Urban Atmospheric Environment Quality Assessment by Naturally Growing Bryophytes in Central China
Abstract
1. Introduction
2. Materials and Methods
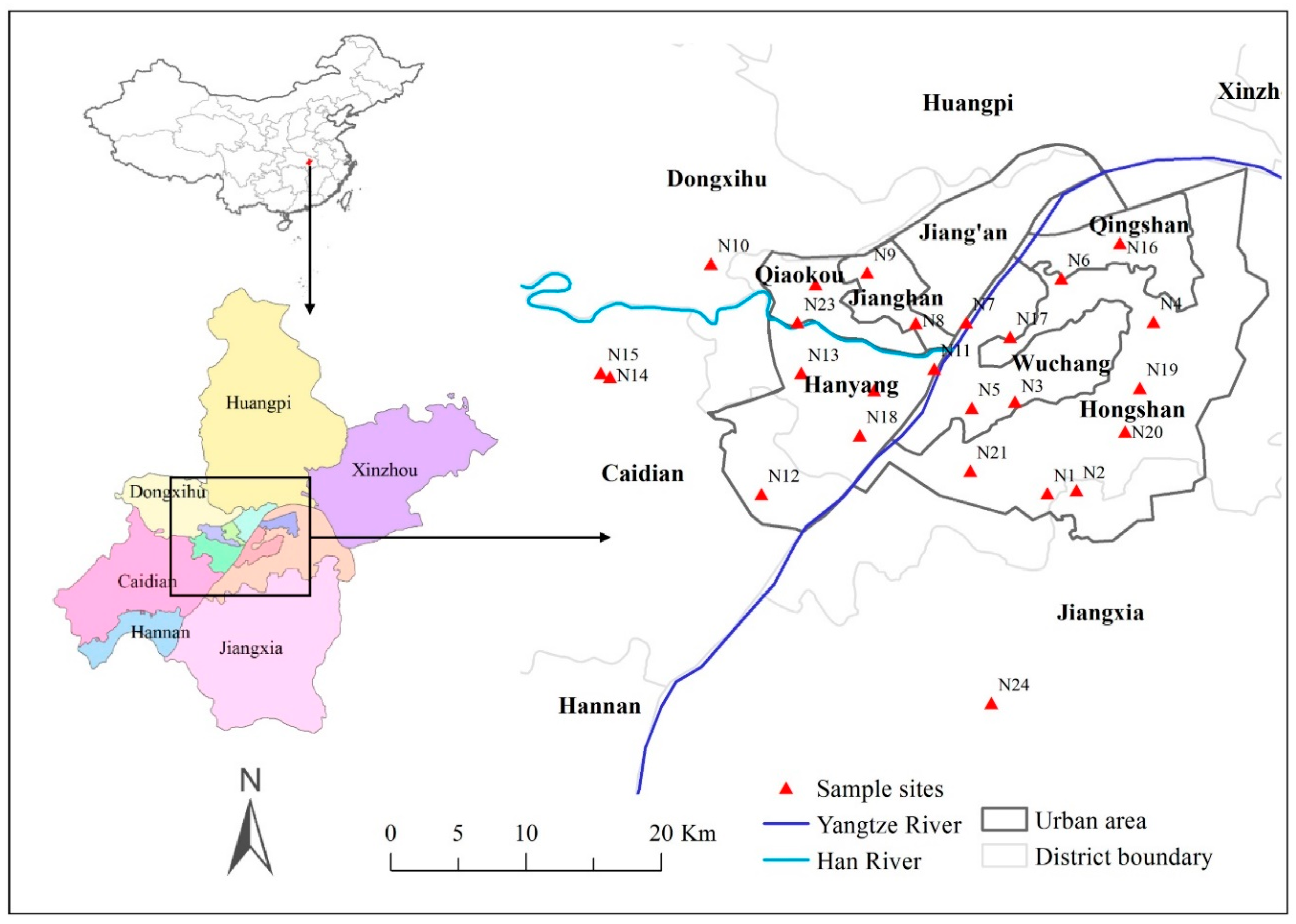
2.1. Study Area and Sampling
2.2. Sample Preparation and Chemical Analysis
2.3. Data Analyses
2.3.1. Diversity and Ordination Analyses for Bryophyte Species
2.3.2. Atmospheric Environment Assessment
2.3.3. Statistical Analyses
3. Results
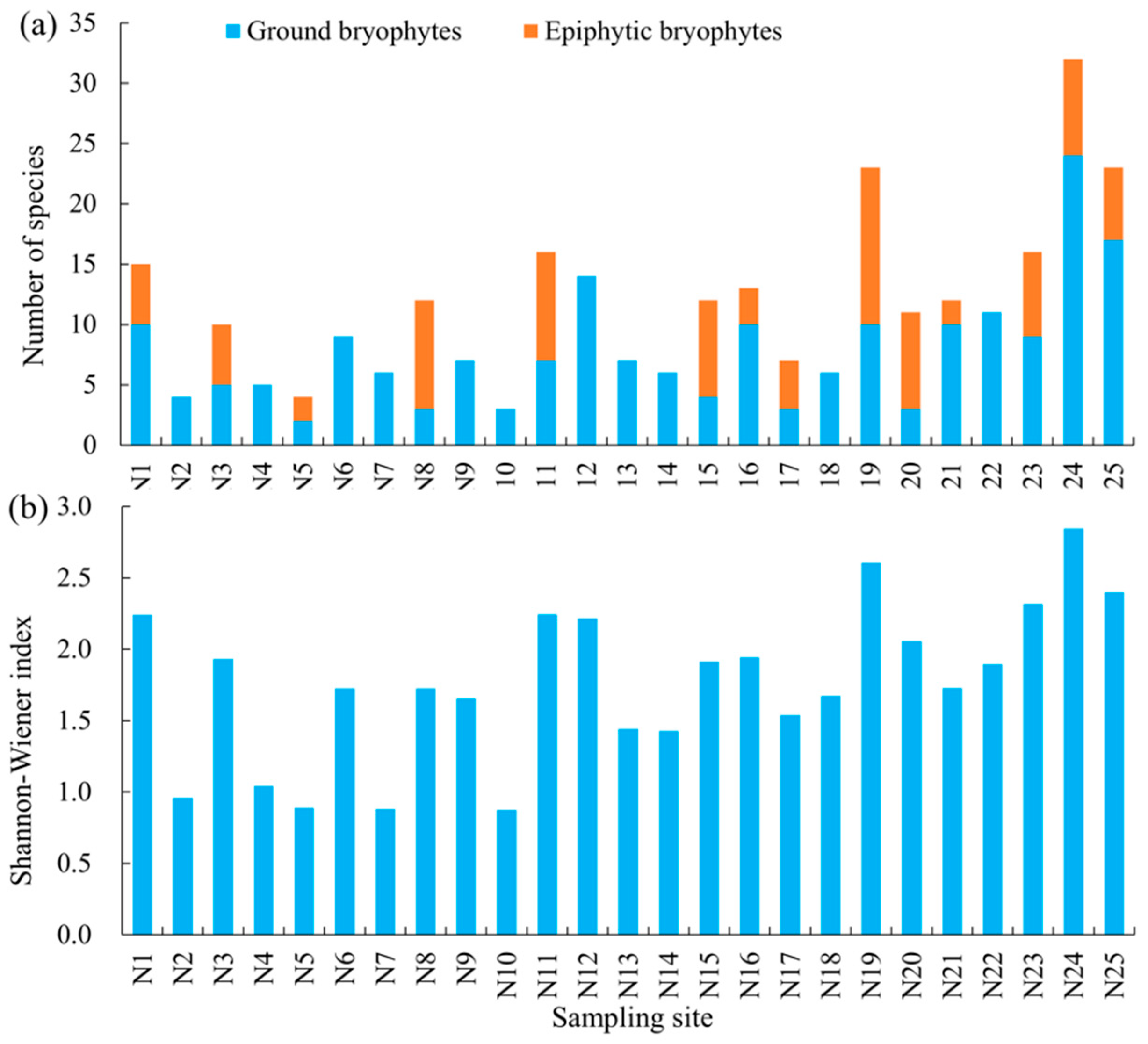
3.1. Diversity and Distribution of Bryophytes in the Urban Areas of Wuhan
3.2. Trace Elements Present in H. angustifolium and Underlying Soil
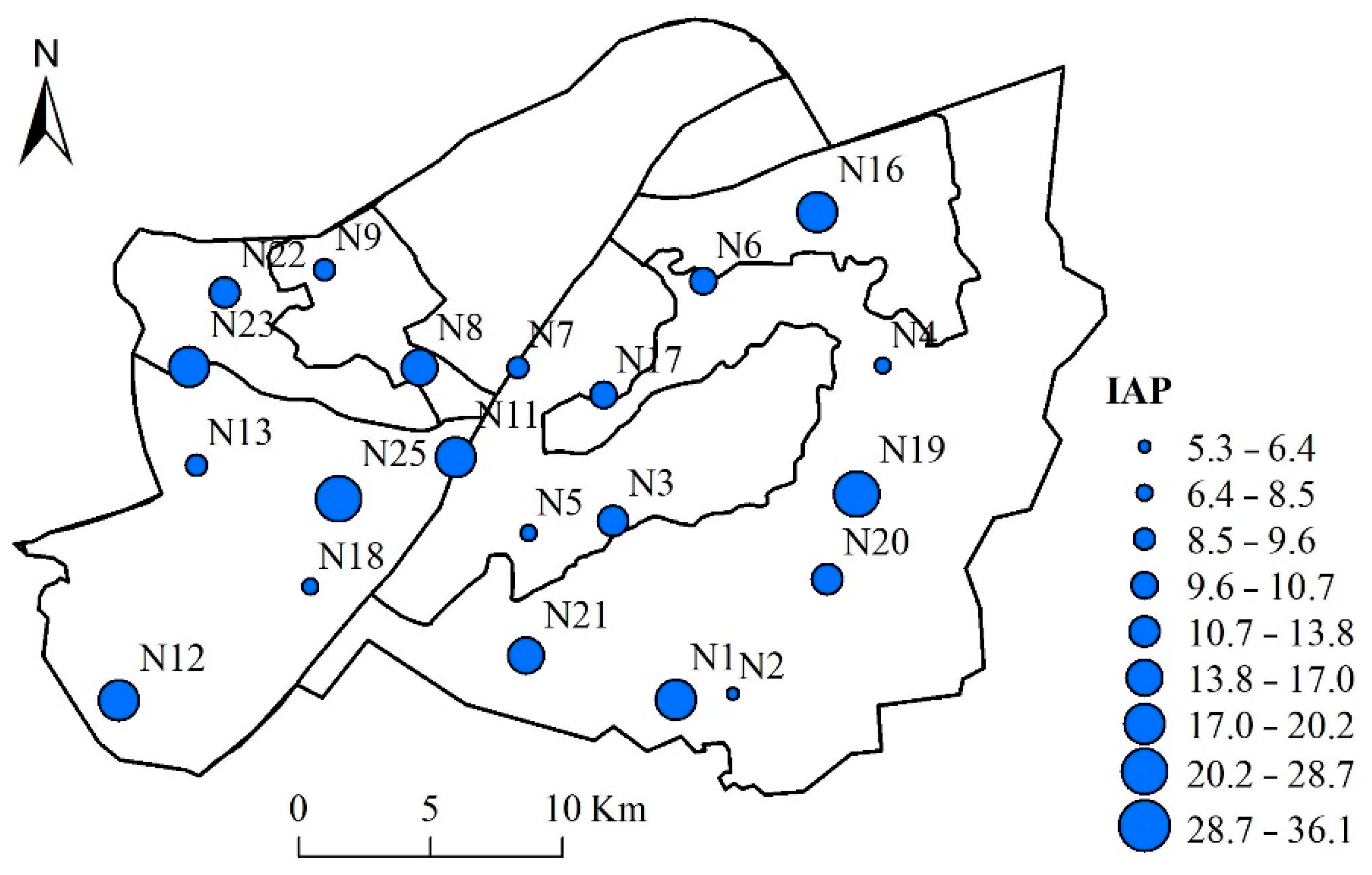
3.3. Assessment of Atmospheric Quality of the Urban Areas of Wuhan by IAP and RI through Bryophytes
4. Discussion
4.1. Suitable Bryophyte Species as Biomonitors in Central China
4.2. Variations in Trace Element Accumulation in Moss Species
4.3. Using IAP and RI to Assess Environmental Quality by Bryophytes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klemm, R. Assessing the effects of air pollution on human health: Impacts of lagging pollutants. Epidemiology 2007, 18, S98. [Google Scholar] [CrossRef]
