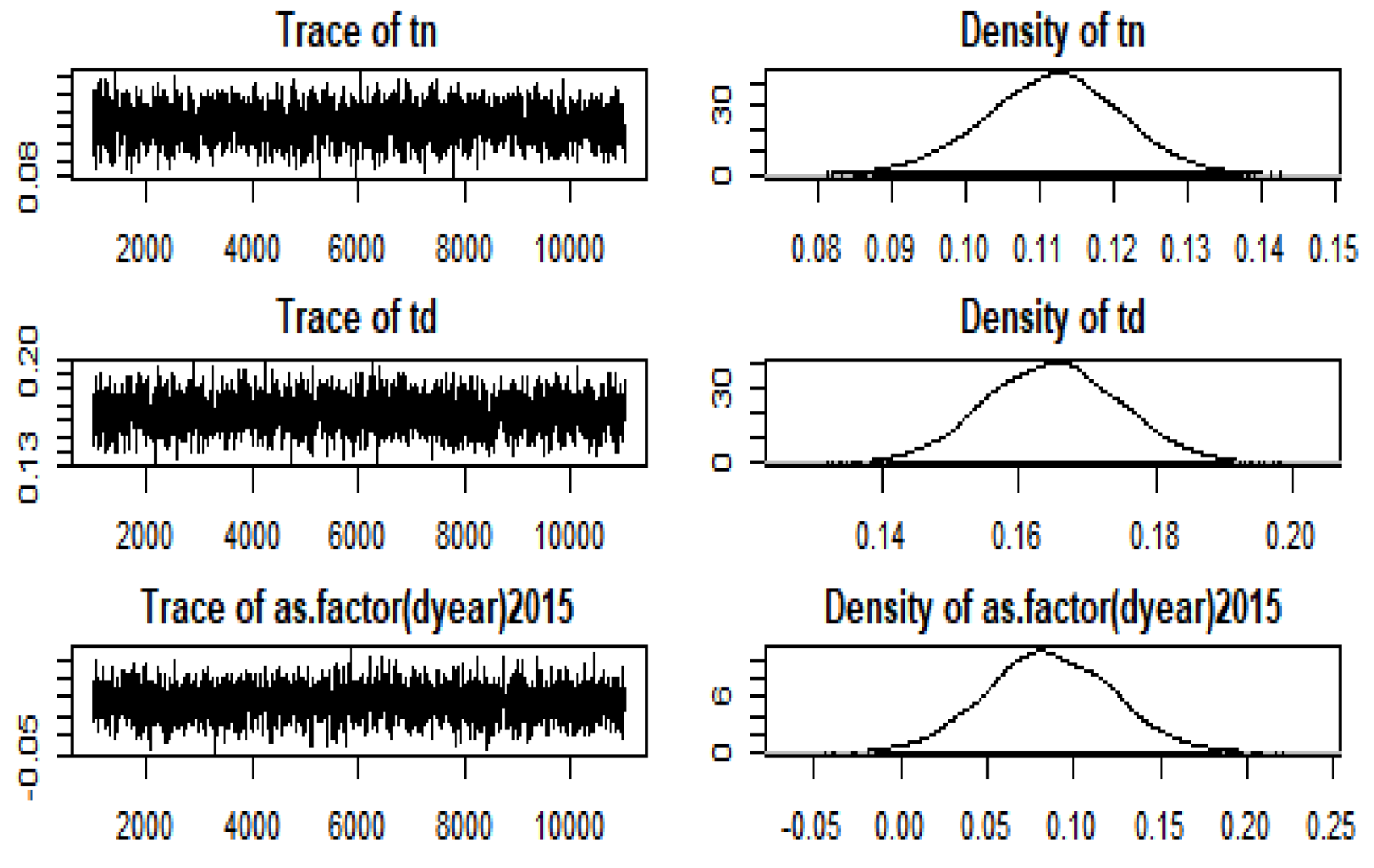
2.1.2. Bayesian Approach
2.1.3. Computation of NB Using Bayesian Estimation
- for draw from its PG ~ distribution
- for define ,
- update from distribution,
- update using a random-walk Metropolis-Hastings algorithm.
