Incidence, Prevalence, and Risk Factors of Hemiplegic Shoulder Pain: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction and Assessment of the Risk of Bias
2.5. Summary Statistics and Synthesis of Results
3. Results
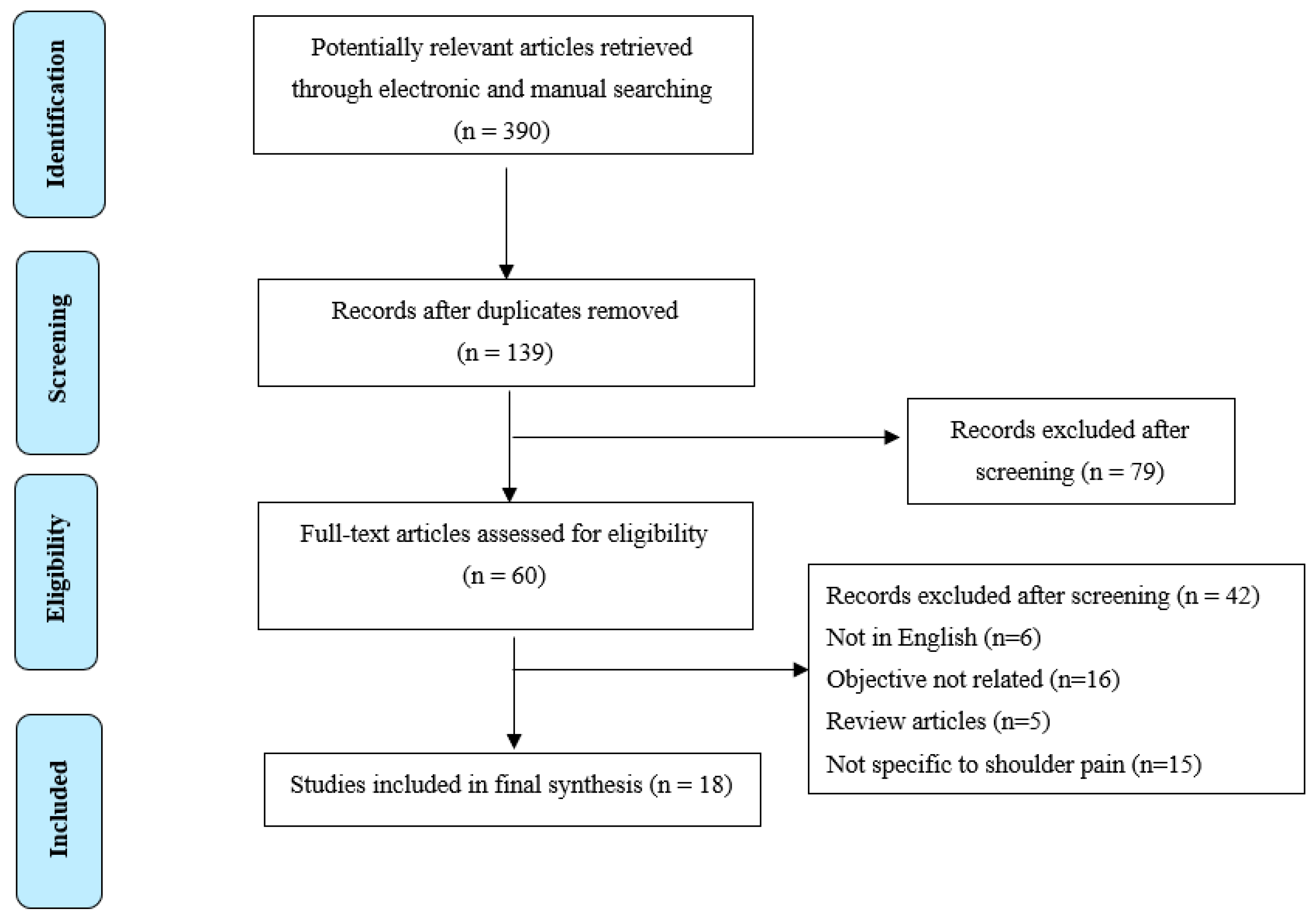
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Methodological Quality
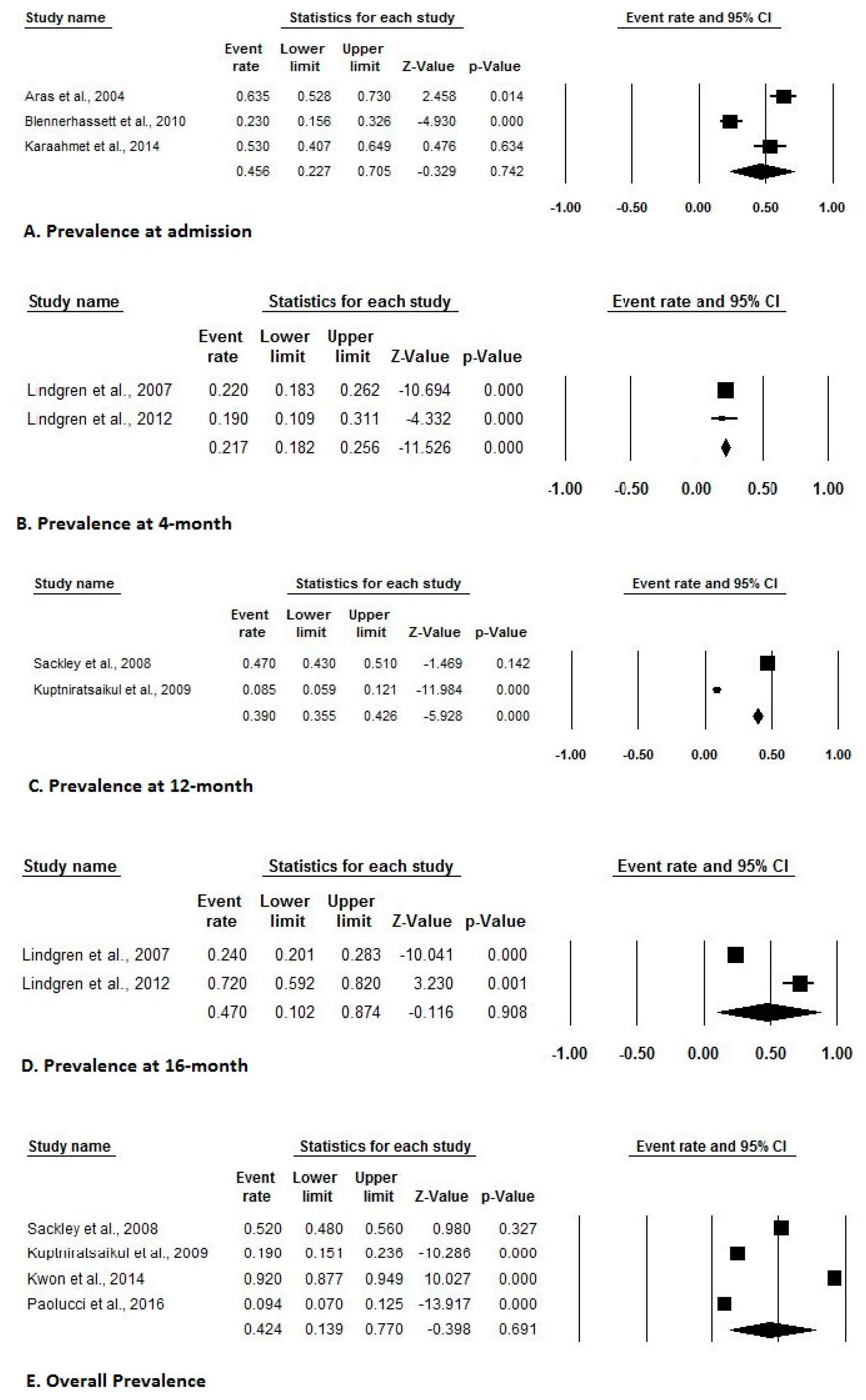
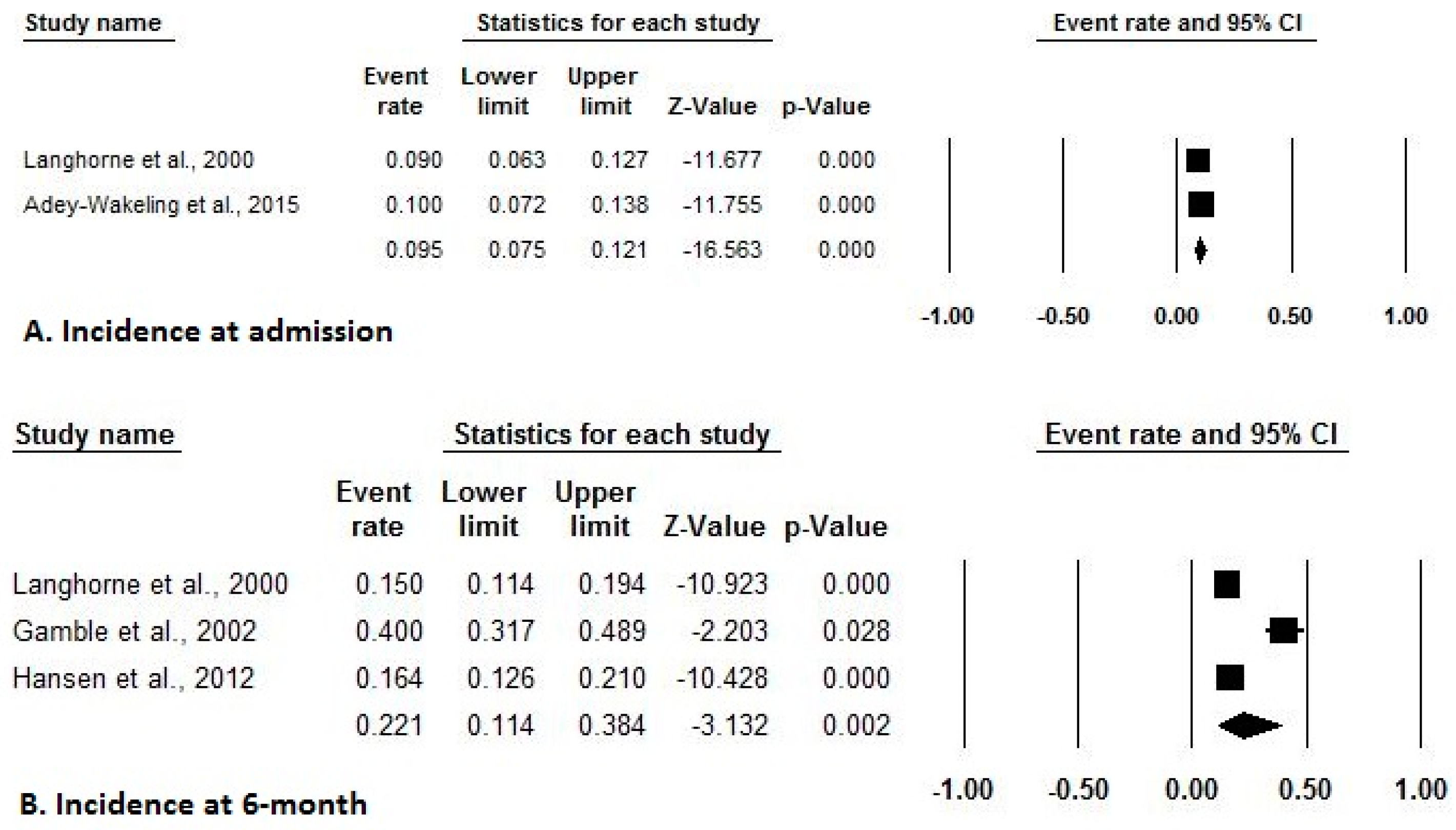
3.4. Incidence and Prevalence of HSP after Stroke
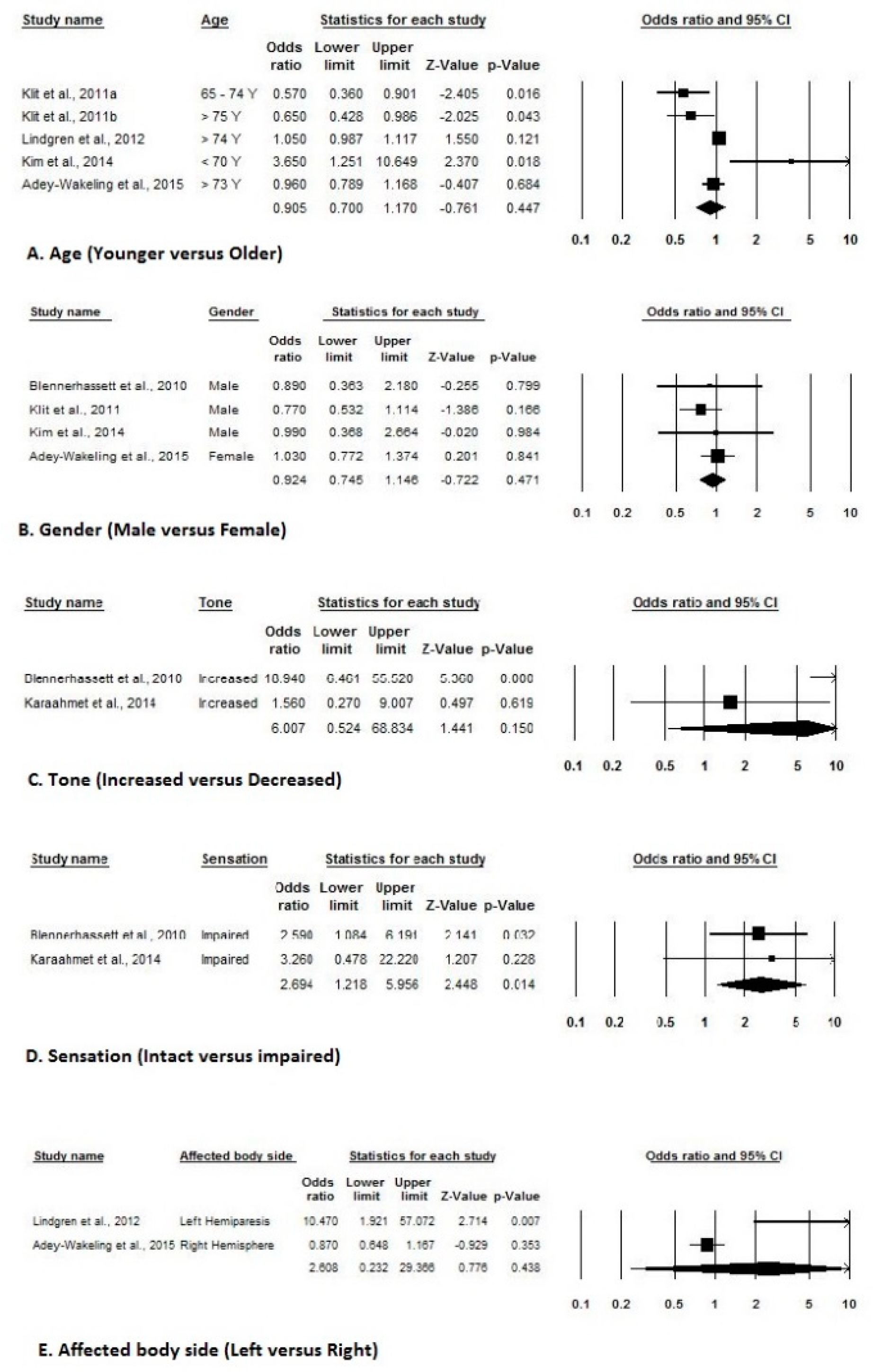
3.5. Risk Factors of HSP after Stroke
3.6. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, R.; Zhou, M.; Cai, H.; Guo, Y.; Zhan, L.; Li, M.; Yang, Z.; Zhu, L.; Zhan, J.; Chen, H. A randomized controlled trial of a modified wheelchair arm-support to reduce shoulder pain in stroke patients. Clin. Rehabil. 2017. [Google Scholar] [CrossRef] [PubMed]
- Poduri, K.R. Shoulder pain in stroke patients and its effects on rehabilitation. J. Stroke Cerebrovasc. Dis. 1993, 3, 261–266. [Google Scholar] [CrossRef]
- Adey-Wakeling, Z.; Arima, H.; Crotty, M.; Leyden, J.; Kleinig, T.; Anderson, C.S.; Newbury, J. Incidence and associations of hemiplegic shoulder pain poststroke: Prospective population-based study. Arch. Phys. Med. Rehabil. 2015, 96, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, J.M.; Vasudevan, S.V. Hemiplegic shoulder pain: Diagnosis and management. Crit. Rev. Phys. Rehabil. Med. 2008, 20, 207–220. [Google Scholar] [CrossRef]
- Murie-Fernández, M.; Carmona Iragui, M.; Gnanakumar, V.; Meyer, M.; Foley, N.; Teasell, R. Painful hemiplegic shoulder in stroke patients: Causes and management. Neurologia 2012, 27, 234–244. [Google Scholar] [CrossRef]
- Lindgren, I.; Jonsson, A.C.; Norrving, B.; Lindgren, A. Shoulder pain after stroke: A prospective population-based study. Stroke 2007, 38, 343–348. [Google Scholar] [CrossRef]
- Koog, Y.H.; Jin, S.S.; Yoon, K.; Min, B.I. Interventions for hemiplegic shoulder pain: Systematic review of randomized controlled trials. Disabil. Rehabil. 2010, 32, 282–291. [Google Scholar] [CrossRef]
- Bender, L.; McKenna, K. Hemiplegic shoulder pain: Defining the problem and its management. Disabil. Rehabil. 2001, 23, 698–705. [Google Scholar]
- Aras, M.; Gokkaya, N.K.; Comert, D.; Kaya, A.; Cakci, A. Shoulder pain in hemiplegia - results from a national rehabilitation hospital in Turkey. Am. J. Phys. Med. Rehabil. 2004, 83, 713–719. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.E. Medical complications experienced by a cohort of stroke survivors during inpatient, tertiary-level stroke rehabilitation. Arch. Phys. Med. Rehabil. 2004, 85, 466–469. [Google Scholar] [CrossRef]
- Blennerhassett, J.M.; Gyngell, K.; Crean, R. Reduced active control and passive range at the shoulder increase risk of shoulder pain during inpatient rehabilitation post-stroke: An observational study. J. Physiother. 2010, 56, 195–199. [Google Scholar] [CrossRef]
- Dromerick, A.W.; Edwards, D.F.; Kumar, A. Hemiplegic shoulder pain syndrome: Frequency and characteristics during inpatient stroke rehabilitation. Arch. Phys. Med. Rehabil. 2008, 89, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Barlak, A.; Unsal, S.; Kaya, K.; Sahin-Onat, S.; Ozel, S. Poststroke shoulder pain in Turkish stroke patients: Relationship with clinical factors and functional outcomes. Int. J. Rehabil. Res. 2009, 32, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Stott, D.J.; Robertson, L.; Macdonald, J.; Jones, L.; McAlpine, C.; Dick, F.; Taylor, G.S.; Murray, G. Medical complications after stroke: A multicenter study. Stroke 2000, 31, 1223–1229. [Google Scholar] [CrossRef]
- Ratnasabapathy, Y.; Broad, J.; Baskett, J.; Pledger, M.; Marshall, J.; Bonita, R. Shoulder pain in people with a stroke: A population-based study. Clin. Rehabil. 2003, 17, 304–311. [Google Scholar] [CrossRef]
- Gamble, G.E.; Barberan, E.; Laasch, H.U.; Bowsher, D.; Tyrrell, P.J.; Jones, A.K. Post stroke shoulder pain: A prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. Eur. J. Pain 2002, 6, 467–474. [Google Scholar] [CrossRef]
