Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Subjects
2.2. Measurements
2.3. Extracranial Carotid Artery Ultrasound Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/health–topics/cardiovascular–diseases/#tab=tab_1 (accessed on 2 March 2020).
- Blankenhorn, D.H.; Hodis, H.N. George lyman duff memorial lecture. Arterial imaging and atherosclerosis reversal. Arter. Thromb. 1994, 14, 177–192. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F. Intima–media thickness: A tool for atherosclerosis imaging and event prediction. Am. J. Cardiol. 2002, 90, 18l–21l. [Google Scholar] [CrossRef] [PubMed]
- Nambi, V.; Chambless, L.; Folsom, A.R.; He, M.; Hu, Y.; Mosley, T.; Volcik, K.; Boerwinkle, E.; Ballantyne, C.M. Carotid intima–media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The ARIC (Atherosclerosis Risk In Communities) study. J. Am. Coll. Cardiol. 2010, 55, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, D.; Hamsten, A.; Veglia, F.; de Faire, U.; Humphries, S.E.; Smit, A.J.; Giral, P.; Kurl, S.; Rauramaa, R.; Mannarino, E.; et al. Measurements of carotid intima–media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: Results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT–Progression as Predictors of Vascular Events in a High Risk European Population) study. J. Am. Coll. Cardiol. 2012, 60, 1489–1499. [Google Scholar] [CrossRef]
