An Evaluation of the Process and Quality Improvement Measures of the University of Virginia Cancer Center Tobacco Treatment Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Approach
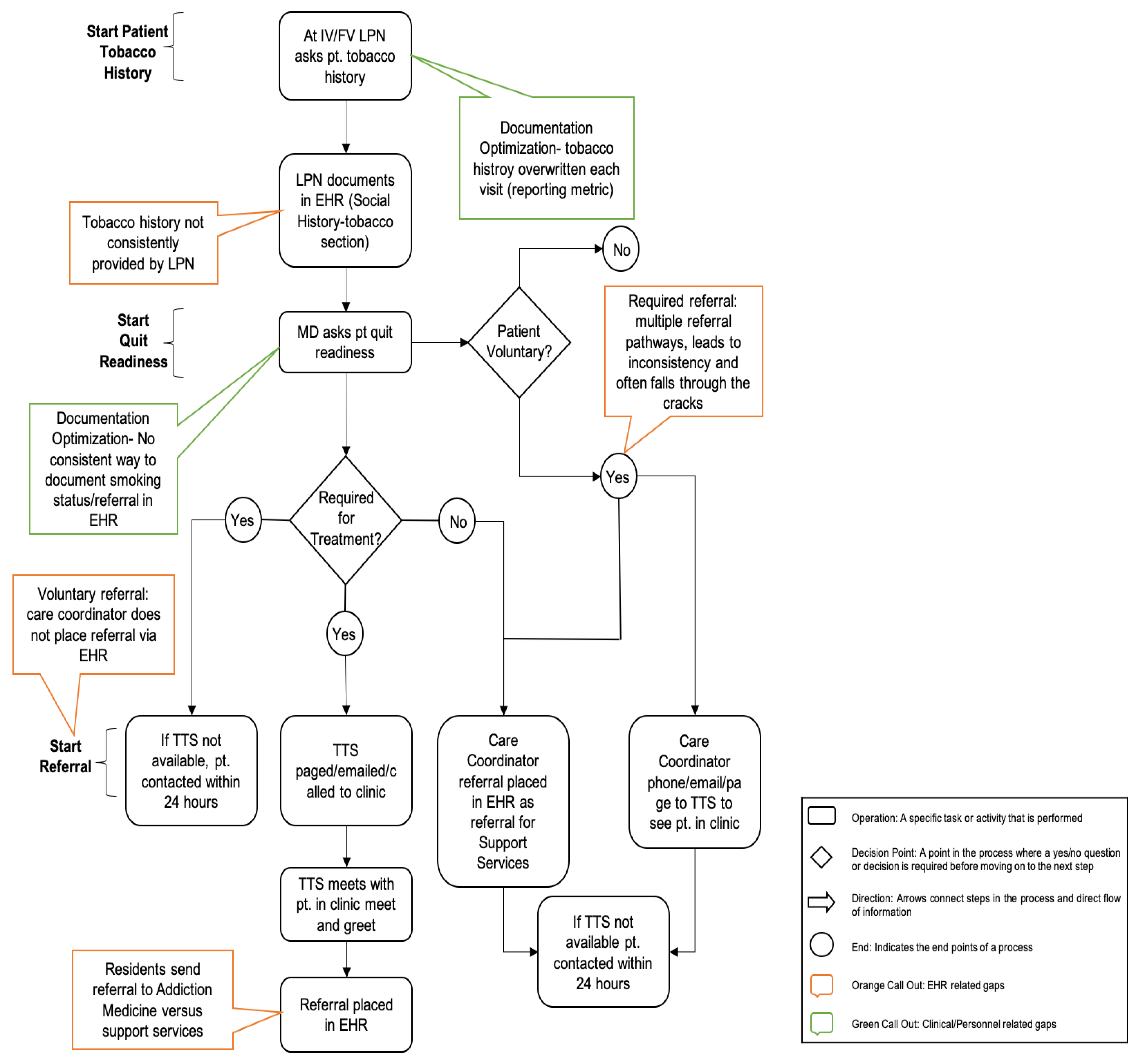
2.2.1. Map the Process
2.2.2. Create Flow
2.3. Data and Analyses
3. Results
3.1. Gap Analysis and Prioritization
3.2. Development of Solutions and Implementation of New Program
3.2.1. Tobacco Use Documentation
3.2.2. Triaging
3.2.3. Flowsheets and Smart Phrases
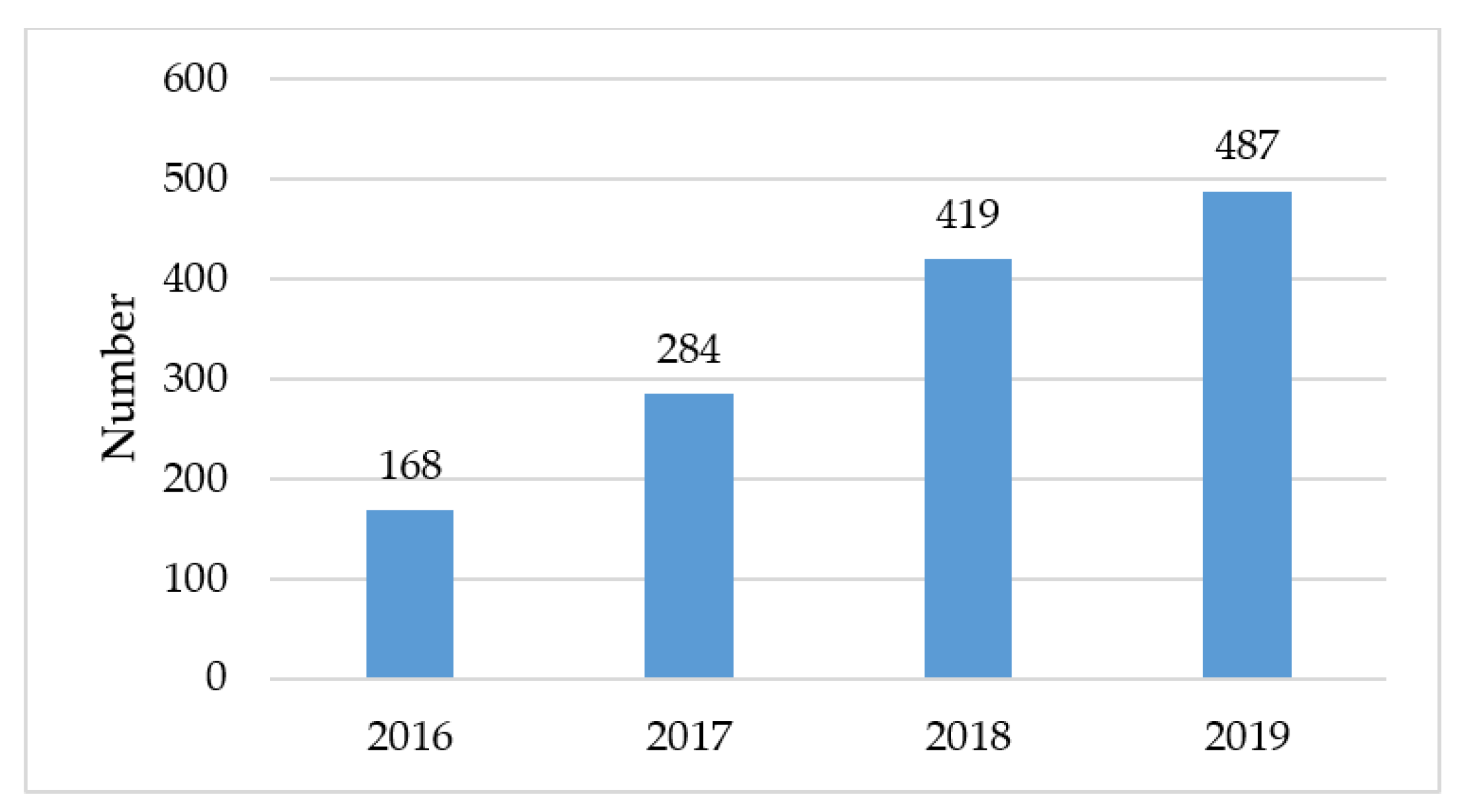
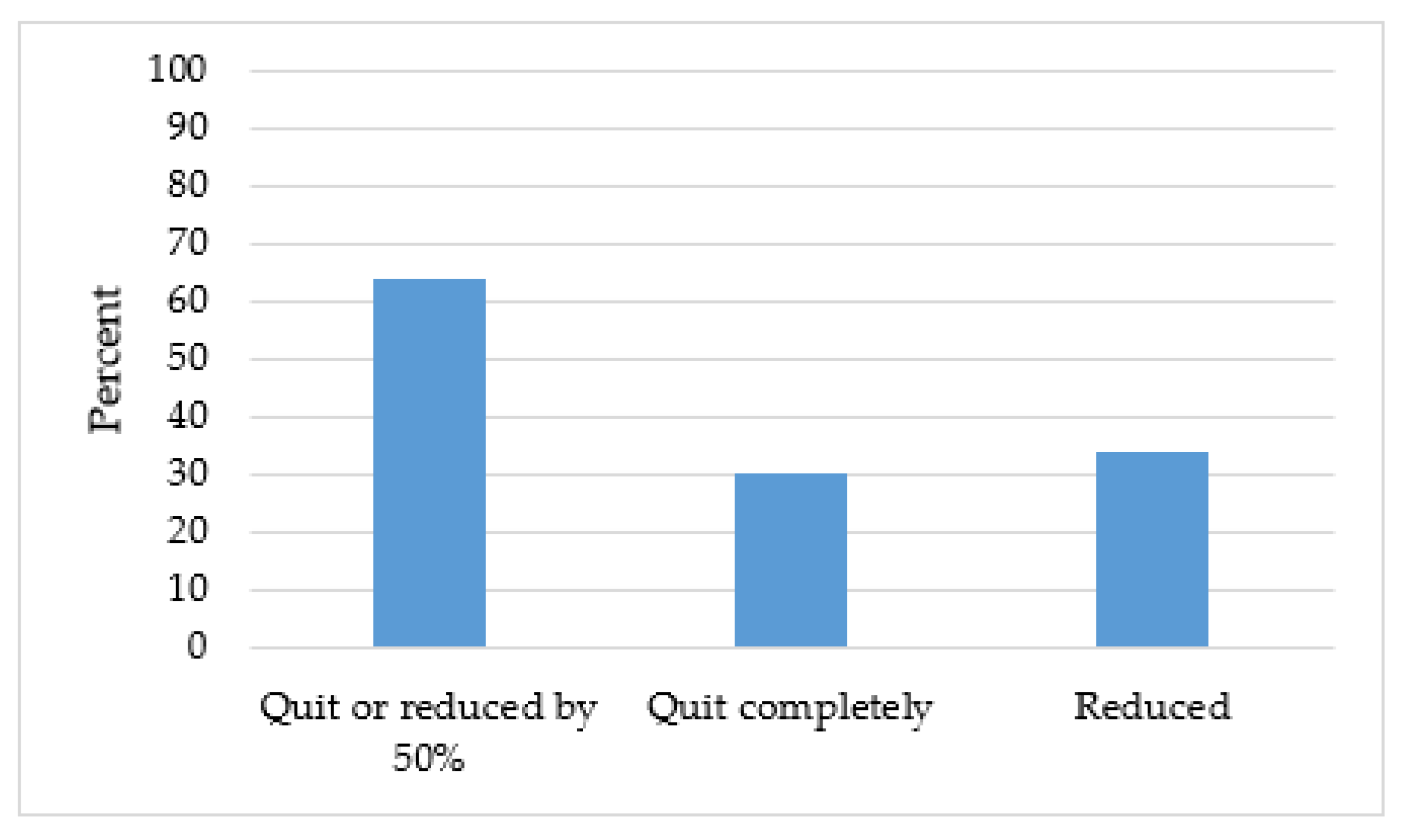
3.3. Implementation of New Tobacco Treatment Program
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robert Wood Johnson Foundation. County Health Rankings and Roadmaps: Virginia. Available online: https://www.countyhealthrankings.org/app/virginia/2019/measure/factors/9/datasource?sort=desc-2 (accessed on 2 March 2020).