- Demirci, A.; Öcek, B.; Köseoglu, F. Shoulder pain in hemiplegic patients. J. Phys. Med. Rehabil. Sci. 2007, 1, 25–30. [Google Scholar]
- Ikai, T.; Tei, K.; Yoshida, K.; Miyano, S.; Yonemoto, K. Evaluation and treatment of shoulder subluxation in hemiplegia: Relationship between subluxation and pain. Am. J. Phys. Med. Rehabil. 1998, 77, 421–426. [Google Scholar] [CrossRef]
- Hanger, H.C.; Whitewood, P.; Brown, G.; Ball, M.C.; Harper, J.; Cox, R.; Sainsbury, R. A randomized controlled trial of strapping to prevent post-stroke shoulder pain. Clin. Rehabil. 2000, 14, 370–380. [Google Scholar] [CrossRef]
- Gustafsson, L.; McKenna, K. A programme of static positional stretches does not reduce hemiplegic shoulder pain or maintain shoulder range of motion—A randomized controlled trial. Clin. Rehabil. 2006, 20, 277–286. [Google Scholar] [CrossRef]
- Andrews, A.W.; Bohannon, R.W. Decreased shoulder range of motion on paretic side after stroke. Phys. Ther. 1989, 69, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Sher, J.S.; Andersen, W.K.; Garretson, R.; Uribe, J.W.; Hechtman, K.; Neviaser, R.J. Glenohumeral motion in patients with rotator cuff tears: A comparison of asymptomatic and symptomatic shoulders. J. Shoulder Elbow Surg. 2000, 9, 6–11. [Google Scholar] [CrossRef]
- Chantraine, A.; Baribeault, A.; Uebelhart, D.; Gremion, G. Shoulder pain and dysfunction in hemiplegia: Effects of functional electrical stimulation. Arch. Phys. Med. Rehabil. 1999, 80, 328–331. [Google Scholar] [CrossRef]
- Vuagnat, H.; Chantraine, A. Shoulder pain in hemiplegia revisited: Contribution of functional electrical stimulation and other therapies. J. Rehabil. Med. 2003, 35, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Meskers, C.G.; Koppe, P.A.; Konijnenbelt, M.H.; Veeger, D.H.; Janssen, T.W. Kinematic alterations in the ipsilateral shoulder of patients with hemiplegia due to stroke. Am. J. Phys. Med. Rehabil. 2005, 84, 97–105. [Google Scholar] [CrossRef][Green Version]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Ottawa Health Research Institute: Ottawa, ON, Canada, 2010. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Gamble, G.E.; Barberan, E.; Bowsher, D.; Tyrrell, P.J.; Jones, A.K. Post stroke shoulder pain: More common than previously realized. Eur. J. Pain 2000, 4, 313–315. [Google Scholar] [CrossRef]
- Sackley, C.; Brittle, N.; Patel, S.; Ellins, J.; Scott, M.; Wright, C.; Dewey, M. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke 2008, 39, 3329–3334. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Kovindha, A.; Suethanapornkul, S.; Manimmanakorn, N.; Archongka, Y. Complications during the rehabilitation period in Thai patients with stroke: A multicenter prospective study. Am. J. Phys. Med. Rehabil. 2009, 88, 92–99. [Google Scholar] [CrossRef]
- Klit, H.; Finnerup, N.B.; Overvad, K.; Andersen, G.; Jensen, T.S. Pain following stroke: A population-based follow-up study. PLoS ONE 2011, 6, e27607. [Google Scholar] [CrossRef]
- Hansen, A.P.; Marcussen, N.S.; Klit, H.; Andersen, G.; Finnerup, N.B.; Jensen, T.S. Pain following stroke: A prospective study. Eur. J. Pain 2012, 16, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, I.; Lexell, J.; Jönsson, A.C.; Brogårdh, C. Left-sided hemiparesis, pain frequency, and decreased passive shoulder range of abduction are predictors of long-lasting poststroke shoulder pain. PMR 2012, 4, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Kuptniratsaikul, V.; Kovindha, A.; Suethanapornkul, S.; Manimmanakorn, N.; Archongka, Y. Long-term morbidities in stroke survivors: A prospective multicenter study of Thai stroke rehabilitation registry. BMC Geriatr. 2013, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Hsu, H.; Chang, C.-H.; Lin, C.-H.; Chen, K.-H.; Hsieh, W.-C.; Chang, W.-M. Age-based prediction of incidence of complications during inpatient stroke rehabilitation: A retrospective longitudinal cohort study. BMC Geriatr. 2014, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jung, S.J.; Yang, E.J.; Paik, N.J. Clinical and sonographic risk factors for hemiplegic shoulder pain: A longitudinal observational study. J. Rehabil. Med. 2014, 46, 81–87. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kwon, J.W.; Lee, N.K.; Kang, K.W.; Son, S.M. Prevalence and determinants of pain in the ipsilateral upper limb of stroke patients. Percept. Mot. Skills 2014, 119, 799–810. [Google Scholar] [CrossRef]