- Cao, J.J.; Arnold, A.M.; Manolio, T.A.; Polak, J.F.; Psaty, B.M.; Hirsch, C.H.; Kuller, L.H.; Cushman, M. Association of carotid artery intima–media thickness, plaques, and C–reactive protein with future cardiovascular disease and all–cause mortality: The Cardiovascular Health Study. Circulation 2007, 116, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Stork, S.; Feelders, R.A.; van den Beld, A.W.; Steyerberg, E.W.; Savelkoul, H.F.; Lamberts, S.W.; Grobbee, D.E.; Bots, M.L. Prediction of mortality risk in the elderly. Am. J. Med. 2006, 119, 519–525. [Google Scholar] [CrossRef]
- Roumeliotis, A.; Roumeliotis, S.; Panagoutsos, S.; Theodoridis, M.; Argyriou, C.; Tavridou, A.; Georgiadis, G.S. Carotid intima–media thickness is an independent predictor of all–cause mortality and cardiovascular morbidity in patients with diabetes mellitus type 2 and chronic kidney disease. Ren. Fail. 2019, 41, 131–138. [Google Scholar] [CrossRef]
- Clemens, R.K.; Annema, W.; Baumann, F.; Roth-Zetzsche, S.; Seifert, B.; von Eckardstein, A.; Amann-Vesti, B.R. Cardiac biomarkers but not measures of vascular atherosclerosis predict mortality in patients with peripheral artery disease. Clin. Chim. Acta; Int. J. Clin. Chem. 2019, 495, 215–220. [Google Scholar] [CrossRef]
- Hanna, D.B.; Moon, J.Y.; Haberlen, S.A.; French, A.L.; Palella, F.J., Jr.; Gange, S.J.; Witt, M.D.; Kassaye, S.; Lazar, J.M.; Tien, P.C.; et al. Carotid artery atherosclerosis is associated with mortality in HIV–positive women and men. Aids (Lond. Engl.) 2018, 32, 2393–2403. [Google Scholar] [CrossRef]