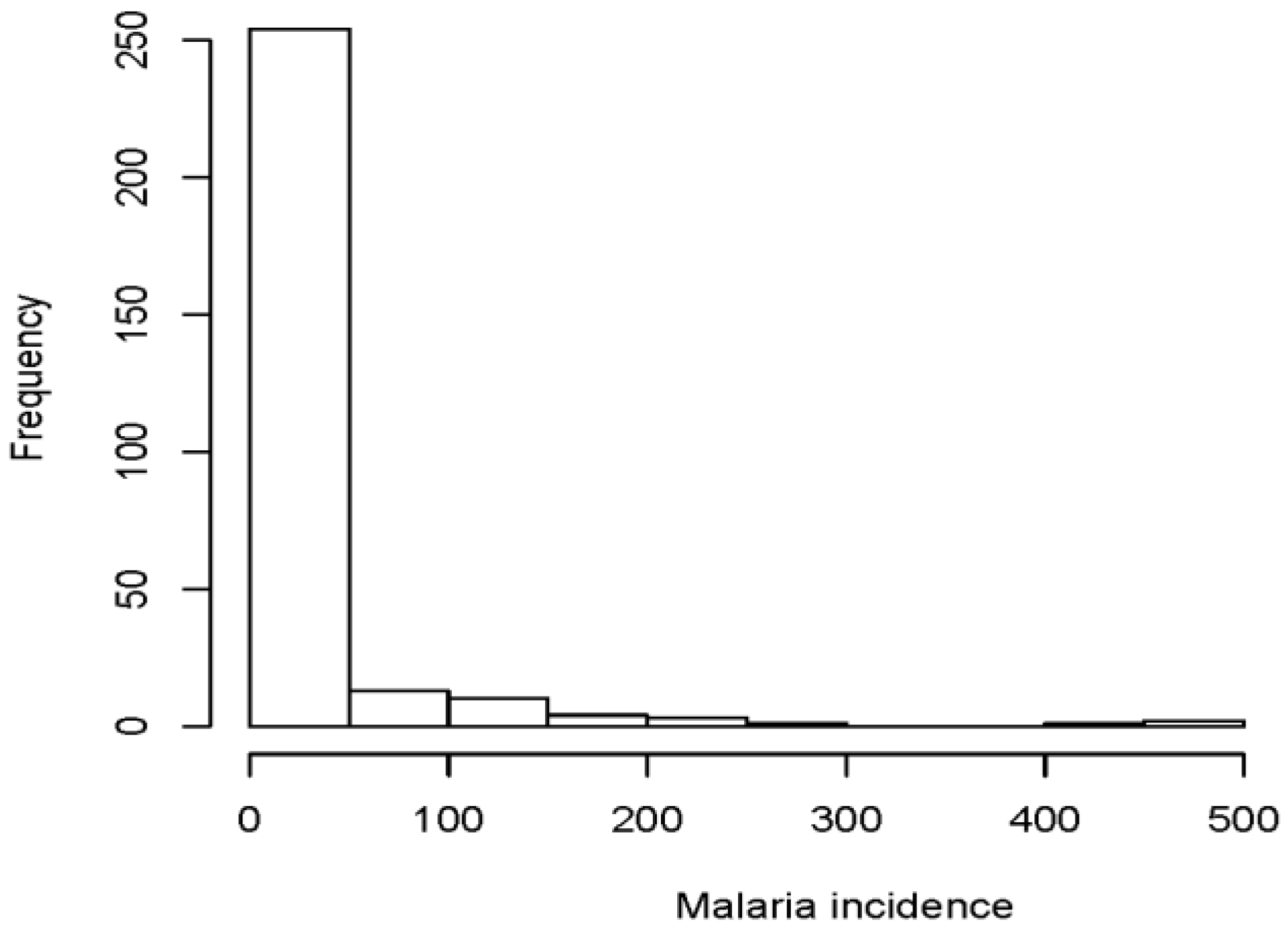
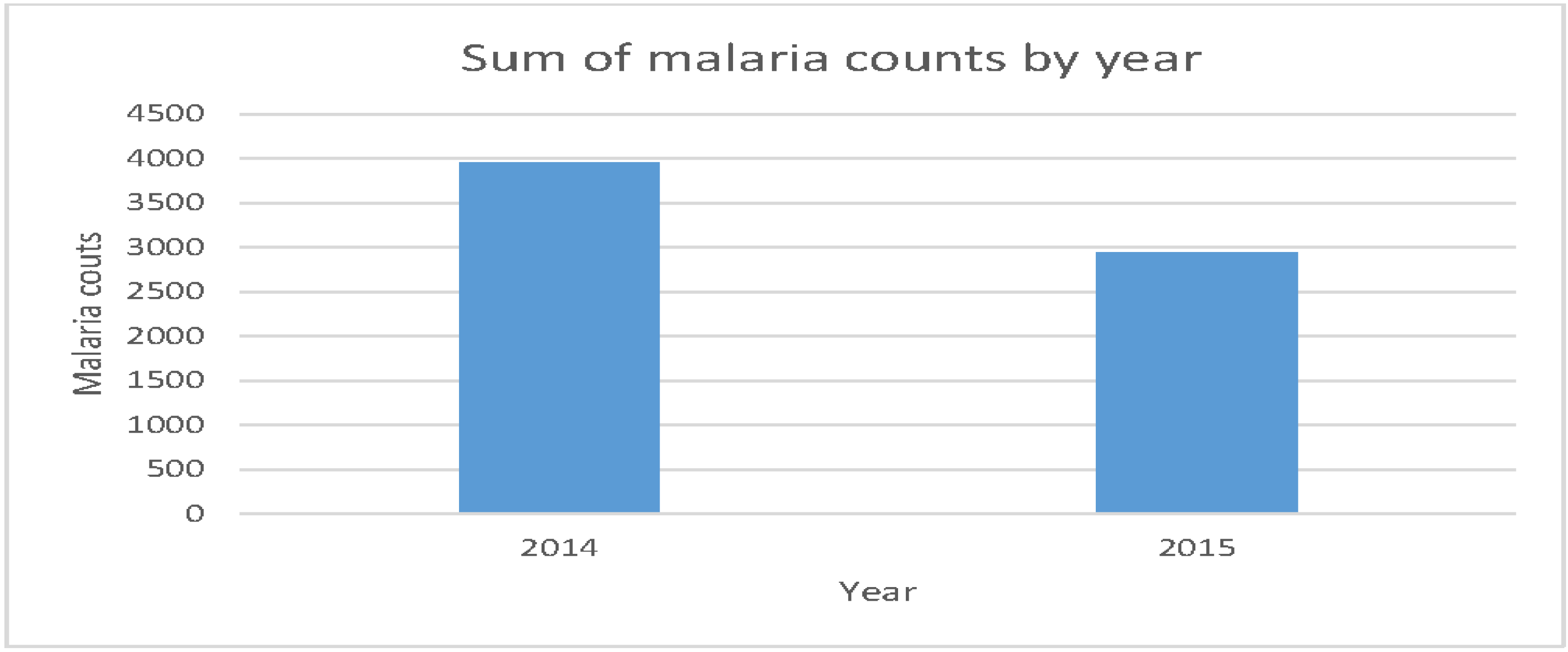
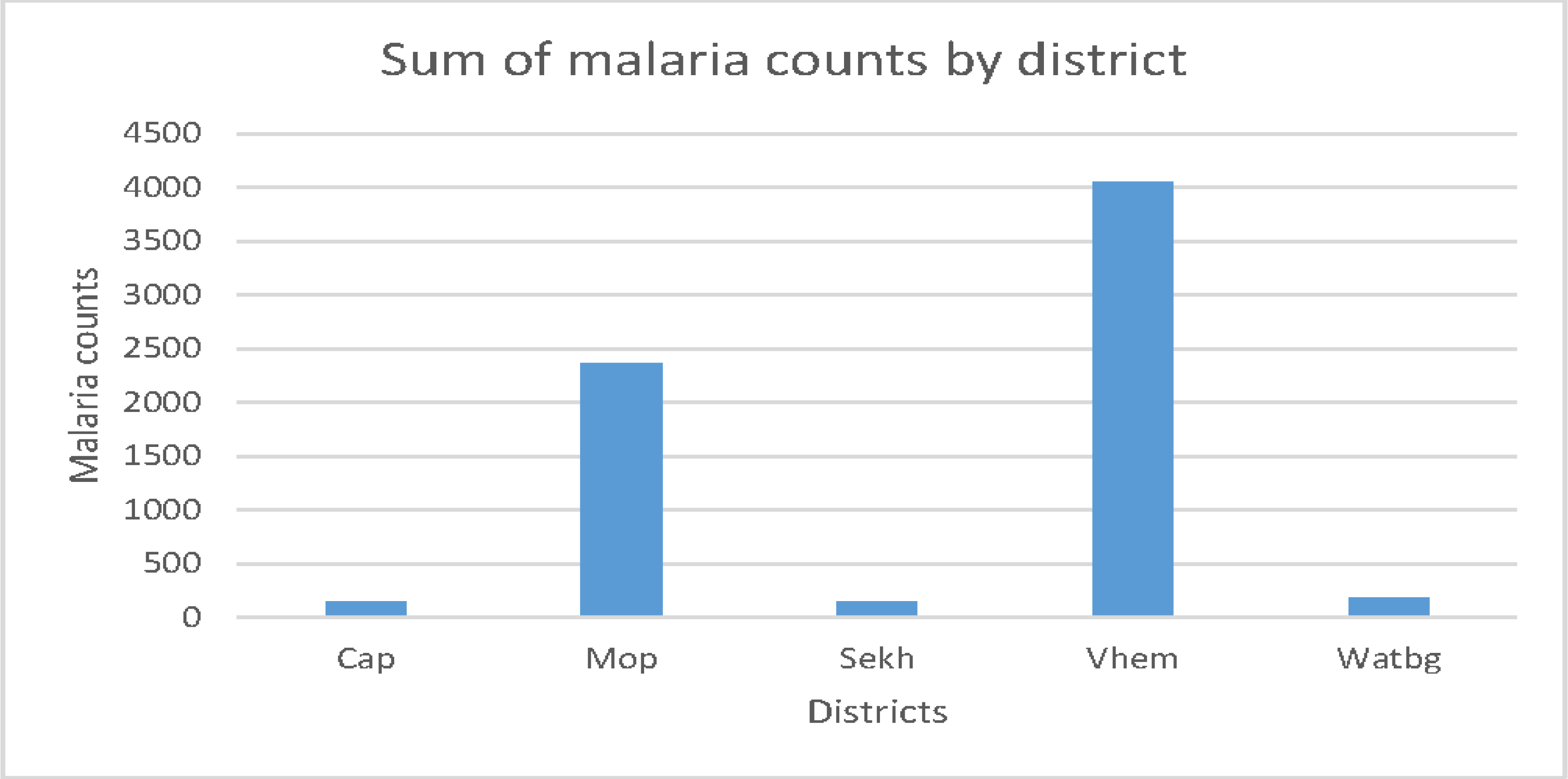
3. Results
Detection of Over-Dispersion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Snow, R.W. Global malaria eradication and the importance of Plasmodium falciparum epidemiology in Africa. BMC Med. 2015, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.N.; Guidera, K.E.; Simon, M.J.; Nonyane, B.A.; Brieger, W.; Bornman, M.S.; Kruger, P.S. Interactive malaria education intervention and its effect on community participant knowledge: The malaria awareness program in Vhembe district, Limpopo, South Africa. Int. Q. Community Health Educ. 2018, 38, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S. Travel to malaria areas. What’s the fuss about the buzz? SA Pharm. Assist. 2017, 17, 27–30. [Google Scholar]
- Sachs, J.; Malaney, P. The economic and social burden of malaria. Nature 2002, 415, 680. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Snow, R.W.; Rogers, D.J. From predicting mosquito habitat to malaria seasons using remotely sensed data: Practice, problems and perspectives. Parasitol. Today 1998, 14, 306–313. [Google Scholar] [CrossRef]