- Karaahmet, O.Z.; Eksioglu, E.; Gürçay, E.; Karsli, P.B.; Tamkan, U.; Bal, A.; Cakcı, A.; Cakci, A. Hemiplegic shoulder pain: Associated factors and rehabilitation outcomes of hemiplegic patients with and without shoulder pain. Top. Stroke Rehabil. 2014, 21, 237–245. [Google Scholar] [CrossRef]
- Paolucci, S.; Iosa, M.; Barbanti, P.; Bovi, P.; Candeloro, E.; Mancini, A.; Monaco, S.; Pieroni, A.; Truini, A.; Toni, D.; et al. Prevalence and Time Course of Post-Stroke Pain: A Multicenter Prospective Hospital-Based Study. Pain Med. 2016, 17, 924–930. [Google Scholar] [CrossRef]
- Griffin, J.W. Hemiplegic shoulder pain. Phys. Ther. 1986, 66, 1884–1893. [Google Scholar] [CrossRef]
- Roy, C.W. Shoulder pain in hemiplegia: A literature review. Clin. Rehabil. 1988, 2, 35–44. [Google Scholar] [CrossRef]
- Bruton, J.D. Shoulder pain in stroke patients with hemiplegia or hemiparesis following a cerebrovascular accident. Physiotherapy 1985, 71, 2–4. [Google Scholar]
- Krotenberg, R. Shoulder pain in hemiplegia. Adv. Clin. Rehabil. 1990, 3, 189–196. [Google Scholar] [PubMed]
- Wanklyn, P. The painful hemiplegic shoulder: Pathogenesis, diagnosis and management. Rev. Clin. Gerontol. 1994, 4, 245–251. [Google Scholar] [CrossRef]
- Griffin, J.; Reddin, G. Shoulder pain in patients with hemiplegia. A literature review. Phys. Ther. 1981, 61, 1041–1045. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Larkin, A.P.; Smith, M.B.; Horton, M.G. Shoulder pain in hemiplegia: Statistical relationship with five variables. Arch. Phys. Med. Rehabil. 1986, 67, 514–516. [Google Scholar]
- Coskun Benlidayi, I.; Basaran, S. Hemiplegic shoulder pain: A common clinical consequence of stroke. Pract. Neurol. 2014, 14, 88–91. [Google Scholar] [CrossRef]
- Kalichman, L.; Ratmansky, M. Underlying pathology and associated factors of hemiplegic shoulder pain. Am. J. Phys. Med. Rehabil. 2011, 90, 768–780. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Jackson, D. Shoulder pain after stroke: A review of the evidence base to inform the development of an integrated care pathway. Clin. Rehabil. 2002, 16, 276–298. [Google Scholar] [CrossRef]
- Wanklyn, P.; Forster, A.; Young, J. Hemiplegic shoulder pain (HSP): Natural history and investigation of associated features. Disabil. Rehabil. 1996, 18, 497–501. [Google Scholar] [CrossRef]
- Hadianfard, H.; Hadianfard, M.J. Predictor factors of hemiplegic shoulder pain in a group of stroke patients. Iran Red Crescent Med. J. 2008, 10, 215–219. [Google Scholar]
- Vasudevan, J.M.; Browne, B.J. Hemiplegic shoulder pain: An approach to diagnosis and management. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Roosink, M.; Renzenbrink, G.J.; Buitenweg, J.R.; Van Dongen, R.T.; Geurts, A.C.; IJzerman, M.J. Persistent shoulder pain in the first 6 months after stroke: Results of a prospective cohort study. Arch. Phys. Med. Rehabil. 2011, 92, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Van Ouwenaller, C.; Laplace, P.M.; Chantraine, A. Painful shoulder in hemiplegia. Arch. Phys. Med. Rehabil. 1986, 67, 23–25. [Google Scholar]
- Gillen, G. Upper Extremity Function and Management In Stroke Rehabilitation: A Function-Based Approach; Gillen, G., Burkhardt, A., Eds.; Mosby: St. Louis, MO, USA, 1998; pp. 109–151. [Google Scholar]
- O’Sullivan, S.B.; Schmitz, T.J. Physical Rehabilitation: Assessment and Treatment, 3rd ed.; FA Davis: Philadelphia, PA, USA, 1995; pp. 327–360. [Google Scholar]
- Iannotti, J.P.; Williams, G.R. Disorders of the Shoulder: Diagnosis and Management; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Mao, C.Y.; Jaw, W.C.; Cheng, H.C. Frozen shoulder: Correlation between the response to physical therapy and follow-up shoulder arthrography. Arch. Phys. Med. Rehabil. 1997, 78, 857–859. [Google Scholar] [CrossRef]