- Chang, C.-S.; Kuo, C.-L.; Huang, C.-S.; Cheng, Y.-S.; Lin, S.-S.; Liu, C.-S. Association of cyclophilin A level and pulse pressure in predicting recurrence of cerebral infarction. Kaohsiung J. Med. Sci. 2019. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, X.; Hua, Y.; Tang, Z.; Guan, S.; Wu, X.; Liu, H.; Liu, B.; Wang, C.; Zhang, Z.; et al. Carotid Artery Plaques, Carotid Intima–Media Thickness, and Risk of Cardiovascular Events and All–Cause Death in Older Adults: A 5–Year Prospective, Community–Based Study. Angiology 2018, 69, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Brevetti, G.; Schiano, V.; Chiariello, M. Endothelial dysfunction: A key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 2008, 197, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hillege, H.L.; Janssen, W.M.; Bak, A.A.; Diercks, G.F.; Grobbee, D.E.; Crijns, H.J.; Van Gilst, W.H.; De Zeeuw, D.; De Jong, P.E. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 2001, 249, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Tighiouart, H.; Amin, M.G.; Stark, P.C.; MacLeod, B.; Griffith, J.L.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Chronic kidney disease as a risk factor for cardiovascular disease and all–cause mortality: A pooled analysis of community–based studies. J. Am. Soc. Nephrol. Jasn 2004, 15, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E.; Mann, J.F.; Schumacher, H.; Gao, P.; Mancia, G.; Weber, M.A.; McQueen, M.; Koon, T.; Yusuf, S. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J. Am. Soc. Nephrol. Jasn 2011, 22, 1353–1364. [Google Scholar] [CrossRef]