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health: Atlanta, GA, USA, 2014.
- Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality. In Results from the 2016 National Survey on Drug Use and Health: Detailed Tables; SAMHSA: Rockville, MD, USA, 2017.
- Roberts, M.E.; Doogan, N.J.; Stanton, C.A. Rural Versus Urban Use of Traditional and Emerging Tobacco Products in the United States, 2013–2014. Am. J. Public Health 2017, 107, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- American Lung Association. Cutting Tobacco’s Rural Roots: Tobacco Use in Rural Communities. Available online: https://www.lung.org/getmedia/429eb03a-9196-47bb-9912-e47e835d4466/cutting-tobaccos-rural-roots.pdf.pdf (accessed on 3 March 2020).
- Croyle, R.T.; Morgan, G.D.; Fiore, M.C. Addressing a Core Gap in Cancer Care—The NCI Moonshot Program to Help Oncology Patients Stop Smoking. N. Engl. J. Med. 2019, 380, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.W.; Cummings, K.M. Tobacco and Lung Cancer: Risks, Trends, and Outcomes in Patients with Cancer. Am. Soc. Clin. Oncol. 2013, 33, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Gritz, E.R.; Demark-Wahnefried, W. Health Behaviors Influence Cancer Survival. J. Clin. Oncol. 2009, 27, 1930–1932. [Google Scholar] [CrossRef]
- Gritz, E.R.; Fingeret, M.C.; Vidrine, D.J.; Lazev, A.B.; Mehta, N.V.; Reece, G.P. Successes and failures of the teachable moment. Cancer. 2006, 106, 17–27. [Google Scholar] [CrossRef] [PubMed]
- American Society of Clinical Oncology. Tobacco Cessation Guide for Oncology Providers. Available online: http://www.asco.org/sites/default/files/tobacco_cessation_guide.pdf (accessed on 29 May 2020).
