Estimation of Soil Organic Carbon Storage in Palustrine Wetlands, China
Abstract
1. Introduction
2. Database and Methods
2.1. Data Collection
2.2. Methodology
2.3. Statistical Analysis
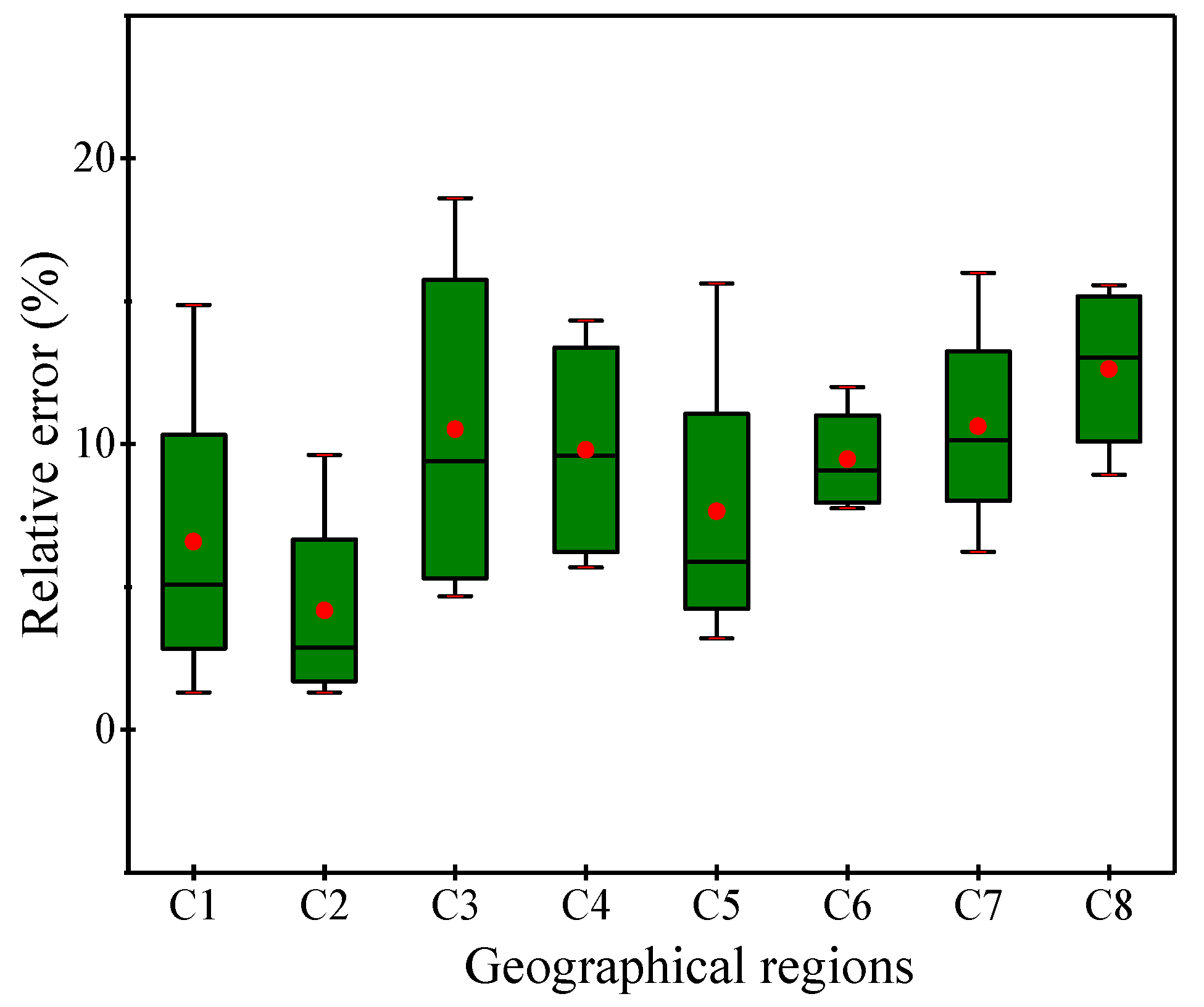
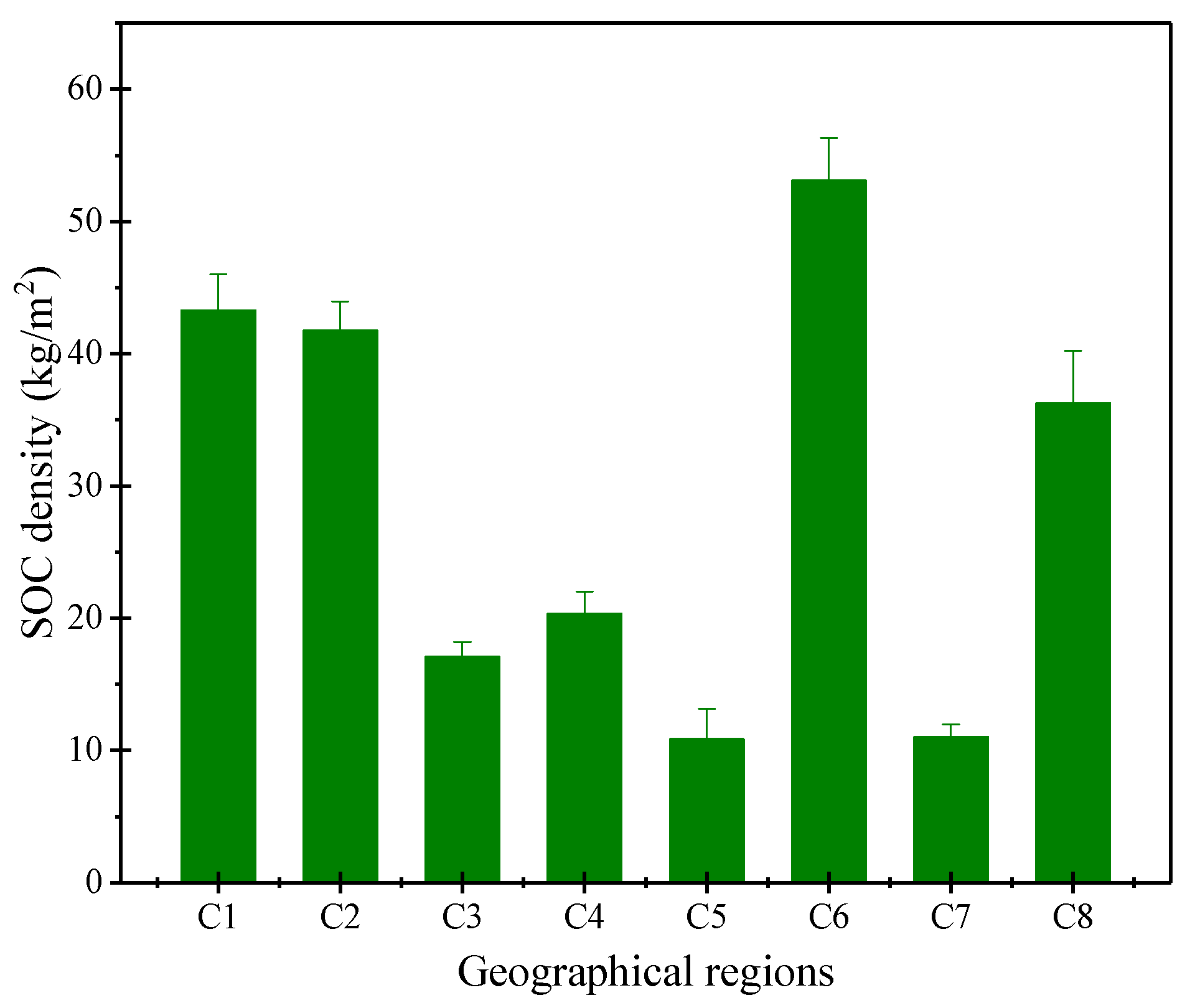
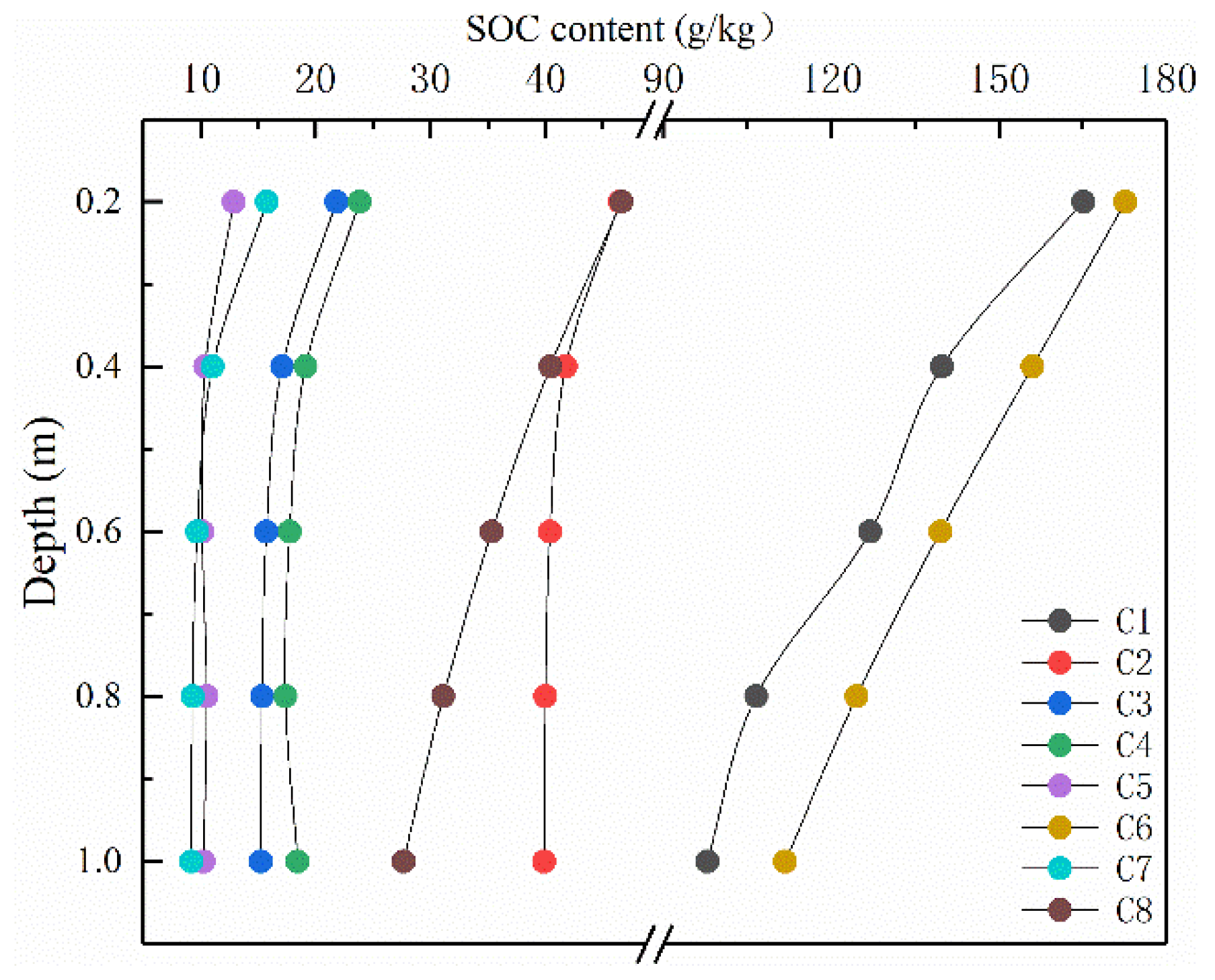
3. Results and Analysis
3.1. Simulated SOC Storage
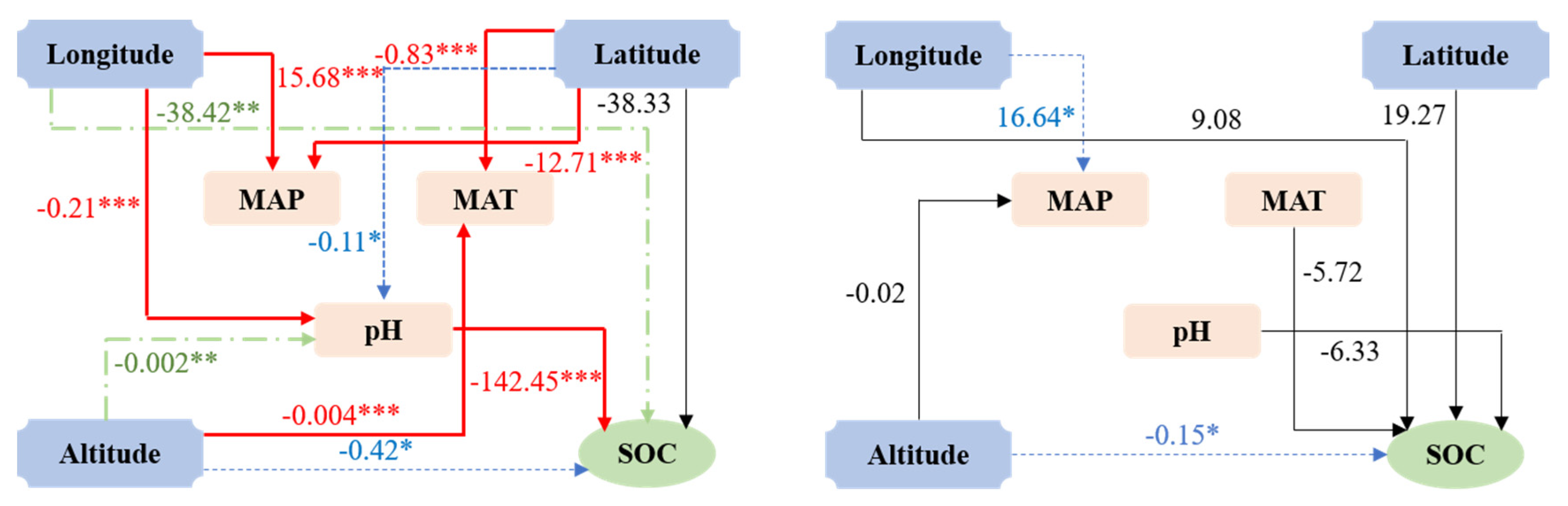
3.2. Environmental Controls on SOC Content
4. Discussion
4.1. Spatial Distribution and Characteristics of SOC on a National-Scale
4.2. Environmental Factors Controlling SOC Content
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitra, S.; Wassmann, R.; Vlek, P.L.G. An appraisal of global wetland area and its organic carbon stock. Curr. Sci. 2005, 88, 25–35. [Google Scholar]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, U.; Zhang, L.; Anderson, C.J.; Jorgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Megonigal, J.P.; Keller, J.K.; Bliss, N.B.; Trettin, C. The carbon balance of North American wetlands. Wetlands 2006, 26, 889–916. [Google Scholar] [CrossRef]
- Roulet, N.T. Peatlands, carbon storage, greenhouse gases, and the Kyoto Protocol: Prospects and significance for Canada. Wetlands 2000, 20, 605–615. [Google Scholar] [CrossRef]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. B-Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Lehner, B.; Döll, P. Development and validation of a global database of lakes, reservoirs and wetlands. J. Hydrol. 2004, 296, 1–22. [Google Scholar] [CrossRef]
- Mu, C.; Han, S.; Luo, J.; Wang, X. Biomass distribution patterns of ecotones between forest and swamp in Changbai Mountain. J. For. Res. 2000, 11, 198–202. [Google Scholar]
- Mu, C.; Lu, H.; Wang, B.; Bao, X.; Cui, W. Short-term effects of harvesting on carbon storage of boreal Larix gmelinii—Carex schmidtii forested wetlands in Daxing’anling, northeast China. Ecol. Manag. 2013, 293, 140–148. [Google Scholar] [CrossRef]