- Nájera, J.A.; Kouznetsov, R.L.; Delacollette, C.; World Health Organization. Malaria Epidemics: Detection and Control, Forecasting and Prevention; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Abiodun, G.J.; Makinde, O.S.; Adeola, A.M.; Njabo, K.Y.; Witbooi, P.J.; Djidjou-Demasse, R.; Botai, J.O. A dynamical and zero-inflated negative binomial regression modelling of malaria incidence in Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2019, 16, 2000. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, G.J.; Adebiyi, B.; Abiodun, R.O.; Oladimeji, O.; Oladimeji, K.E.; Adeola, A.M.; Makinde, O.S.; Okosun, K.O.; Djidjou-Demasse, R.; Semegni, Y.J.; et al. Investigating the resurgence of malaria prevalence in South Africa between 2015 and 2018: A scoping review. Open Public Health J. 2020, 13, 119–125. [Google Scholar] [CrossRef]
- Blumberg, L.; Frean, J. Malaria reduces globally but rebounds across southern Africa. S. Afr. J. Infect. Dis. 2017, 32, 3–4. [Google Scholar] [CrossRef]
- Raman, J.; Morris, N.; Frean, J.; Brooke, B.; Blumberg, L.; Kruger, P.; Mabusa, A.; Raswiswi, E.; Shandukani, B.; Misani, E.; et al. Reviewing South Africa’s malaria elimination strategy (2012–2018): Progress, challenges and priorities. Malar. J. 2016, 15, 438. [Google Scholar] [CrossRef] [PubMed]
- Yé, Y.; Louis, V.R.; Simboro, S.; Sauerborn, R. Effect of meteorological factors on clinical malaria risk among children: An assessment using village-based meteorological stations and community-based parasitological survey. BMC Public Health 