- Price, S.N.; Studts, J.L.; Hamann, H.A. Tobacco Use Assessment and Treatment in Cancer Patients: A Scoping Review of Oncology Care Clinician Adherence to Clinical Practice Guidelines in the U.S. Oncologist 2019, 24, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.W.; Dibaj, S.; Hutson, A.; Cummings, K.M.; Dresler, C.; Marshall, J.R. Identifying targeted strategies to improve smoking cessation support for cancer patients. J. Thorac. Oncol. 2015, 10, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute, National Institute of Health. Cancer Center Cessation Initiative. Available online: https://cancercontrol.cancer.gov/brp/tcrb/cessation-initiative.html (accessed on 29 May 2020).
- Pinkney, J.; Rance, S.; Benger, J. How Can Frontline Expertise and New Models of Care Best Contribute to Safely Reducing Avoidable Acute Admissions? A Mixed-Methods Study of Four Acute Hospitals. Available online: https://www.ncbi.nlm.nih.gov/books/NBK338859/ (accessed on 29 May 2020).
- Maijala, R.; Eloranta, S.; Reunanen, T.; Ikonen, T.S. Successful Implementation of Lean as a Managerial Principle in Health Care: A Conceptual Analysis from Systematic Literature Review. Int. J. Technol. Assess. Health Care. 2018, 34, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Aij, K.H.; Teunissen, M. Lean leadership attributes: A systematic review of the literature. J. Health Organ Manag. 2017, 31, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Kanbanize. Gemba Walk: Where the Real Work Happens. Available online: https://kanbanize.com/lean-management/improvement/gemba-walk. (accessed on 31 May 2020).
- Bassuk, J.A.; Washington, I.M. The A3 problem solving report: A 10-step scientific method to execute performance improvements in an academic research vivarium. PLoS ONE 2013, 8, e76833. [Google Scholar] [CrossRef] [PubMed]
- Asfar, T.; Ebbert, J.O.; Klesges, R.C.; Relyea, G.E. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict. Behav. 2011, 36, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerström, K.-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Land, S.R.; Warren, G.W.; Crafts, J.L. Cognitive testing of tobacco use items for administration to patients with cancer and cancer survivors in clinical research. Cancer 2016, 122, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Mitra, S.; Ruebush, E.; Sisler, L.; Wang, K.; Goldstein, A.O. A lean quality improvement initiative to enhance tobacco use treatment in a cancer hospital. Int. J. Environ. Res. Public Health. 2020, 17, 2165. [Google Scholar] [CrossRef] [PubMed]
- Sisler, L.; Omofoye, O.; Paci, K.; Hadar, E.; Goldstein, A.O.; Ripley-Moffitt, C. Using lean quality improvement tools to increase delivery of evidence-based tobacco use treatment in hospitalized neurosurgical patients. Jt. Comm. J. Qual. Patient Saf. 2017, 43, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Pew Research Center. Digital Gap between Rural and Nonrural America Persists. Available online: https://www.pewresearch.org/fact-tank/2019/05/31/digital-gap-between-rural-and-nonrural-america-persists. (accessed on 31 May 2020).