- Bai, J.; Ouyang, H.; Deng, W.; Zhu, Y.; Zhang, X.; Wang, Q. Spatial distribution characteristics of organic matter and total nitrogen of marsh soils in river marginal wetlands. Geoderma 2004, 124, 181–192. [Google Scholar] [CrossRef]
- Neher, D.A.; Barbercheck, M.E.; El-Allaf, S.M.; Anas, O. Effects of disturbance and ecosystem on decomposition. Appl. Soil Ecol. 2003, 23, 165–179. [Google Scholar] [CrossRef]
- Wang, G.; Guan, D.; Peart, M.R.; Chen, Y.; Peng, Y. Ecosystem carbon stocks of mangrove forest in Yingluo Bay, Guangdong Province of South China. Ecol. Manag. 2013, 310, 539–546. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chang, S.X.; Jiang, P.; Zhou, G.; Fu, S.; Yan, E.; Wu, J.; Lin, L. Long-term intensive management effects on soil organic carbon pools and chemical composition in Moso bamboo (Phyllostachys pubescens) forests in subtropical China. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Bai, J.; Ouyang, H.; Xiao, R.; Gao, J.; Gao, H.; Cui, B.; Huang, L. Spatial and variability of soil carbon, nitrogen, and phosphorus content and storage in an alpine wetland in the Qinghai-Tibet Plateau, China. Austrilian J. Soil Res. 2010, 48, 730–736. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, J.; Wang, L.; Guo, J.; Feng, J.; Wu, H.; Lin, G. Distribution patterns and controlling factors for the soil organic carbon in four mangrove forests of China. Glob. Ecol. Conserv. 2019, 17, e00575. [Google Scholar] [CrossRef]
- Mao, D.H.; Wang, Z.M.; Li, L.; Miao, Z.H.; Ma, W.H.; Song, C.C.; Ren, C.Y.; Jia, M.M. Soil organic carbon in the Sanjiang Plain of China: Storage, distribution and controlling factors. Biogeosciences 2015, 12, 1635–1645. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Adams, D.E.; Wild, A. Model estimates of CO2 emissions from soil in response to global warming. Nature 1991, 351, 304–306. [Google Scholar] [CrossRef]
- Armentano, T.V.; Menges, E.S. Patterns of change in the carbon balance of organic soil-wetlands of the temperate zone. J. Ecol. 1986, 74, 755–774. [Google Scholar] [CrossRef]
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef]