2007, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Shimaponda-Mataa, N.M.; Tembo-Mwase, E.; Gebreslasie, M.; Achia, T.N.; Mukaratirwa, S. Modelling the influence of temperature and rainfall on malaria incidence in four endemic provinces of Zambia using semiparametric Poisson regression. Acta Trop. 2017, 1, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Omonijo, A.G.; Matzarakis, A.; Oguntoke, O.; Adeofun, C.O. Influence of weather and climate on malaria occurrence based on human-biometeorological methods in Ondo State, Nigeria. J. Environ. Sci. Eng. 2011, 1, 5. [Google Scholar]
- Zayeri, F.; Salehi, M.; Pirhosseini, H. Geographical mapping and Bayesian spatial modeling of malaria incidence in Sistan and Baluchistan province, Iran. Asian Pac. J. Trop. Med. 2011, 4, 985–992. [Google Scholar] [CrossRef]
- Kleinschmidt, I.; Sharp, B.L.; Clarke, G.P.; Curtis, B.; Fraser, C. Use of generalized linear mixed models in the spatial analysis of small-area malaria incidence rates in KwaZulu Natal, South Africa. Am. J. Epidemiol. 2001, 153, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Kazembe, L.N. Spatial modelling and risk factors of malaria incidence in northern Malawi. Acta Trop. 2007, 102, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Ramalata, A. Modeling the Incidence of Malaria Cases in the Limpopo Province, South Africa, 2014–2017. Master’s Dissertation, School of Mathematical and Computer Sciences, University of Limpopo, Polokwane, South Africa, 2017. [Google Scholar]