| PubMed | Web of Science | Scopus | |
|---|---|---|---|
| Date | 19-Nov-2019 | 19-Nov-2019 | 19-Nov-2019 |
| “Stroke” OR “Hemiplegia” | 291,950 | 296,060 | 395,489 |
| “Shoulder” OR “Arm” OR “Shoulder joint” | 213,412 | 228,005 | 450,679 |
| “Shoulder pain” OR “Pain” | 678,816 | 538,019 | 1,038,105 |
| “Prevalence” OR “Incidence” | 1,323,666 | 1,288,045 | 1,882,741 |
| Combined search | 94 | 115 | 181 |
| Total minus duplicates | 139 | ||
| Studies | Selection | Comparability | Outcome | Quality Score † | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of Interest Was Not Present at Start of Study | Comparability of cohorts based on the design or analysis | Assessment of outcome | Follow-Up Long Enough for Outcome to Occur (Median Duration of Follow-Up ≥ 6 Months) | Adequacy of follow up of cohorts | ||
| Langhorne et al. (2000) [14] | Three major hospitals in the West of Scotland were participated. Two hospitals provided acute stroke patient care and one hospital provide acute stroke rehabilitation care after one-week discharge. ★ | No non-exposed cohort | Weekly assessments in hospitals by 3 research nurses ★ | Yes ★ | Complication subdivided by their baseline level of dependency and compared using Chi-square test. ★ | Questionnaire based structured interview ★ | Yes ★ | 100% participated at the 6-month follow-up, 99% at the 18-month follow-up, and 93% at the 30-month. ★ | Good |
| Gamble et al. (2000) [29] | Consecutive cohort of stroke patients admitted to a single hospital. | Yes ★ | Patients underwent interview about shoulder pain ★ | Yes ★ | Age, sex, level of anxiety, disability score, or moderate to severe motor weakness were adjusted in chi-square test. ★ | Questionnaire based structured interview ★ | No | Not reported | Poor |
| Gamble et al. (2002) [16] | Consecutive cohort of stroke patients admitted to a single hospital. | Yes ★ | Patients underwent interview about shoulder pain ★ | Yes ★ | Age, sex, depression and anxiety scores, and functional scores were adjusted in chi-square test. ★ | Questionnaire based structured interview ★ | Yes ★ | 97% participated at the 6-month follow-up. ★ | Good |
| Aras et al. (2004) [9] | Consecutive cohort of stroke patients admitted to a single hospital. | Yes ★ | Patients underwent interview about shoulder pain ★ | No | The presence of spasticity, thalamic pain, neglect, and comorbidities were compared between groups with and without shoulder pain. ★ | Questionnaire based structured interview ★ | No | No statement | Poor |
| Lindgren et al. (2007) [6] | Participants were representative of the Lund Stroke Register which covers the population of Lund-Orup, including 8 municipalities representing the local geographical area of Lund University Hospital. ★ | Yes ★ | Patients underwent interview about shoulder pain ★ | Yes ★ | Univariate analyses were used to compare arm motor function, disability, self-perceived health, subluxation, and sensory disturbance, between patients with and without shoulder pain. ★ | Questionnaire based structured interview ★ | Yes ★ | 79% participated at the 4-month follow-up, and 73% at the 12-month follow-up. ★ | Good |
| Sackley et al. (2008) [30] | Potential participants were representative of the Nottingham Stroke Register, which includes all stroke admissions to Nottingham City Hospital and Queens Medical Centre, Nottingham, UK. ★ | No non-exposed cohort | Patients underwent interview about shoulder pain ★ | Yes ★ | confounders were not reported. | Questionnaire based structured interview ★ | Yes ★ | 84% participated at the 3-month follow-up, 61% at the 6-month follow-up, and 50% at the 12-month follow-up. ★ | Poor |