- Bohn, H.L. Estimate of Organic Carbon in World Soils. Soil Sci. Soc. Am. J. 1982, 46, 1118–1119. [Google Scholar] [CrossRef]
- Eswaran, H.; Van Den Berg, E.; Reich, P. Organic carbon in soils of the world. Soil Sci. Soc. Am. J. 1993, 57, 192–194. [Google Scholar] [CrossRef]
- Arrouays, D.; Deslais, W.; Badeau, V. The carbon content of topsoil and its geographical distribution in France. Soil Use Manag. 2001, 17, 7–11. [Google Scholar] [CrossRef]
- Krogh, L.; Noergaard, A.; Hermansen, M.; Greve, M.H.; Balstroem, T.; Breuning-Madsen, H. Preliminary estimates of contemporary soil organic carbon stocks in Denmark using multiple datasets and four scaling-up methods. Agric. Ecosyst. Environ. 2003, 96, 19–28. [Google Scholar] [CrossRef]
- Sun, W.; Shi, X.; Yu, D.; Wang, K.; Wang, H. Estimation of Soil organic carbon density and storage of northeast china. Acta Pedol. Sin. 2004, 41, 298–300. [Google Scholar]
- Li, M.; Han, X.; Du, S.; Li, L.J. Profile stock of soil organic carbon and distribution in croplands of Northeast China. Catena 2019, 174, 285–292. [Google Scholar] [CrossRef]
- Meersmans, J.; van Wesemael, B.; De Ridder, F.; Van Molle, M. Modelling the three-dimensional spatial distribution of soil organic carbon (SOC) at the regional scale (Flanders, Belgium). Geoderma 2009, 152, 43–52. [Google Scholar] [CrossRef]
- Nord-Larsen, T.; Vesterdal, L.; Bentsen, N.S.; Larsen, J.B. Ecosystem carbon stocks and their temporal resilience in a semi-natural beech-dominated forest. Ecol. Manag. 2019, 447, 67–76. [Google Scholar] [CrossRef]
- Santini, N.S.; Adame, M.F.; Nolan, R.H.; Miquelajauregui, Y.; Pinero, D.; Mastretta-Yanes, A.; Cuervo-Robayo, A.P.; Eamus, D. Storage of organic carbon in the soils of Mexican temperate forests. Ecol. Manag. 2019, 446, 115–125. [Google Scholar] [CrossRef]
- Du, Y.G.; Zhou, G.; Guo, X.W.; Cao, G.M. Spatial distribution of grassland soil organic carbon and potential carbon storage on the Qinghai Plateau. Grassl. Sci. 2019, 65, 141–146. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, S.Y.; Dong, J.; Ren, M.; Zhang, X.L.; Dong, K.H.; Wang, C.H. Effects of spring fire and slope on the aboveground biomass, and organic C and N dynamics in a semi-arid grassland of northern China. J. Arid Land 2019, 11, 267–279. [Google Scholar] [CrossRef]