- Gosoniu, L.; Vounatsou, P.; Sogoba, N.; Smith, T. Bayesian modelling of geostatistical malaria risk data. Geospat. Health 2006, 1, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, A.A.; Kruger, P.; van der Loeff, M.F.; Grobusch, M.P. Malaria incidence in Limpopo Province, South Africa, 1998–2007. Malar. J. 2008, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Adeola, A.; Botai, J.; Rautenbach, H.; Adisa, O.; Ncongwane, K.; Botai, C.; Adebayo-Ojo, T. Climatic variables and malaria morbidity in Mutale Local Municipality, South Africa: A 19-year data analysis. Int. J. Environ. Res. Public Health 2017, 14, 1360. [Google Scholar] [CrossRef] [PubMed]
- Komen, K.; Olwoch, J.; Rautenbach, H.; Botai, J.; Adebayo, A. Long-run relative importance of temperature as the main driver to malaria transmission in Limpopo Province, South Africa: A simple econometric approach. EcoHealth 2015, 12, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Machimana, G.G. Spatial Distribution of Malaria Cases in Mopani District, Limpopo Province, South Africa 2006–2015. Master’s Dissertation, Faculty of Health Sciences, University of Limpopo, Polokwane, South Africa, 2016. [Google Scholar]
- Statistics South Africa. Available online: www.statssa.gov (accessed on 18 January 2015).