- Warnock, D.G.; Muntner, P.; McCullough, P.A.; Zhang, X.; McClure, L.A.; Zakai, N.; Cushman, M.; Newsome, B.B.; Kewalramani, R.; Steffes, M.W.; et al. Kidney function, albuminuria, and all–cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2010, 56, 861–871. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, M.; Gu, W.; Bi, Y.; Li, X.; Ning, G. Low–grade albuminuria is associated with carotid intima–media thickness in Chinese type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2010, 95, 5122–5128. [Google Scholar] [CrossRef]
- Li, M.F.; Tu, Y.F.; Li, L.X.; Lu, J.X.; Dong, X.H.; Yu, L.B.; Zhang, R.; Bao, Y.Q.; Jia, W.P.; Hu, R.M. Low–grade albuminuria is associated with early but not late carotid atherosclerotic lesions in community–based patients with type 2 diabetes. Cardiovasc. Diabetol. 2013, 12, 110. [Google Scholar] [CrossRef]
- Blekkenhorst, L.C.; Lewis, J.R.; Bondonno, C.P.; Sim, M.; Devine, A.; Zhu, K.; Lim, W.H.; Woodman, R.J.; Beilin, L.J.; Thompson, P.L.; et al. Vegetable diversity in relation with subclinical atherosclerosis and 15–year atherosclerotic vascular disease deaths in older adult women. Eur. J. Nutr. 2019, 59, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, T.Z.; Lee, M.S. Carotid intima–media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014, 7, 1025–1038. [Google Scholar] [CrossRef]
- Kappelle, L.J.; Eliasziw, M.; Fox, A.J.; Sharpe, B.L.; Barnett, H.J. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. The North American Symptomatic Carotid Endarterectomy Trail. Stroke 1999, 30, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Suemotoa, C.K.; Grinbergb, L.T.; Leitea, R.E.P.; Ferretti-Rebustinic, R.E.L.; Jacob-Filhoa, W.; Yaffed, K.; Nitrinie, R.; Pasqualucci, C.A. Morphometric measurements of extracranial and intracranial atherosclerotic disease: A population–based autopsy study. Atherosclerosis 2018, 270, 218–223. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. American Society of Echocardiography Carotid Intima–Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima–Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr 2008, 21, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Fatar, M.; Hernandez, R.H.; et al. Mannheim carotid intima–media thickness consensus (2004–2006): An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cereb. Dis. 2007, 23, 75–80. [Google Scholar] [CrossRef]
- Polak, J.F.; Pencina, M.J.; Meisner, A.; Pencina, K.M.; Brown, L.S.; Wolf, P.A.; D’Agostino, R.B. Associations of carotid artery intima–media thickness (IMT) with risk factors and prevalent cardiovascular disease: Comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. J. Ultrasound Med. 2010, 29, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Pathophysiology and medical treatment of carotid artery stenosis. Int. J. Angiol. 2015, 24, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, O.; Hulthe, J.; Wikstrand, J.; Schmidt, C.; Olofsson, S.O.; Bondjers, G. Effect of controlled release/extended release metoprolol on carotid intima–media thickness in patients with hypercholesterolemia: A 3–year randomized study. Stroke 2002, 33, 572–577. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Variables | Mortality Status n (%) | p-Value | |
|---|---|---|---|
| Alive (N = 2714) | Dead (N = 242) | ||
| Socio-demographic factors | |||
| Men | 1205 (44.4) | 167 (69.01) | <0.001 |
| Age (years) | 54.32 ± 11.27 | 70.05 ± 11.51 | <0.001 |
| Educational attainment | <0.001 | ||
| ≤6 years | 213 (17.15) | 57 (31.32) | |
| 7–12 years | 527 (42.43) | 65 (35.71) | |
| ≥13 years | 502 (40.42) | 60 (32.97) | |
| Married | 2162 (79.66) | 203 (83.88) | 0.14 |
| Body mass index (kg/m2) | 0.61 | ||
| <18.5 | 73 (2.69) | 9 (3.72) | |
| 18.5–25 | 1636 (60.28) | 141 (58.26) | |
| 25–30 | 843 (31.06) | 74 (30.58) | |
| ≥30 | 162 (5.97) | 18 (7.44) | |
| Lifestyle behaviors | |||
| Smoking | 362 (13.34) | 43 (17.77) | 0.07 |
| Alcohol drinking | 483 (17.80) | 56 (23.14) | 0.05 |
| Physical activity | 1698 (62.56) | 167 (69.01) | 0.05 |
| Disease history | |||
| Hypertension | 662 (24.39) | 130 (53.72) | <0.001 |
| Diabetes Mellitus | 245 (9.03) | 63 (26.03) | <0.001 |
| Heart disease | 277 (10.21) | 71 (29.34) | <0.001 |
| Stroke | 37 (1.36) | 25 (10.33) | <0.001 |
| Cancer | 82 (3.02) | 25 (10.33) | <0.001 |
| CKD (eGFR < 60 mL/min/1.73 m2) | 117 (4.31) | 67 (27.69) | <0.001 |
| Blood biochemical indexes † | |||
| Fasting plasma glucose (mg/dL) | 100.85 ± 22.34 | 111.57 ± 33.99 | <0.001 |
| Total cholesterol (mg/dL) | 199.08 ± 35.28 | 190.18 ± 36 | <0.001 |
| Triglyceride (mg/dL) | 121.87 ± 89.75 | 137.55 ± 95.37 | 0.01 |
| High-density lipoprotein (mg/dL) | 48.88 ± 14.08 | 44.47 ± 13.09 | <0.001 |
| Low-density lipoprotein (mg/dL) | 120.94 ± 31.27 | 112.64 ± 31.81 | <0.001 |
| WBC (103/mL) | 5.75 ± 1.56 | 6.12 ± 1.70 | <0.001 |
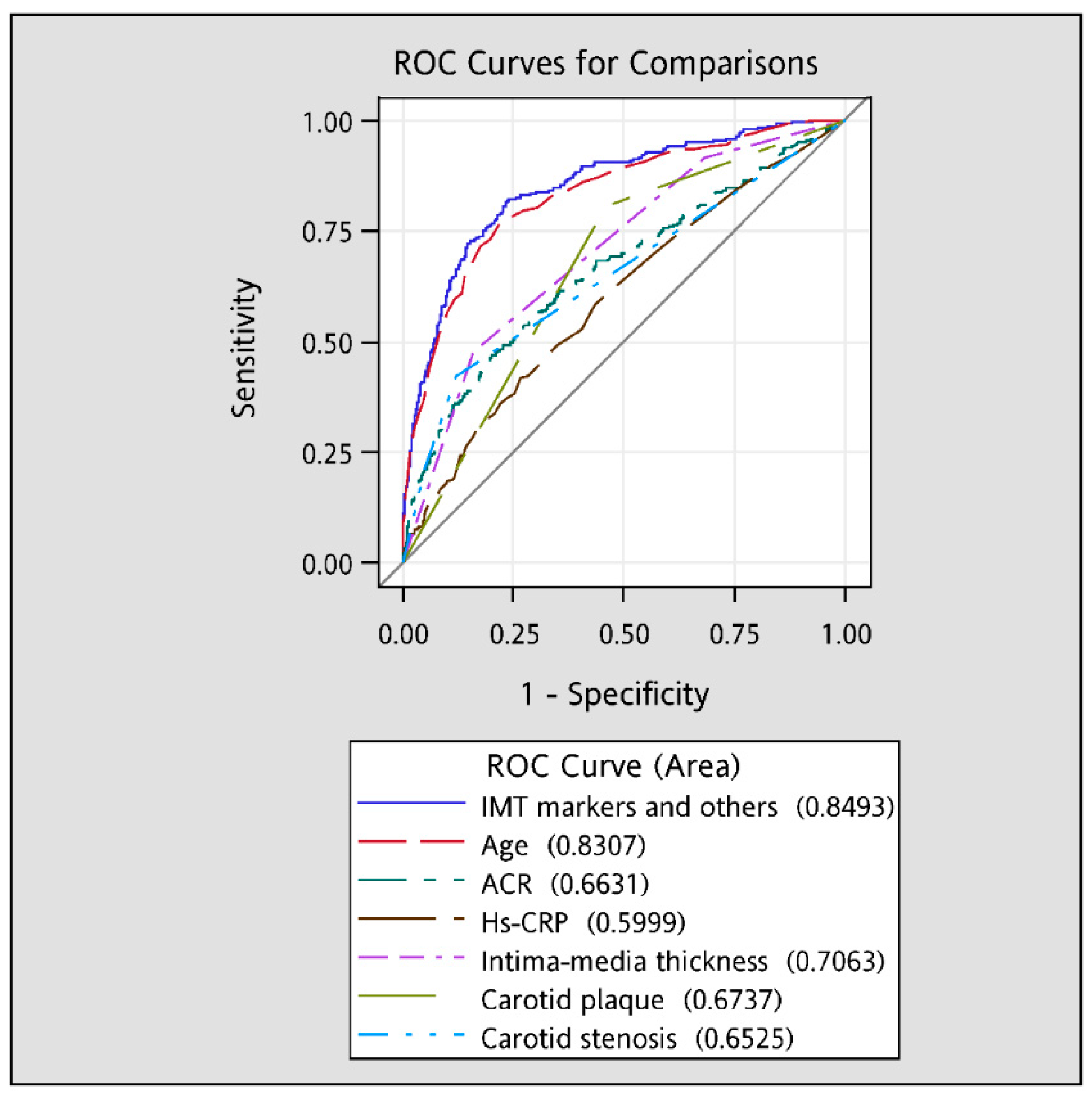
