End-of-Life Cancer Care Resource Utilisation in Rural Versus Urban Settings: A Systematic Review
Abstract
1. Introduction
Background
2. Materials and Methods
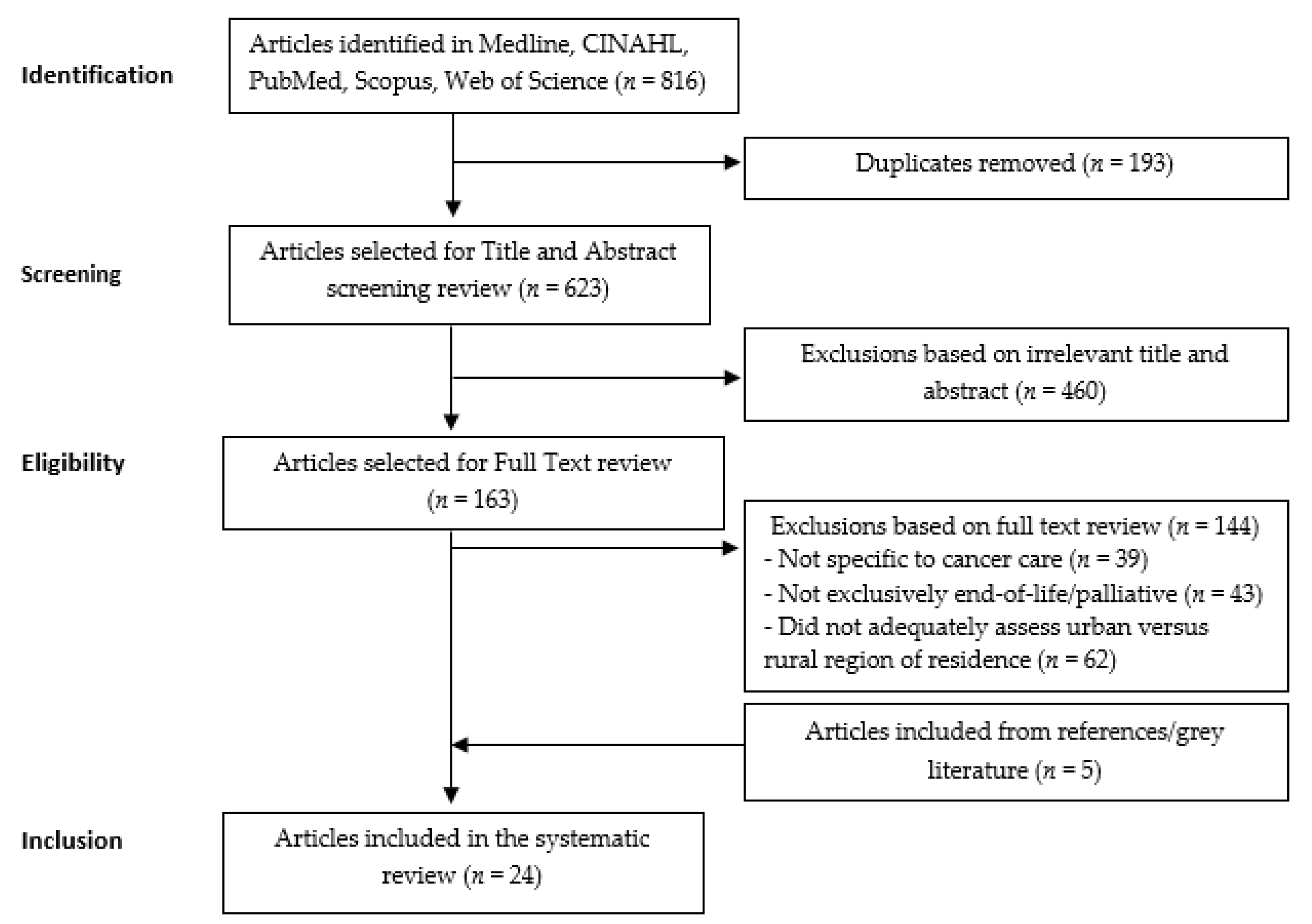
2.1. Selection Protocol
2.2. Search Strategy
2.3. Inclusion/Exclusion Criteria
2.4. Data Extraction and Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. End-of-Life Period Classification
3.3. Urban Versus Rural classification
3.4. Cancer Classification and Tumour Characteristics
3.5. Healthcare Service Utilisation—Treatment Intent
3.6. Study Quality
3.7. Results by Primary Outcome
3.7.1. Urban-Rural Effect on Acute and Life-Sustaining Healthcare Services Use
3.7.2. Urban–Rural Effect on Community-Based Healthcare Services Use
3.7.3. Urban-Rural Effect on Palliative Healthcare Services Use
3.7.4. Covariate Effect
4. Discussion
4.1. Relevance to Prior Knowledge
4.2. Strengths and Limitations
4.3. Implications for Practice
4.4. Implications for Science
4.5. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Connor SRB, M.C.S. Global Atlas of Palliative Care at the End of Life; World Health Organisation (WHO): Geneva, Switzerland, 2014. [Google Scholar]
- Voda, A.I.; Bostan, I. Public Health Care Financing and the Costs of Cancer Care: A Cross-National Analysis. Cancers (Basel) 2018, 10, 117. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer. New Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Australia’s Health 2016; AIHW: Canberra, Australia, 2016. [Google Scholar]
- Mandelblatt, J.S.; Yabroff, K.R.; Kerner, J.F. Equitable access to cancer services: A review of barriers to quality care. Cancer 1999, 86, 2378–2390. [Google Scholar] [CrossRef]
- Beccaro, M.; Costantini, M.; Merlo, D.F.; The ISG. Inequity in the provision of and access to palliative care for cancer patients. Results from the Italian survey of the dying of cancer (ISDOC). BMC Public Health 2007, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Luckett, T.; Phillips, J.; Agar, M.; Virdun, C.; Green, A.; Davidson, P.M. Elements of effective palliative care models: A rapid review. BMC Health Serv. Res. 2014, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, K.; Bullock, C. Urban-Rural Differences in the Quality of Care for Medicare Patients With Acute Myocardial Infarction. Arch. Intern. Med. 2001, 161, 737–743. [Google Scholar] [CrossRef]
- Barbera, L.; Paszat, L.; Chartier, C. Indicators of poor quality end-Of-Life cancer care in Ontario. J. Palliat Care 2006, 22, 12–17. [Google Scholar] [CrossRef]
- Burge, F.; Lawson, B.; Johnston, G. Family physician continuity of care and emergency department use in end-of-life cancer care. Medical Care 2003. [Google Scholar] [CrossRef]
- Ho, T.H.; Barbera, L.; Saskin, R.; Lu, H.; Neville, B.A.; Earle, C.C. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J. Clin. Oncol. 2011, 29, 1587–1591. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Hall, J.; Pollack, C.E.; Adelson, K.; Bradley, E.H.; Long, J.B.; Gross, C.P. Trends in end-Of-Life cancer care in the Medicare program. J. Geriatr. Oncol. 2016, 7, 116–125. [Google Scholar] [CrossRef]