- Bernal, B.; Mitsch, W.J. Comparing carbon sequestration in temperate freshwater wetland communities. Glob. Chang. Biol. 2012, 18, 1636–1647. [Google Scholar] [CrossRef]
- Acutis, M.; Donatelli, M. SOILPAR 2.00: Software to estimate soil hydrological parameters and functions. Eur. J. Agron. 2003, 18, 373–377. [Google Scholar] [CrossRef]
- Heuscher, S.A.; Brandt, C.C.; Jardine, P.M. Using soil physical and chemical properties to estimate bulk density. Soil Sci. Soc. Am. J. 2005, 69, 51–56. [Google Scholar]
- Benites, V.M.; Machado, P.; Fidalgo, E.C.C.; Coelho, M.R.; Madari, B.E. Pedotransfer functions for estimating soil bulk density from existing soil survey reports in Brazil. Geoderma 2007, 139, 90–97. [Google Scholar] [CrossRef]
- Yang, R.M. Mechanisms of soil organic carbon storage response to Spartina alterniflora invasion and climate change. Sci. Total Environ. 2019, 690, 7–15. [Google Scholar] [CrossRef]
- Han, G.Z.; Zhang, G.L.; Gong, Z.T.; Wang, G.F. Pedotransfer Functions for Estimating Soil Bulk Density in China. Soil Sci. 2012, 177, 158–164. [Google Scholar] [CrossRef]
- Han, G.Z.; Wang, D.C.; Xie, D.J. Study on soil bulk density transfer function of main soil types in China. Acta Pedol. Sin. 2016, 53, 93–102. [Google Scholar]
- Minasny, B.; McBratney, A.B.; Mendonça-Santos, M.L.; Odeh, I.O.A.; Guyon, B. Prediction and digital mapping of soil carbon storage in the Lower Namoi Valley. Soil Res. 2006, 44, 233–244. [Google Scholar] [CrossRef]
- Cambule, A.H.; Rossiter, D.G.; Stoorvogel, J.J.; Smaling, E.M.A. Soil organic carbon stocks in the Limpopo National Park, Mozambique: Amount, spatial distribution and uncertainty. Geoderma 2014, 213, 46–56. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Li, B. Determining the influence factors of soil organic carbon stock in opencast coal-mine dumps based on complex network theory. Catena 2019, 173, 433–444. [Google Scholar] [CrossRef]