| ACR (mg/g) | 21.82 ± 103.34 | 127.66 ± 532.54 | 0.002 |
| Fasting insulin (IU/L) | 6.74 ± 4.79 | 7.73 ± 8.47 | 0.08 |
| Hs-CRP (mg/L) | 0.16 ± 0.28 | 0.27 ± 0.44 | <0.001 |
| Measurements of the carotid arteries | |||
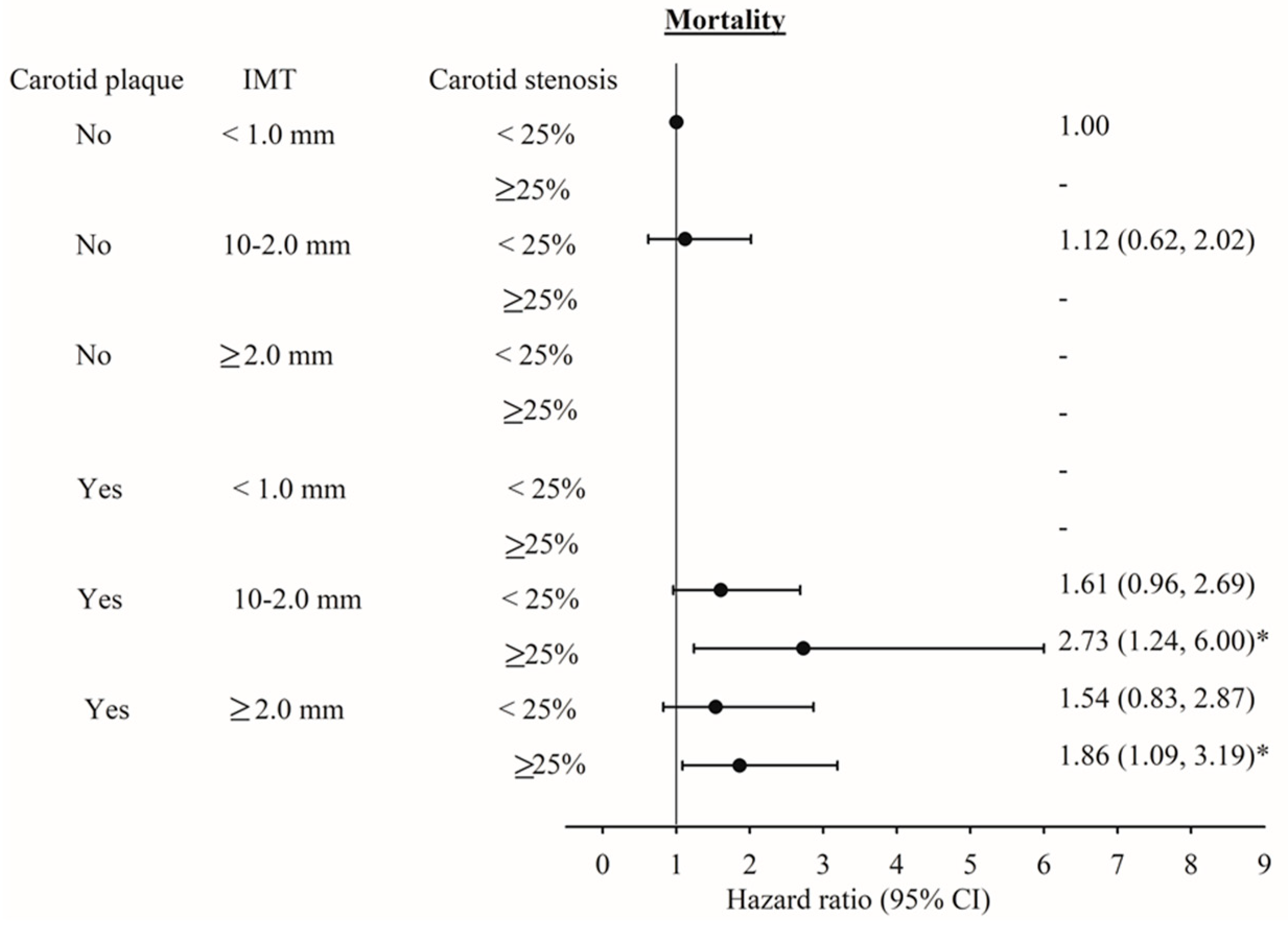
| Total carotid intima–media thickness | <0.001 | ||
| <1.0 mm | 857 (31.58) | 20 (8.26) | |
| 1.0–2.0 mm | 1426 (52.54) | 107 (44.21) | |
| ≥2.0 mm | 431 (15.88) | 115 (47.52) | |
| Carotid plaque | <0.001 | ||
| No | 1470 (54.16) | 47 (19.42) | |
| Yes | 1244 (45.84) | 195 (80.58) | |
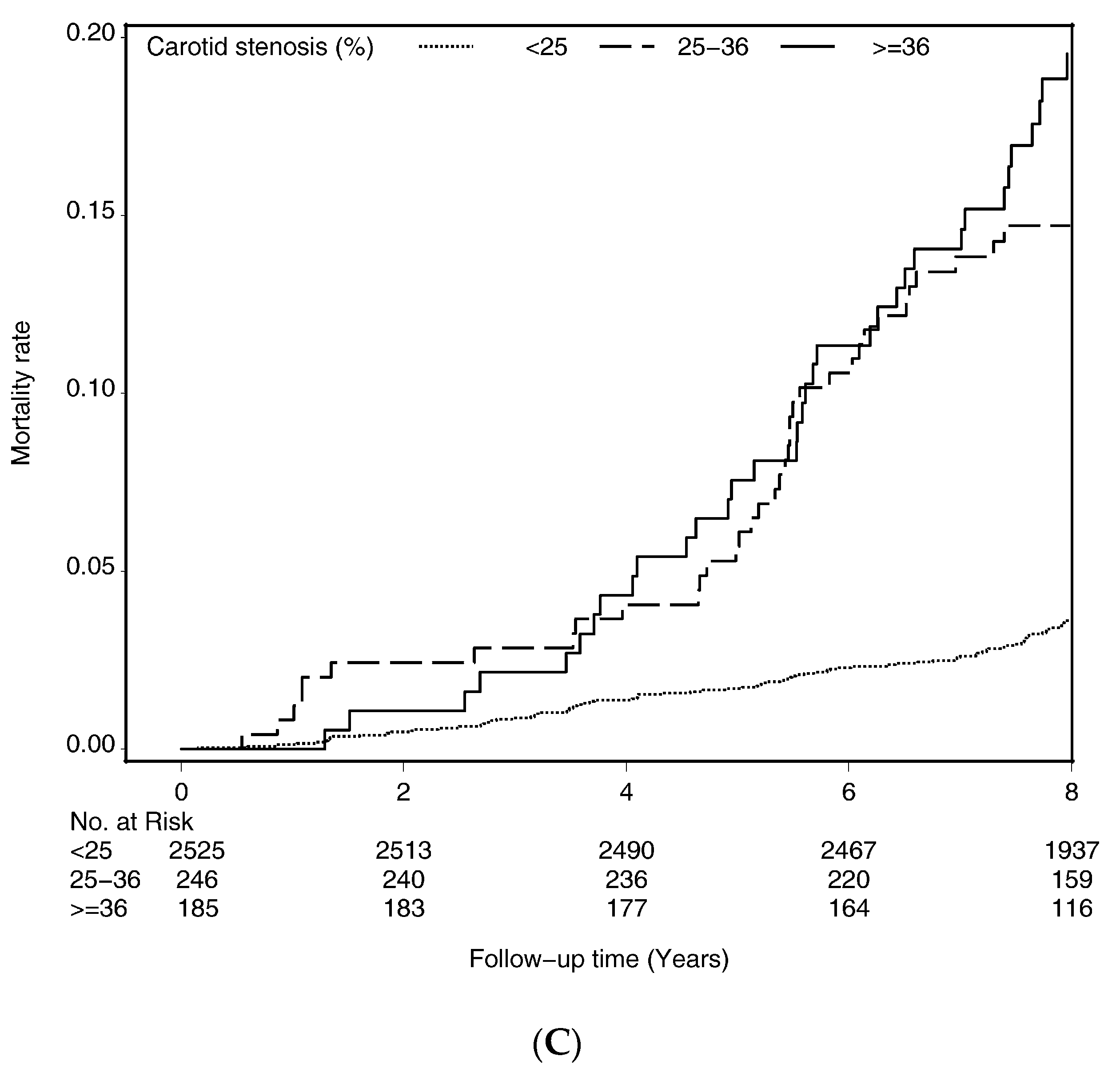
| Carotid stenosis | <0.001 | ||
| <25% | 2385 (87.88) | 140 (57.85) | |
| 25%–36% | 195 (7.18) | 51 (21.07) | |
| ≥36% | 134 (4.94) | 51 (21.07) | |
| Variables | n | Cases | Person-Years | Incidence Rate | Age and Sex-Adjusted | Multivariate-Adjusted 1 | Multivariate-Adjusted 2 |
|---|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |||||
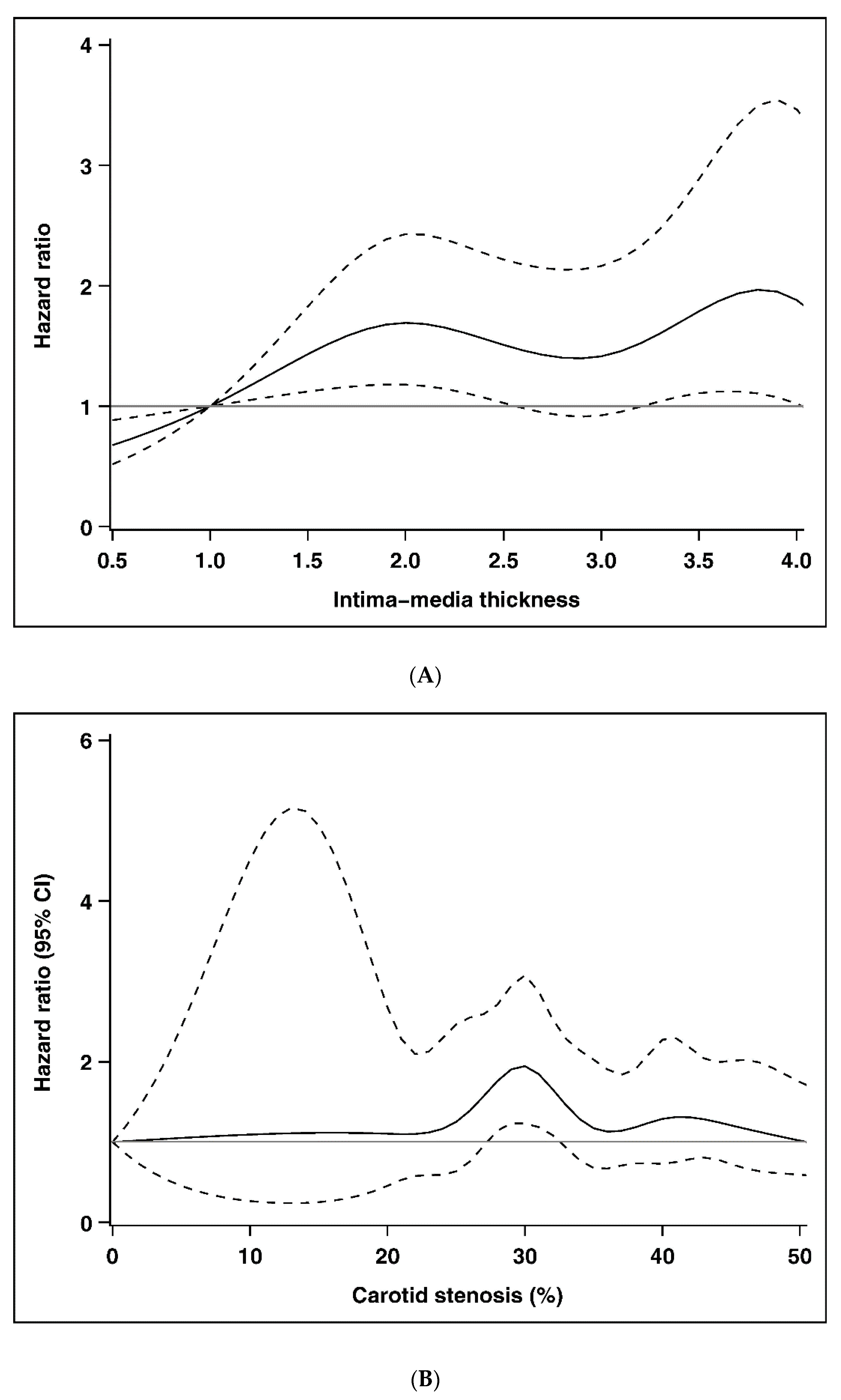
| Intima–media thickness | |||||||
| <1.0 mm | 877 | 20 | 8140.94 | 2.46 | 1.00 | 1.00 | 1.00 |
| 1.0–2.0 mm | 1533 | 107 | 14,714.21 | 7.27 | 1.58 (0.97, 2.57) | 1.53 (0.94, 2.49) | 1.53 (0.94, 2.50) |
| ≥2.0 mm | 546 | 115 | 4955.72 | 23.21 | 2.22 (1.34, 3.68) ** | 2.06 (1.24, 3.42) ** | 1.79 (1.07, 3.00) * |
| p for trend | <0.001 | 0.002 | 0.003 | ||||
| Carotid plaque | |||||||
| No | 1517 | 47 | 14,453.17 | 3.25 | 1.00 | 1.00 | 1.00 |
| Yes | 1439 | 195 | 13,357.71 | 14.60 | 1.89 (1.35, 2.64) *** | 1.82 (1.30, 2.55) *** | 1.65 (1.17, 2.32) ** |
| Carotid stenosis | |||||||