- Walter, J.; Tufman, A.; Leidl, R.; Holle, R.; Schwarzkopf, L. Rural versus urban differences in end-of-life care for lung cancer patients in Germany. Support Care Cancer 2018, 26, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, K.M.; Boyd, C.A.; Benarroch-Gampel, J.; Kuo, Y.F.; Cooksley, C.D.; Riall, T.S. End-Of-Life care in Medicare beneficiaries dying with pancreatic cancer. Cancer 2011, 117, 5003–5012. [Google Scholar] [CrossRef] [PubMed]
- Shugarman, L.R.; Bird, C.E.; Schuster, C.R.; Lynn, J. Age and gender differences in Medicare expenditures at the end of life for colorectal cancer decedents. J. Womens Health (Larchmt) 2007, 16, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Shugarman, L.R.; Bird, C.E.; Schuster, C.R.; Lynn, J. Age and Gender Differences in Medicare Expenditures and Service Utilization at the End of Life for Lung Cancer Decedents. Women’s Health Issues 2008, 18, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; Landrum, M.B.; Guadagnoli, E.; Winer, E.P.; Ayanian, J.Z. Care in the months before death and hospice enrollment among older women with advanced breast cancer. J. Gen. Intern. Med. 2008, 23, 11–18. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.P.; Burns, R.B.; Davis, R.B.; Phillips, R.S. Barriers to hospice care among older patients dying with lung and colorectal cancer. J. Clin. Oncol. 2003, 21, 728–735. [Google Scholar] [CrossRef]
- Jorgensen, M.L.; Young, J.M.; Dobbins, T.A.; Solomon, M.J. Predictors of variation in colorectal cancer care and outcomes in New South Wales: A population-Based health data linkage study. Med. J. Aust. 2014, 200, 403–407. [Google Scholar] [CrossRef]
- Virgilsen, L.F.; Møller, H.; Vedsted, P. Cancer diagnostic delays and travel distance to health services: A nationwide cohort study in Denmark. Cancer Epidemiol. 2019, 59, 115–122. [Google Scholar] [CrossRef]
- Bakitas, M.A.; Elk, R.; Astin, M.; Ceronsky, L.; Clifford, K.N.; Dionne-Odom, J.N.; Emanuel, L.L.; Fink, R.M.; Kvale, E.; Levkoff, S.; et al. Systematic Review of Palliative Care in the Rural Setting. Cancer Control 2015, 22, 450–464. [Google Scholar] [CrossRef]
- Parikh, R.B.; Kirch, R.A.; Smith, T.J.; Temel, J.S. Early specialty palliative care--Translating data in oncology into practice. New Engl. J. Med. 2013, 369, 2347–2351. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-Analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Cochrane: London, UK, 2011. [Google Scholar]
- Dzhambov, A.M.; Dimitrova, D.D.; Dimitrakova, E.D. Association between residential greenness and birth weight: Systematic review and meta-analysis. Urban For. Urban Green. 2014, 13, 621–629. [Google Scholar] [CrossRef]
- National Heart Lung and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-sectional Studies 2014. Available online: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (accessed on 8 November 2019).