- Meersmans, J.; Van Wesemael, B.; De Ridder, F.; Dotti, M.F.; De Baets, S.; Van Molle, M. Changes in organic carbon distribution with depth in agricultural soils in northern Belgium, 1960-2006. Glob. Chang. Biol. 2019, 15, 2739–2750. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, G.L.; Sun, Y.J.; Zhao, Y.G.; Li, D.C. Mapping the three-dimensional distribution of soil organic matter across a subtropical hilly landscape. Soil Sci. Soc. Am. J. 2013, 77, 1241–1253. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Chimner, R.A.; Ewel, C. A tropical freshwater wetland: II. production, decomposition, and peat formation. Wetl. Ecol. Manag. 2005, 13, 671–684. [Google Scholar] [CrossRef]
- Liu, K.; Huang, J.; Li, D.M.; Yu, X.C.; Ye, H.C.; Hu, H.W.; Hu, Z.H.; Huang, Q.H.; Zhang, H.M. Comparison of carbon sequestration efficiency in soil aggregates between upland and paddy soils in a red soil region of China. J. Integr. Agr. 2019, 18, 1348–1359. [Google Scholar] [CrossRef]
- Gu, Z.; Xie, Y.; Gao, Y.; Ren, X.; Cheng, C.; Wang, S. Quantitative assessment of soil productivity and predicted impacts of water erosion in the black soil region of northeastern China. Sci. Total Environ. 2018, 637–638, 706–716. [Google Scholar] [CrossRef]
- Bonner, M.T.L.; Herbohn, J.; Gregorio, N.; Pasa, A.; Avela, M.S.; Solano, C.; Moreno, M.O.M.; Almendras-Ferraren, A.; Wills, J.; Shoo, L.P.; et al. Soil organic carbon recovery in tropical tree plantations may depend on restoration of soil microbial composition and function. Geoderma 2019, 353, 70–80. [Google Scholar] [CrossRef]
- Pérez-Rojas, J.; Moreno, F.; Quevedo, J.C.; Villa, J. Soil organic carbon stocks in fluvial and isolated tropical wetlands from Colombia. Catena 2019, 179, 138–149. [Google Scholar] [CrossRef]
- Zhong, Q.; Wang, K.; Nie, M.; Zhang, G.; Zhang, W.; Zhu, Y.; Fu, Y.; Zhang, Q.; Gao, Y. Responses of wetland soil carbon and nutrient pools and microbial activities after 7 years of experimental warming in the Yangtze Estuary. Ecol. Eng. 2019, 136, 68–78. [Google Scholar] [CrossRef]