- Jose, T.; Ohde, J.W.; Hays, J.T.; Burke, M.V.; Warner, D.O. Design and pilot implementation of an electronic health record-based system to automatically refer cancer patients to tobacco use treatment. Int. J. Environ. Res. Public Health. 2020, 17, 4054. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.; Sharee, K.; Boreta, L.; Silveira, W.R.; Braunstein, S.; Fogh, S. Quality improvement initiative to improve tobacco cessation efforts in radiation oncology. J. Oncol. Pract. 2019, 15, e382–e388. [Google Scholar] [CrossRef] [PubMed]
| Gap Number | Gap Description | Domain | Priority | Solution |
|---|---|---|---|---|
| 1 | Lack of LPN clarity on how to enter tobacco use information | EHR | High | Education/Training |
| 2 | “Mark as Reviewed” EHR button applied to all sections of Alcohol, Tobacco, and Other Drug Use Page, not just the Tobacco Use section and tobacco use updates overwrote previous entries (impacted by #1) | EHR | High | EHR |
| 3 | Inconsistent orientation to tobacco use assessment among LPNs | Center | High | Education/Training |
| 4 | No validation of process flow for current smokers (impacted by #1) | Center | High | Education/Training |
| 5 | No way to trigger automatic referral to TTS through the EHR | EHR | High | EHR |
| 6 | Fractured Ask, Advise, Connect (Impacted by #1) | Clinician/Center | High | Education/Training |
| 7 | Inconsistent use of TTP referral process using triage protocol (impacted by #1) | Center | High | Education/Training |
| 8 | Inconsistent orientation for nurse coordinators and residents/fellows for TTP referral standard work | Center | High | Education/Training |
| 9 | Accessibility of TTS (impacted by #7) | Clinician | High | Education/Training Workflow |
| 10 | Survivor information and progress within the TTP are manually entered in a non-standardized way | EHR | High | EHR |
| 11 | Non-optimized triaging (impacted by #7) | Center | High | Workflow Education/Training |
| 12 | Access to tobacco use history dependent on access level of staff | Center | Med | EHR |
| 13 | “Ready to Quit” EHR button “Yes” response not saved | EHR | Med | EHR |
| 14 | Referral data not available for validation or reporting to stakeholders (Clinical care team/ Administration/Grantors) | EHR | Med | EHR |
| 15 | Multiple referral queues (impacted by #8) | Center | Med | Education/Training, EHR |
| 16 | Lack of survivor communication tools (Telehealth) | Center | Med | Workflow |
| 17 | Outdated survivor education materials and standard work documentation | Center | Low | Workflow |
| 18 | No automated reminders to survivors for follow-up within TTP | EHR | Low | EHR |
| 19 | No automated referrals to State quit line or SmokefreeTXT | EHR | Low | EHR |
| 20 | Reports created manually | EHR | Low | EHR |
| Tier | Criteria | Tobacco Treatment Services Received |
|---|---|---|
| 1 | Impending surgery, or treatment requires cessation (survivor is not eligible for surgery if not tobacco free), mental health diagnoses, or substance abuse history | 1 h initial visit with TTS 8-10 phone or in-person visits (~30 min each) Pharmacotherapy (collaboration with UVA pharmacy) Provided information about community resources TTS meets with survivor post-surgery for continued treatment (if applicable) |
| 2 | Tobacco user who would like to participate in the TTP and is ready to quit now | 1 h initial visit with TTS 4 phone or in-person visits (~30 min each) Pharmacotherapy (collaboration with UVA pharmacy) Provided information about community resources |
| 3 | Tobacco user who would like to participate in the TTP but is not ready to quit now | 30 min initial visit with TTS TTS provides their contact information Provided information about community resources |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiseman, K.P.; Hauser, L.; Clark, C.; Odumosu, O.; Dahl, N.; Peregoy, J.; Sheffield, C.W.; Klesges, R.C.; Anderson, R.T. An Evaluation of the Process and Quality Improvement Measures of the University of Virginia Cancer Center Tobacco Treatment Program. Int. J. Environ. Res. Public Health 2020, 17, 4707. https://doi.org/10.3390/ijerph17134707
Wiseman KP, Hauser L, Clark C, Odumosu O, Dahl N, Peregoy J, Sheffield CW, Klesges RC, Anderson RT. An Evaluation of the Process and Quality Improvement Measures of the University of Virginia Cancer Center Tobacco Treatment Program. International Journal of Environmental Research and Public Health. 2020; 17(13):4707. https://doi.org/10.3390/ijerph17134707
Chicago/Turabian StyleWiseman, Kara P., Lindsay Hauser, Connie Clark, Onyiyoza Odumosu, Neely Dahl, Jennifer Peregoy, Christina W. Sheffield, Robert C. Klesges, and Roger T. Anderson. 2020. "An Evaluation of the Process and Quality Improvement Measures of the University of Virginia Cancer Center Tobacco Treatment Program" International Journal of Environmental Research and Public Health 17, no. 13: 4707. https://doi.org/10.3390/ijerph17134707
APA StyleWiseman, K. P., Hauser, L., Clark, C., Odumosu, O., Dahl, N., Peregoy, J., Sheffield, C. W., Klesges, R. C., & Anderson, R. T. (2020). An Evaluation of the Process and Quality Improvement Measures of the University of Virginia Cancer Center Tobacco Treatment Program. International Journal of Environmental Research and Public Health, 17(13), 4707. https://doi.org/10.3390/ijerph17134707