- Qureshi, D.; Tanuseputro, P.; Perez, R.; Pond, G.R.; Seow, H.Y. Early initiation of palliative care is associated with reduced late-life acute-hospital use: A population-Based retrospective cohort study. Palliat. Med. 2019, 33, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, D.; Seow, H.; Sussman, J.; Pond, G. Factors associated with acute care use among nursing home residents dying of cancer: A population-Based study. Int. J. Palliat. Nurs. 2015, 21, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.S.C.; Caswell, J.M.; Santi, S.A.; Ballantyne, B.; Meigs, M.L.; Knight, A.; Earle, C.; Hartman, M. Access to Palliative Care for Cancer Patients Living in a Northern and Rural Environment in Ontario, Canada: The Effects of Geographic Region and Rurality on End-of-Life Care in a Population-Based Decedent Cancer Cohort. Clin. Med. Insights Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Soo, J.; French, J.; McGahan, C.E.; Duncan, G.; Sonca, L. A retrospective study on accessibility of palliative radiation therapy in the management of prostate cancer in British Columbia. J. Radiother. Pract. 2011, 10, 159–172. [Google Scholar] [CrossRef][Green Version]
- Rosenwax, L.; McNamara, B. Who receives specialist palliative care in Western Australia-and who misses out. Palliat. Med. 2006, 20, 439–445. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, Y.-C.; Tseng, Y.-H.; Lin, M.-H.; Hwang, S.-J.; Chen, T.-J.; Chou, L.-F. Trend of Urban-Rural Disparities in Hospice Utilization in Taiwan. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Kao, Y.H.; Liu, Y.T.; Koo, M.; Chiang, J.K. Factors associated with emergency services use in Taiwanese advanced cancer patients receiving palliative home care services during out-of-hours periods: A retrospective medical record study. BMC Palliat. Care 2018, 17, 46. [Google Scholar] [CrossRef]
- Lavergne, M.R.; Johnston, G.M.; Gao, J.; Dummer, T.J.B.; Rheaume, D.E. Variation in the use of palliative radiotherapy at end of life: Examining demographic, clinical, health service, and geographic factors in a population-based study. Palliat. Med. 2011, 25, 101–110. [Google Scholar] [CrossRef]
- Hu, W.; Yasui, Y.; White, J.; Winget, M. Aggressiveness of end-Of-Life care for patients with colorectal cancer in Salberta, Canada: 2006–2009. J. Pain Symptom Manag. 2014, 47, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-M.; Wu, C.-C.; Yin, B.-Y.; Juang, S.-Y.; Yu, C.-H.; Lee, C.-C. Low Socioeconomic Status Is Associated With More Aggressive End-of-Life Care for Working-Age Terminal Cancer Patients. Oncologist 2014, 19, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Burge, F.I.; Lawson, B.J.; Johnston, G.M.; Grunfeld, E. A population-based study of age inequalities in access to palliative care among cancer patients. Med Care 2008, 46, 1203. [Google Scholar] [CrossRef] [PubMed]
- Forst, D.; Adams, E.; Nipp, R.; Martin, A.; El-Jawahri, A.; Aizer, A.; Jordan, J.T. Hospice utilization in patients with malignant gliomas. Neuro-Oncol. 2017, 20, 538–545. [Google Scholar] [CrossRef]
- Hunt, R.; McCaul, K. Coverage of cancer patients by hospice services, South Australia, 1990 to 1993. Aust. New Zealand J. Public Health 1998, 22, 45–48. [Google Scholar] [CrossRef]