- Vicente-Vicente, J.L.; Gómez-Muñoz, B.; Hinojosa-Centeno, M.B.; Smith, P.; Garcia-Ruiz, R. Carbon saturation and assessment of soil organic carbon fractions in Mediterranean rainfed olive orchards under plant cover management. Agric. Ecosyst. Environ. 2017, 245, 135–146. [Google Scholar] [CrossRef]
- Wang, W.; Akhtar, K.; Ren, G.; Yang, G.; Feng, Y.; Yuan, L. Impact of straw management on seasonal soil carbon dioxide emissions, soil water content, and temperature in a semi-arid region of China. Sci. Total Environ. 2019, 652, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Bai, J.; Wang, W.; Zhang, G.; Jia, J.; Cui, B.; Liu, X. Effects of soil moisture on carbon mineralization in floodplain wetlands with different flooding frequencies. J. Hydrol. 2019, 574, 1074–1084. [Google Scholar] [CrossRef]
- Tu, C.; He, T.; Lu, X.; Luo, Y.; Smith, P. Extent to which pH and topographic factors control soil organic carbon level in dry farming cropland soils of the mountainous region of Southwest China. Catena 2018, 163, 204–209. [Google Scholar] [CrossRef]
- Martinsen, V.; Munera-Echeverri, J.L.; Obia, A.; Cornelissen, G.; Mulder, J. Significant build-up of soil organic carbon under climate-smart conservation farming in Sub-Saharan Acrisols. Sci. Total Environ. 2019, 660, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Xue, X.; You, Q.; Huang, C.; Dong, S.; Liao, J.; Duan, H.; Tsunekawa, A.; Wang, T. Changes of soil properties regulate the soil organic carbon loss with grassland degradation on the Qinghai-Tibet Plateau. Ecol. Indic. 2018, 93, 572–580. [Google Scholar] [CrossRef]
- Epron, D.; Farque, L.; Lucot, É.; Badot, P.M. Soil CO2 efflux in a beech forest: Dependence on soil temperature and soil water content. Ann. Sci. 1999, 56, 221–226. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J.B. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Pendall, E.; Pei, J.; Noh, N.J.; He, J.S.; Li, B.; Nie, M.; Fang, C. Depth dependence of soil carbon temperature sensitivity across Tibetan permafrost regions. Soil Biol. Biochem. 2018, 126, 82–90. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Organic matter accumulation in submerged soils. Adv. Agron. 2004, 81, 169–201. [Google Scholar]