| Kuptniratsaikul et al. (2009) [31] | Participants were representative of Thai Stroke Rehabilitation Registry, which maintain the record of patients with stroke who underwent rehabilitation in Thailand. ★ | Yes ★ | Patients underwent interview about shoulder pain ★ | No | Confounders were compared using the multivariate analysis. ★ | Questionnaire based structured interview ★ | No | No statement | Poor |
| Blennerhassett et al. (2010) [11] | The 94 retrospective histories of patients admitted for inpatient rehabilitation were audited which represented 63% of stroke patients in a 3-year period. | Yes ★ | Medical report | No | Confounders were compared using the logistic regression analysis. ★ | Retrospective data | No | No statement | Poor |
| Klit et al. (2011) [32] | Participants were representative of National Indicator Project database which records all hospitalized acute stroke patients in Denmark. ★ | Yes ★ | Patients reported shoulder pain | Yes ★ | Confounders were compared using the multiple logistic regression analysis. ★ | Questionnaire-based survey | No | No statement | Poor |
| Hansen et al. (2012) [33] | Consecutive cohort of stroke patients admitted to a single hospital. | No | Patients underwent a structured interview. ★ | No | Age and gender were adjusted in chi-square test. ★ | Questionnaire based structured interview ★ | Yes ★ | 97% participated at the 3-month follow-up, and 92% at the 6-month follow-up. ★ | Poor |
| Lindgren et al. (2012) [34] | Participants were representative of the Lund Stroke Register which covers the population of Lund-Orup, including 8 municipalities representing the local geographical area of Lund University Hospital. ★ | Yes ★ | Patients underwent interview about shoulder pain ★ | Yes ★ | Univariate analyses were used to compare age, pain frequency, affected side, motor function, and passive range of abduction between patients with and without shoulder pain. ★ | Questionnaire based structured interview ★ | Yes ★ | 79% participated at the 4-month follow-up, and 73% at the 12-month follow-up. ★ | Good |
| Kuptniratsaikul et al. (2013) [35] | Participants were representative of Thai Stroke Rehabilitation Registry, which maintain the record of patients with stroke who underwent rehabilitation in Thailand. ★ | Yes ★ | Patients underwent interview about shoulder pain ★ | No | Confounders were compared using the multivariate analysis. ★ | Questionnaire based structured interview ★ | Yes ★ | 65% participated at the 12-month follow-up. ★ | Good |
| Chen et al. (2014) [36] | The medical records of patients consecutively admitted to a single hospital. | No | Retrospective data | No | Confounders were compared using the Chi-square test. ★ | Retrospective medical record data | No | No statement | Poor |
| Kim et al. (2014) [37] | Consecutive cohort of stroke patients admitted to a single hospital. | Yes ★ | Patients underwent interview about shoulder pain ★ | Yes ★ | Age, gender and significant variables from the univariate analysis were included in the final multivariate logistic regression model. ★ | Questionnaire based structured interview ★ | Yes ★ | 78% participated at the 3-month follow-up, and 62% at the 6-month follow-up. ★ | Good |
| Kwon et al. (2014) [38] | Participants were representative of eight rehabilitation units situated in three different large local catchment area in the Republic of Korea. ★ | Yes ★ | Patients underwent interview about shoulder pain ★ | No | Age, sex, the Motricity Index of the upper and lower limbs, and ambulatory types were included in the multivariate logistic regression model. ★ | Questionnaire based structured interview ★ | No | No statement | Poor |
| Karaahmet et al. (2014) [39] | Consecutive cohort of stroke patients admitted to a single physical medicine and rehabilitation clinic. | Yes ★ | Patients underwent interview about shoulder pain ★ | No | Disease duration, neglect, sensory disturbance, spasticity, immobilization, late rehabilitation, and motor function were included in backward stepwise multinomial logistic regression analysis. ★ | Questionnaire based structured interview ★ | Yes ★ | No statement | Fair |