- Lackan, N.A.; Ostir, G.V.; Freeman, J.L.; Kuo, Y.F.; Zhang, D.D.; Goodwin, J.S. Hospice use by Hispanic and non-Hispanic white cancer decedents. Health Serv. Res. 2004, 39, 969–983. [Google Scholar] [CrossRef]
- Langton, J.M.; Blanch, B.; Drew, A.K.; Haas, M.; Ingham, J.M.; Pearson, S.A. Retrospective studies of end-Of-Life resource utilization and costs in cancer care using health administrative data: A systematic review. Palliat Med. 2014, 28, 1167–1196. [Google Scholar] [CrossRef]
- Tedder, T.; Elliott, L.; Lewis, K. Analysis of common barriers to rural patients utilizing hospice and palliative care services: An integrated literature review. J. Am. Assoc. Nurse Pract. 2017, 29, 356–362. [Google Scholar] [CrossRef]
- Van Vorst, R.F.; Crane, L.A.; Barton, P.L.; Kutner, J.S.; Kallail, K.J.; Westfall, J.M. Barriers to quality care for dying patients in rural communities. J. Rural. Health Off. J. Am. Rural Health Assoc. Natl. Rural. Health Care Assoc. 2006, 22, 248–253. [Google Scholar] [CrossRef]
- Currow, D.C.; Allingham, S.; Bird, S.; Yates, P.; Lewis, J.; Dawber, J.; Eagar, K. Referral patterns and proximity to palliative care inpatient services by level of socio-Economic disadvantage. A national study using spatial analysis. BMC Health Serv. Res. 2012, 12, 424. [Google Scholar] [CrossRef]
- Gao, J.; Johnston, G.M.; Lavergne, M.R.; McIntyre, P. Identifying population groups with low palliative care program enrolment using classification and regression tree analysis. J. Palliat. Care 2011, 27, 98–106. [Google Scholar] [CrossRef]
- Hawley, P. Barriers to Access to Palliative Care. Palliat Care 2017. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.B.; Humphreys, J.S.; Wilson, M.G.A. Addressing the health disadvantage of rural populations: How does epidemiological evidence inform rural health policies and research? Aust. J. Rural Health 2008, 16, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.G.; Larson, E.H.; Lishner, D.M. Rural Definitions for Health Policy and Research. Am. J. Public Health 2005, 95, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Dickerson, J.B.; Wendel, M.L.; Ahn, S.; Pulczinski, J.C.; Drake, K.N.; Ory, M.G. The utility of rural and underserved designations in geospatial assessments of distance traveled to healthcare services: Implications for public health research and practice. J. Environ. Public Health 2013, 2013, 960157. [Google Scholar] [CrossRef]
- Yang, W.; Williams, J.H.; Hogan, P.F.; Bruinooge, S.S.; Rodriguez, G.I.; Kosty, M.P.; Bajorin, D.F.; Hanley, A.; Muchow, A.; Hanley, A.; et al. Projected Supply of and Demand for Oncologists and Radiation Oncologists Through 2025: An Aging, Better-Insured Population Will Result in Shortage. J. Oncol. Pract. 2014, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Seow, H.; Snyder, C.F.; Mularski, R.A.; Shugarman, L.R.; Kutner, J.S.; Lorenz, K.A.; Wu, A.W.; Dy, S.M. A framework for assessing quality indicators for cancer care at the end of life. J. Pain Symptom. Manag. 2009, 38, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Craigs, C.L.; West, R.M.; Hurlow, A.; Bennett, M.I.; Ziegler, L.E. Access to hospital and community palliative care for patients with advanced cancer: A longitudinal population analysis. PLoS ONE 2018, 13, e0200071. [Google Scholar] [CrossRef] [PubMed]