- Zhan, C.L.; Cao, J.J.; Han, Y.M.; Wang, P.; Huang, R.J.; Wei, C.; Hu, W.G.; Zhang, J.Q. Spatial patterns, storages and sources of black carbon in soils from the catchment of Qinghai Lake, China. Eur. J. Soil Sci. 2015, 66, 525–534. [Google Scholar] [CrossRef]
- Heikkinen, J.E.P.; Elsakov, V.; Martikainen, P.J. Carbon dioxide and methane dynamics and annual carbon balance in tundra wetland in NE Europe, Russia. Glob. Biogeochem. Cycles 2002, 16, 62-1-62-15. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, J.; Cui, L.; Feng, W.; Wang, Y.; Zhang, J. Soil organic carbon stabilization mechanisms in a subtropical mangrove and salt marsh ecosystems. Sci. Total Environ. 2019, 673, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Feagin, R.A.; Hu, B.; He, M.; Li, H. The spatial distribution of blue carbon in the coastal wetlands of China. Estuar. Coast. Shelf Sci. 2019, 222, 13–20. [Google Scholar] [CrossRef]


| Geographical Region | Palustrine Wetlands Area × 104 km2 | Altitude m | MAP mm | MAT °C | Climatic Characteristic |
|---|---|---|---|---|---|
| C1 | 7.5204 | 327.1 | 550 | 1.4 | Temperate monsoon climate |
| C2 | 0.0329 | 1847.2 | 898 | 18 | Subtropical monsoon climate Tropical monsoon climate |
| C3 | 0.1595 | 25.5 | 1320 | 16.6 | Subtropical monsoon climate |
| C4 | 0.0046 | 43.6 | 2283 | 21.3 | Subtropical monsoon climate |
| C5 | 0.1710 | 7.2 | 879 | 14.1 | Temperate monsoon climate Subtropical monsoon climate Tropical monsoon climate |
| C6 | 9.9865 | 3539.1 | 655 | 3.3 | Plateau mountain climate |
| C7 | 0.2493 | 11.7 | 510 | 13.5 | Temperate monsoon climate |
| C8 | 3.6089 | 2730 | 87 | 4.5 | Temperate continental climate |
| Item | AX 1 | AX 2 |
|---|---|---|
| Eigenvalues | 0.579 | 0.421 |
| Species-environment correlation | 0.761 | 0.000 |
| Cumulative % variance of species | 57.90 | 100.00 |