| Adey-Wakeling et al. (2015) [3] | A population-based stroke incidence study conducted in a specific region of the western suburbs of Adelaide, South Australia. ★ | Yes ★ | Patients underwent interview about shoulder pain ★ | No | logistic regression models were used to analyses different confounders. ★ | Questionnaire based structured interview ★ | Yes ★ | 78% participated at the 4-month follow-up, and 75% at the 12-month follow-up. ★ | Good |
| Paolucci et al. (2016) [40] | Consecutive cohort of stroke patients admitted to at eight Italian hospitals. ★ | No | Patients underwent interview about shoulder pain ★ | No | Age, gender, type of stroke, and severity of stroke were included in regression analysis. ★ | Questionnaire based structured interview ★ | No | No statement | Poor |
| Setting/Country | Sample Size | Targeted Population | Time since Stroke (Months) at Recruitment | Average Age at Recruitment | Outcome Measurement | Design | |
|---|---|---|---|---|---|---|---|
| Langhorne et al. (2000) [14] | Multicenter hospital-based study/Scotland, UK. | N = 311 | People with stroke, Hemiplegia | up to 30 months after stroke | 76 years (interquartile range 70 to 82 years) | VAS | A prospective study |
| Gamble et al. (2000) [29] | Hospital-based study/UK | N = 123 | Patients with a diagnosis of acute stroke | up to 6 months | 70.6 years | VAS | A prospective study |
| Gamble et al. (2002) [16] | Hospital-based study/UK | N = 123 | Patients with a diagnosis of acute stroke | up to 6 months | 70.6 years (range 29–93) | VAS | A prospective study |
| Aras et al. (2004) [9] | Hospital-based study/Turkey | N = 85 | Patients with hemiplegia | 64.8 days from the onset | 58.7 years | Physical examination | A prospective study |
| Lindgren et al. (2007) [6] | Population-Based Study/Sweden | N = 416 | First-ever stroke patients | up to 16 months | 73.1 years (range 17–102 years) | VAS | A prospective study |
| Sackley et al. (2008) [30] | Hospital-based study/UK | N = 600 | 3-months post stroke | 3-months from the onset up to 12-months | 76 years (range 31–98 years) | VAS | A prospective study |
| Kuptniratsaikul et al. (2009) [31] | Multicenter hospital-based study/Thailand | N = 327 | Patients with stroke | more than two months | 62.2 years (SD 12.1) | Physical examination | A prospective study |
| Blennerhassett et al. (2010) [11] | Hospital-based data/Australia | N = 94 | Patients with stroke | More than 2 months | 59 years (range 17–80 years) | Physical examination | Retrospective observational study |
| Klit et al. (2011) [32] | Population-based study/Denmark | N = 608 (stroke patients), 519 (reference subjects) | Patients with stroke | Median days from stroke 794.5 (range 588–1099) | Median age, 72.6 years | NRS | A prospective study |
| Hansen et al. (2012) [33] | Hospital-based study/Denmark | N = 299 | Patients with stroke | up to 6 months post stroke | 65.6 years (24–92 years) | Interview | A prospective study |
| Lindgren et al. (2012) [34] | Hospital-based study/Sweden | N = 58 | First-ever stroke patients | up to 16 months | 71 years | VAS | A prospective study |
| Kuptniratsaikul et al. (2013) [35] | Multicenter hospital-based study/Thailand | N = 327 | Patients with stroke | 12 months of onset | 62.1 years (SD 12.5 years) | Physical examination | A prospective study |
| Chen et al. (2014) [36] | Hospital-based study/Taiwan | N = 568 | First-time stroke patients | Not reported | 65.7 years (SD 13.3 years) | Medical records | A retrospective longitudinal cohort study |
| Kim et al. (2014) [37] | Hospital-based study/Korea | N = 94 | Patients with first-ever unilateral stroke lesion | up to 6 months post-stroke | 65.6 years | NRS | A prospective study |
| Kwon et al. (2014) [38] | Hospital-based study/Korea | N = 229 | Patients with stroke | More than 2 months | 59.0 years (SD 12.4) | University of Alabama’s Pain Behaviors Scale | A prospective study |
| Karaahmet et al. (2014) [39] | Hospital-based study/Turkey | N = 63 | Patients with stroke | More than 2 months | 61 years (range, 39–85 years) | Physical examination | A prospective study |