- Life IoMCoCatEo. Approaching Death: Improving Care at the End of Life; Field, M.J., Cassel, C.K., Eds.; National Academies Press (US): Washington DC, USA, 1997. [Google Scholar]
| Study Characteristic | Group | Number of Studies i |
|---|---|---|
| Year of publication | 1998–2004 | 4 |
| 2005–2012 | 10 | |
| 2013–2019 | 10 | |
| Study setting (country) | Canada | 9 |
| USA | 9 | |
| Taiwan | 3 | |
| Australia | 2 | |
| Germany | 1 | |
| Study design | Case Control study (CCS) | 1 |
| Cross-sectional study (CSS) | 2 | |
| Cohort/Longitudinal study (CS) | 21 | |
| Statistical analysis methods | Linear Regression | 5 |
| Univariate logistic regression | 3 | |
| Binary logistic regression | 4 | |
| Multivariate logistic regression | 15 | |
| Negative binomial regression | 1 | |
| Hierarchical non-linear regression | 1 | |
| Cos proportional hazards regression | 1 | |
| Poisson Regression | 1 | |
| No comprehensive analysis techniques used | 1 | |
| Quality assessment iii | High | 14 |
| Fair | 7 | |
| Low | 3 | |
| Inclusion criteria (minimum age) | Any age | 6 |
| 18–20 | 9 | |
| 65–70 | 7 | |
| Not Reported | 2 | |
| End-of-life period | ≤1 month | 9 |
| 6 months | 3 | |
| 9 months | 1 | |
| 12 months | 4 | |
| >12 months | 1 | |
| Variable ii | 2 | |
| Not Reported | 4 | |
| Cancer types | Lung | 15 |
| Colorectal | 14 | |
| Prostate | 12 | |
| Breast | 11 | |
| Haematological | 9 | |
| Gynaecological | 4 | |
| Pancreatic | 4 | |
| Head and Neck | 4 | |
| Upper gastrointestinal | 4 | |
| Liver | 4 | |
| Melanoma | 2 | |
| Central Nervous System | 2 | |
| Healthcare services | Chemotherapy | 7 |
| ED/ER visit | 10 | |
| Hospital admission | 8 | |
| ICU admission | 6 | |
| SPC service/s | 15 | |
| Palliative Radiotherapy | 2 | |
| Home doctor/physician visit/s | 3 | |
| Prescription medication/s | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerni, J.; Rhee, J.; Hosseinzadeh, H. End-of-Life Cancer Care Resource Utilisation in Rural Versus Urban Settings: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4955. https://doi.org/10.3390/ijerph17144955
Cerni J, Rhee J, Hosseinzadeh H. End-of-Life Cancer Care Resource Utilisation in Rural Versus Urban Settings: A Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(14):4955. https://doi.org/10.3390/ijerph17144955
Chicago/Turabian StyleCerni, Jessica, Joel Rhee, and Hassan Hosseinzadeh. 2020. "End-of-Life Cancer Care Resource Utilisation in Rural Versus Urban Settings: A Systematic Review" International Journal of Environmental Research and Public Health 17, no. 14: 4955. https://doi.org/10.3390/ijerph17144955
APA StyleCerni, J., Rhee, J., & Hosseinzadeh, H. (2020). End-of-Life Cancer Care Resource Utilisation in Rural Versus Urban Settings: A Systematic Review. International Journal of Environmental Research and Public Health, 17(14), 4955. https://doi.org/10.3390/ijerph17144955