- Paoli, L.; Guttova, A.; Grassi, A.; Lackovicova, A.; Senko, D.; Loppi, S. Biological effects of airborne pollutants released during cement production assessed with lichens (SW Slovakia). Ecol. Indic. 2014, 40, 127–135. [Google Scholar] [CrossRef]
- Bokwa, A. Environmental impacts of long-term air pollution changes in Krakow, Poland. Pol. J. Environ. Stud. 2008, 17, 673–686. [Google Scholar]
- Cujic, M.; Dragovic, S.; Dordevic, M.; Dragovic, R.; Gajic, B. Environmental assessment of heavy metals around the largest coal fired power plant in Serbia. Catena 2016, 139, 44–52. [Google Scholar] [CrossRef]
- Stankovic, S.; Kalaba, P.; Stankovic, A.R. Biota as toxic metal indicators. Environ. Chem. Lett. 2014, 12, 63–84. [Google Scholar] [CrossRef]
- Pesch, R.; Schroeder, W. Mosses as bioindicators for metal accumulation: Statistical aggregation of measurement data to exposure indices. Ecol. Indic. 2006, 6, 137–152. [Google Scholar] [CrossRef]
- Szczepaniak, K.; Biziuk, M. Aspects of the biomonitoring studies using mosses and lichens as indicators of metal pollution. Environ. Res. 2003, 93, 221–230. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, H.Y.; Guan, H.; Long, C.J. Monitoring atmospheric nitrogen pollution in Guiyang (SW China) by contrasting use of Cinnamomum camphora leaves, branch bark and bark as biomonitors. Environ. Pollut. 2018, 233, 1037–1048. [Google Scholar] [CrossRef]