| Adey-Wakeling et al. (2015) [3] | Population-Based Study/Australia | N = 318 | Patients with stroke | Average 8.7 days post onset up to 12 years | 72.5 years | VAS | A prospective study |
| Paolucci et al. (2016) [40] | Hospital-based multicenter study/Italy | N = 443 | Patients with stroke | more than 90 days onset of stroke | 67.1 years | Neuropathic Pain Symptom Inventory | A prospective study |
| Study | Incidence [Proportion (95% CI)] | Prevalence [Proportion (95% CI)] | Prevalence in Defined Group |
|---|---|---|---|
| L | Incidence at admission: 9% (6–12%) | Weekly point prevalence: 6% (5–7%) | |
| Discharge to 6-month incidence: 15% (9–21%) | |||
| 6-months to 18-months incidence: 11% (6–16%) | |||
| 18-months to 30-months incidence: 12% (6–17%) | |||
| Gamble et al. (2000) [29] | Incidence at 2-week: 25% | ||
| Gamble et al. (2002) [16] | Incidence at 6-months: 40% | ||
| Aras et al. (2004) [9] | Prevalence at admission: 63.5% | ||
| Lindgren et al. (2007) [6] | Prevalence at 4-months: 22% Prevalence at 16-months: 24% | Functional status independence: 37% | |
| Moderate dependence: 31% | |||
| Major dependence: 32% | |||
| Self-perceived ill health: 23% | |||
| Arm motor function | |||
| No function: 27% | |||
| Reduced function: 56% | |||
| Normal function: 17% | |||
| Sensory disturbance for light touch: 31% | |||
| Shoulder Subluxation: 41% | |||
| Sackley et al. (2008) [30] | Overall prevalence: 52% | ||
| Prevalence at 3-months: 36% | |||
| Prevalence at 6-months: 42% | |||
| Prevalence at 12-months: 47% | |||
| Kuptniratsaikul et al. (2009) [31] | Overall prevalence: 19% | Hemorrhagic stroke, prevalence of shoulder pain: 26.1% | |
| Infarction stroke, prevalence of shoulder pain: 16.2% | |||
| Blennerhassett et al. (2010) [11] | Incidence during inpatient: 11.7% | Prevalence at admission: 23% | |
| Prevalence during inpatient: 35% | |||
| Klit et al. (2011) [32] | Two-year incidence: 15.1% | ||
| Hansen et al. (2012) [33] | Incident at onset: 1.5% | Shoulder pain in stroke-affected side at onset: 1.1% | |
| Incident at 3-months: 13.1% | at 3-months: 10.2% | ||
| Incident at 6-months: 16.4% | at 6-months:12.0% | ||
| Lindgren et al. (2012) [34] | Prevalence at 4 and 16-months: 19% and 72%, respectively | ||
| Kuptniratsaikul et al. (2013) [35] | Prevalence at 12-months: 8.5% | ||
| Chen et al. (2014) [36] | Incidence in acute ward: 2.6% | Incidence in rehabilitation ward (age group wise) | |
| Incidence in rehabilitation ward:23.2% | < 65 years: 23.4% | ||
| 65–75 years: 22.1% | |||
| ≥ 75 years: 24.5% | |||
| Kim et al. (2014) [37] | Not reported | Not reported | Not reported |
| Kwon et al. (2014) [38] | Overall prevalence: 91.9% | Prevalence of shoulder pain based on ambulatory mode | |
| Independent: 93.3% | |||
| Cane: 89.2% | |||
| Wheelchair: 70% | |||
| Karaahmet et al. (2014) [39] | Prevalence at admission: 53% Prevalence at discharge: 62% | Prevalence of HSP with other complications | |
| Neglect: 90% | |||
| Aphasia: 55.6% | |||
| Depression: 65% | |||
| Spasticity: 78.9% | |||
| Sensory disturbance: 40% | |||
| Subluxation: 77.8% | |||
| Adey-Wakeling et al. (2015) [3] | Incidence at admission: 10% Incidence at 4 months: 21% Incidence at 12 months: 21% Overall incidence: 29% | Female: 46% | |
| Medical history | |||
| Previous stroke:12% | |||
| Previous MI: 17% | |||
| Hypertension: 71% | |||
| Diabetes: 28% | |||
| History of shoulder pain: 27% | |||
| Stroke subtype | |||
| Total ischemic: 88% | |||
| Large artery: 14% | |||
| Cardio embolic: 34% | |||
| Lacunar: 9% | |||
| Other/unknown ischemic: 32% | |||
| Hemorrhagic: 9% | |||
| Unknown: 2% | |||
| Left side: 52% | |||
| High NIHSS score (> median): 5% | |||
| Motor arm | |||
| Reduced function: 38% | |||
| No function: 31% | |||
| Paolucci et al. (2016) [40] | Overall mean prevalence: 9.41% | ||
| Acute phase prevalence: 0.63% | |||
| Sub-acute phase prevalence: 17.27% | |||
| Chronic phase prevalence:10.34% |
| Study | Risk Factors of Shoulder Pain Which Were Assessed | Odd Ratios (OR) [95% Confidence Interval (CI)] |