- Cameron, A.C.; Trivedi, P.K. Regression Analysis of Count Data; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
| Variable | Description | Data Set Code |
|---|---|---|
| Malaria | The number of malaria cases. | mal |
| Population | The population size. | pop |
| Districts | The five districts of Limpopo province: Capricorn, Mopani, Sekhukhune, Vhembe and Waterberg. | dist |
| Years | The data for which the data were collected: 2014 and 2015. | dyear |
| Elevation | The elevation above sea level measured in meters. | ele |
| Rainfall | The rainfall measured in millimetres. | rain |
| NDVI | The difference between near-infrared reflected by the vegetation and red light which is absorbed by the vegetation, it ranges from −1 to 1. | ndvi |
| Temperature during the day | The maximum temperature in degrees Celsius. | td |
| Temperature during the night | Minimum temperature in degrees Celsius. | tn |
| Statistics | Ele | Tn | Td | NDVI | Rain | Mal |
|---|---|---|---|---|---|---|
| Min | 19.690 | 4.920 | 20.990 | 0.210 | 0.000 | 0.000 |
| Max | 822.241 | 25.600 | 40.240 | 0.660 | 159.200 | 4820.00 |
| Mean | 325.270 | 16.250 | 31.660 | 0.410 | 32.591 | 23.95 |
| Median | 242.200 | 16.710 | 32.441 | 0.400 | 23.910 | 3.000 |
| Std. Error | 216.090 | 4.450 | 4.151 | 0.110 | 38.420 | 61.830 |
| 1st Quartile | 182.431 | 13.170 | 28.720 | 0.320 | 0.520 | 1.000 |
| 3rd Quartile | 491.181 | 19.550 | 34.640 | 0.490 | 47.021 | 12.250 |
| Coefficient | Estimate | Std. Error | 95% Confidence Intervals |
|---|---|---|---|
| Intercept | −17.850 | 0.288 | [−18.414, −17.288] ** |
| Rainfall | −0.008 | 0.000 | [−0.009, −0.007] ** |
| Province (Ref: Capricorn) | - | - | - |
| Mopane | 2.213 | 0.089 | [2.048, 2.385] ** |
| Sekhukhune | 0.537 | 0.122 | [0.297, 0.777] ** |
| Vhembe | 2.458 | 0.084 | [2.297, 2.627] ** |
| Waterberg | 0.489 | 0.111 | [0.272, 0.708] ** |
| Year (Ref: 2014) | - | - | - |
| 2015 | −0.170 | 0.029 | [−0.227, −0.114] ** |
| Temperature during the night | 0.276 | 0.007 | [0.263, 0.290] ** |
| Temperature during the day | 0.064 | 0.008 | [0.049, 0.080] ** |
| Elevation | −0.001 | 0.000 | [−0.001, −0.001] ** |
| Normalized Difference Vegetation Index | 0.521 | 0.228 | [0.074, 0.969] ** |
| Deviance | DF | ||
| Null Deviance | 18541.3 | - | 287 |
| Residual Deviance | 3532.6 | - | 277 |
| Dispersion parameter () | 12.753 | - | - |
| AIC | 4421.4 | - | - |
| Coefficient | Estimate | Std. Error | 95% Confidence Intervals |
|---|---|---|---|
| Intercept | −1.3300 | 1.0380 | [−17.4827, –13.1860] ** |
| Rainfall | −0.0051 | 0.0020 | [−0.0095, −0.0006] ** |
| Province (Ref: Capricorn) | - | - | - |
| Mopani | 2.2150 | 0.2086 | [1.7890, 2.6425] ** |