- Aboal, J.R.; Fernandez, J.A.; Boquete, T.; Carballeira, A. Is it possible to estimate atmospheric deposition of heavy metals by analysis of terrestrial mosses? Sci. Total Environ. 2010, 408, 6291–6297. [Google Scholar] [CrossRef]
- Maxhuni, A.; Lazo, P.; Kane, S.; Qarri, F.; Marku, E.; Harmens, H. First survey of atmospheric heavy metal deposition in Kosovo using moss biomonitoring. Environ. Sci. Pollut. R 2016, 23, 744–755. [Google Scholar] [CrossRef]
- Qarri, F.; Lazo, P.; Allajbeu, S.; Bekteshi, L.; Kane, S.; Stafilov, T. The evaluation of air quality in Albania by moss biomonitoring and metals atmospheric deposition. Arch. Environ. Con. Tox. 2019, 76, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Zhang, Z.H.; Wang, Z.H. Bryophyte communities as biomonitors of environmental factors in the Goujiang karst bauxite, southwestern China. Sci. Total Environ. 2015, 538, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.; Aboal, J.R.; Carballeira, A.; Giordano, S.; Adamo, P.; Fernandez, J.A. Moss bag biomonitoring: A methodological review. Sci. Total Environ. 2014, 475, 153–154. [Google Scholar] [CrossRef]