| Cumulative % variance of species-environment | 100.00 | 0.00 |
| Sum of all canonical eigenvalues | 0.579 | |
| Response Variable | Variable | R2 | Direct Path Coefficient | Indirect Path Coefficient | Total Path Coefficient | Residual Path Coefficient | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP | MAT | Latitude | Longitude | Altitude | Mc | pH | ||||||
| SOC | MAP | 0.999 | 0.823 | -- | 0.682 | −0.618 | −0.082 | −0.027 | −0.003 | −0.000 | −0.047 | 0.001 |
| MAT | 0.823 | 0.682 | -- | −0.686 | −0.118 | 0.039 | −0.011 | −0.002 | −0.095 | |||
| Latitude | 0.856 | −0.594 | −0.659 | -- | 0.275 | 0.231 | 0.009 | 0.002 | −0.736 | |||
| Longitude | 0.375 | −0.179 | −0.259 | 0.627 | -- | 0.253 | 0.005 | 0.002 | 0.449 | |||
| Altitude | −0.286 | 0.077 | −0.111 | −0.692 | −0.332 | -- | −0.013 | −0.001 | −1.072 | |||
| MC | 0.025 | −0.088 | −0.354 | 0.322 | 0.078 | 0.144 | -- | 0.001 | 0.104 | |||
| pH | −0.003 | 0.065 | 0.427 | −0.573 | −0.263 | −0.140 | −0.012 | -- | −0.496 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Wan, Z.; Guo, Y.; Song, C.; Jin, S.; Zuo, Y. Estimation of Soil Organic Carbon Storage in Palustrine Wetlands, China. Int. J. Environ. Res. Public Health 2020, 17, 4646. https://doi.org/10.3390/ijerph17134646
Han L, Wan Z, Guo Y, Song C, Jin S, Zuo Y. Estimation of Soil Organic Carbon Storage in Palustrine Wetlands, China. International Journal of Environmental Research and Public Health. 2020; 17(13):4646. https://doi.org/10.3390/ijerph17134646
Chicago/Turabian StyleHan, Lu, Zhongmei Wan, Yuedong Guo, Changchun Song, Shaofei Jin, and Yunjiang Zuo. 2020. "Estimation of Soil Organic Carbon Storage in Palustrine Wetlands, China" International Journal of Environmental Research and Public Health 17, no. 13: 4646. https://doi.org/10.3390/ijerph17134646
APA StyleHan, L., Wan, Z., Guo, Y., Song, C., Jin, S., & Zuo, Y. (2020). Estimation of Soil Organic Carbon Storage in Palustrine Wetlands, China. International Journal of Environmental Research and Public Health, 17(13), 4646. https://doi.org/10.3390/ijerph17134646