|---|---|---|
| Blennerhassett et al. (2010) [11] | Gender (male) | 0.89 (0.36 to 2.16) |
| Altered Tone | 18.94 (6.46 to 55.51) | |
| Subluxation | 19.34 (5.57 to 65.94) | |
| Sensory deficits | 2.59 (1.08 to 6.17) | |
| Inattention/neglect | 1.53 (0.59 to 3.97) | |
| Cognitive impairment | 1.03 (0.44 to 2.40) | |
| Impaired communication | 1.48 (0.62 to 3.50) | |
| Type of stroke | 0.76 (0.26 to 2.17) | |
| Hand dominance | 0.24 (0.03 to 2.05) | |
| Previous shoulder problem | 2.55 (0.63 to 10.22) | |
| Klit et al. (2011) [32] | Males (vs. females) | 0.77 (0.53–1.11) |
| Age 65–74 years (vs. < 65 years) | 0.57 (0.36–0.90) | |
| Age > 75 years (vs. < 65 years) | 0.65 (0.43–0.99) | |
| Diabetes (vs. no diabetes) | 1.08 (0.65–1.78) | |
| Depression (vs. no depression) | 3.43 (2.25–5.25) | |
| Infarction (vs. hemorrhage) | 0.73 (0.43–1.26) | |
| Unspecified (vs. hemorrhage) | 1.09 (0.57–2.09) | |
| Lindgren et al. (2012) [34] | Left-sided hemiparesis | 10.47 (1.92–57.05), p = 0.01 |
| Pain frequency | 6.85 (1.46–32.14), p = 0.02 | |
| Decreased passive abduction | 4.46 (0.99–20.10), p = 0.05 | |
| Age | 1.05 (0.99–1.12), p = 0.07 | |
| Kim et al. (2014) [37] | Young age (< 70 years) | 3.65 (1.250–10.637), p = 0.018 |
| Male | 0.99 (0.370–2.683), p = 0.99 | |
| Poor NIHSS item 5 score (≥ 3) | 2.96 (1.141–7.665), p = 0.026 | |
| Presence of long head of biceps | 2.35 (0.897–6.150), p = 0.082 | |
| tendon effusion | ||
| Presence of supraspinatus tendon | 4.21 (1.372–12.931), p = 0.012 | |
| tendinosis/tear | ||
| Karaahmet et al. (2014) [39] | Neglect | 7.20 (0.840–61.4690), p = 0.071 |
| Sensory disturbance | 3.26 (0.478–22.301), p = 0.228 | |
| Spasticity | 1.56 (0.272–9.002), p = 0.617 | |
| Immobilization | 3.28 (0.527–20.457), p = 0.203 | |
| Late rehabilitation | 0.52 (0.025–10.658), p = 0.669 | |
| Disease duration | 1.05 (0.964–1.134), p = 0.279 | |
| Baseline FMA (Fugl-Meyer Motor Assessment.) | 0.99 (0.905–1.083), p = 0.822 | |
| Baseline FAT (Frenchay Arm Test) | ||
| Baseline FIM (Functional Independence Measure) | 0.66 (0.234–1.872), p = 0.437 | |
| 1.01 (0.970–1.045), p = 0.720 | ||
| Adey-Wakeling et al. (2015) [3] | Mean age (y) | 0.96 (0.79–1.17), p = 0.690 |
| Sex: female | 1.03 (0.77–1.37), p = 0.845 | |
| Medical history | ||
| Previous stroke | 0.47 (0.21–1.07), p = 0.074 | |
| Previous MI | 1.16 (0.53–2.54), p = 0.705 | |
| Hypertension | 0.96 (0.50–1.85), p = 0.907 | |
| Diabetes | 1.20 (0.62–2.30), p = 0.587 | |
| History of shoulder pain | 8.09 (3.16–20.75), p = < 0.0001 | |
| Stroke subtype | ||
| Cardioembolic | 1.10 (0.60–2.01), p = 0.767 | |
| Lacunar | 0.85 (0.36–2.04), p = 0.719 | |
| Other/unknown ischemic | 1.45 (0.78–2.72), p = 0.241 | |
| Hemorrhagic | 1.22 (0.49–3.01), p = 0.670 | |
| Unknown | 0.57 (0.09–3.47), p = 0.541 | |
| Right Hemiparesis | 0.87(0.65–1.17), p = 0.350 | |
| High NIHSS score (> median) | 1.39 (0.78–2.49), p = 0.268 | |
| Motor arm | ||
| Reduced function | 1.20 (0.79–1.83), p = 0.399 | |
| No function | 1.91 (1.20–3.04), p = 0.007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwer, S.; Alghadir, A. Incidence, Prevalence, and Risk Factors of Hemiplegic Shoulder Pain: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4962. https://doi.org/10.3390/ijerph17144962
Anwer S, Alghadir A. Incidence, Prevalence, and Risk Factors of Hemiplegic Shoulder Pain: A Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(14):4962. https://doi.org/10.3390/ijerph17144962
Chicago/Turabian StyleAnwer, Shahnawaz, and Ahmad Alghadir. 2020. "Incidence, Prevalence, and Risk Factors of Hemiplegic Shoulder Pain: A Systematic Review" International Journal of Environmental Research and Public Health 17, no. 14: 4962. https://doi.org/10.3390/ijerph17144962
APA StyleAnwer, S., & Alghadir, A. (2020). Incidence, Prevalence, and Risk Factors of Hemiplegic Shoulder Pain: A Systematic Review. International Journal of Environmental Research and Public Health, 17(14), 4962. https://doi.org/10.3390/ijerph17144962