| <25% | 2525 | 140 | 24,074.52 | 5.82 | 1.00 | 1.00 | 1.00 |
| 25%–36% | 246 | 51 | 2118.61 | 24.07 | 1.75 (1.25, 2.46) ** | 1.71 (1.22, 2.39) ** | 1.57 (1.12, 2.22) ** |
| ≥36% | 185 | 51 | 1617.74 | 31.53 | 1.54 (1.09, 2.18) * | 1.49 (1.05, 2.10) * | 1.15 (0.80, 1.67) |
| p for trend | 0.004 | 0.007 | 0.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-W.; Guo, Y.-C.; Li, C.-I.; Liu, C.-S.; Lin, C.-H.; Liu, C.-H.; Wang, M.-C.; Yang, S.-Y.; Li, T.-C.; Lin, C.-C. Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults. Int. J. Environ. Res. Public Health 2020, 17, 4745. https://doi.org/10.3390/ijerph17134745
Yang C-W, Guo Y-C, Li C-I, Liu C-S, Lin C-H, Liu C-H, Wang M-C, Yang S-Y, Li T-C, Lin C-C. Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults. International Journal of Environmental Research and Public Health. 2020; 17(13):4745. https://doi.org/10.3390/ijerph17134745
Chicago/Turabian StyleYang, Chuan-Wei, Yuh-Cherng Guo, Chia-Ing Li, Chiu-Shong Liu, Chih-Hsueh Lin, Chung-Hsiang Liu, Mu-Cyun Wang, Shing-Yu Yang, Tsai-Chung Li, and Cheng-Chieh Lin. 2020. "Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults" International Journal of Environmental Research and Public Health 17, no. 13: 4745. https://doi.org/10.3390/ijerph17134745
APA StyleYang, C.-W., Guo, Y.-C., Li, C.-I., Liu, C.-S., Lin, C.-H., Liu, C.-H., Wang, M.-C., Yang, S.-Y., Li, T.-C., & Lin, C.-C. (2020). Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults. International Journal of Environmental Research and Public Health, 17(13), 4745. https://doi.org/10.3390/ijerph17134745