| Sekhukhune | 0.4149 | 0.2577 | [−0.0966, 0.9249] |
| Vhembe | 2.8480 | 0.2113 | [2.4123, 3.2851] ** |
| Waterberg | 0.8711 | 0.2291 | [0.3950, 1.3484] ** |
| Year (Ref: 2014) | - | - | - |
| 2015 | 0.2056 | 0.1251 | [−0.0398, 0.4521] |
| Temperature during the night | 0.2537 | 0.0325 | [0.1814, 0.3264] ** |
| Temperature during the day | 0.0000 | 0.0328 | [−0.0705, 0.0704] |
| Elevation | −0.0005 | 0.0005 | [−0.0014, 0.0005] |
| Normalized Vegetation Index | 0.4769 | 1.0140 | [−2.5551, 1.6125] |
| Deviance | DF | ||
| Null Deviance | 1232.31 | - | 287 |
| Residual Deviance | 317.87 | - | 277 |
| Dispersion parameter () | 1.148 | - | - |
| AIC | 1680.9 | - | - |
| Coefficient | Estimate | Naive Std. Error | 95% HPD Credible Intervals |
|---|---|---|---|
| Intercept | −8.179 | 0.005 | [−8.830, −7.516] ** |
| Rainfall | −0.001 | 0.000 | [−0.003, −0.000] ** |
| Province (Ref: Capricorn) | - | - | - |
| Mopani | 2.169 | 0.001 | [1.994, 2.329] ** |
| Sekhukhune | 0.335 | 0.002 | [0.090, 0.562] ** |
| Vhembe | 2.817 | 0.001 | [2.664, 2.992] ** |
| Waterberg | −0.219 | 0.002 | [−0.454, −0.006] |
| Year (Ref: 2014) | - | - | - |
| 2015 | 0.086 | 0.001 | [0.013, 0.157] ** |
| Temperature during the night | 0.111 | 0.000 | [0.093, 0.129] ** |
| Temperature during the day | 0.165 | 0.000 | [0.146, 0.184] ** |
| Elevation | −0.004 | 0.000 | [−0.001, −0.000] ** |
| Normalized Vegetation Index | 4.911 | 0.004 | [4.343, 5.457] ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehlabana, M.A.; Maposa, D.; Boateng, A. Modelling Malaria Incidence in the Limpopo Province, South Africa: Comparison of Classical and Bayesian Methods of Estimation. Int. J. Environ. Res. Public Health 2020, 17, 5016. https://doi.org/10.3390/ijerph17145016
Sehlabana MA, Maposa D, Boateng A. Modelling Malaria Incidence in the Limpopo Province, South Africa: Comparison of Classical and Bayesian Methods of Estimation. International Journal of Environmental Research and Public Health. 2020; 17(14):5016. https://doi.org/10.3390/ijerph17145016
Chicago/Turabian StyleSehlabana, Makwelantle Asnath, Daniel Maposa, and Alexander Boateng. 2020. "Modelling Malaria Incidence in the Limpopo Province, South Africa: Comparison of Classical and Bayesian Methods of Estimation" International Journal of Environmental Research and Public Health 17, no. 14: 5016. https://doi.org/10.3390/ijerph17145016
APA StyleSehlabana, M. A., Maposa, D., & Boateng, A. (2020). Modelling Malaria Incidence in the Limpopo Province, South Africa: Comparison of Classical and Bayesian Methods of Estimation. International Journal of Environmental Research and Public Health, 17(14), 5016. https://doi.org/10.3390/ijerph17145016