- Oishi, Y.; Hiura, T. Bryophytes as bioindicators of the atmospheric environment in urban-forest landscapes. Landsc. Urban Plann. 2017, 167, 348–355. [Google Scholar] [CrossRef]
- González-Miqueo, L.; Elustondo, D.; Lasheras, E.; Santamaría, J. Use of native mosses as biomonitors of heavy metals and nitrogen deposition in the surroundings of two steel works. Chemosphere 2010, 78, 965–971. [Google Scholar] [CrossRef]
- Berisha, S.; Skudnik, M.; Vilhar, U.; Sabovljevic, M.; Zavadlav, S.; Jeran, Z. Trace elements and nitrogen content in naturally growing moss Hypnum cupressiforme in urban and peri-urban forests of the Municipality of Ljubljana (Slovenia). Environ. Sci. Pollut. R 2017, 24, 4517–4527. [Google Scholar] [CrossRef]
- Kosior, G.; Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. Bioindication capacity of metal pollution of native and transplanted Pleurozium schreberi under various levels of pollution. Chemosphere 2010, 81, 321–326. [Google Scholar] [CrossRef]
- Fernandez, J.A.; Aboal, J.R.; Carballeira, A. Use of native and transplanted mosses as complementary techniques for biomonitoring mercury around an industrial facility. Sci. Total Environ. 2000, 256, 151–161. [Google Scholar] [CrossRef]
- Stevenson, C.R.; Hill, M.O. Urban myths exploded: Results of a bryological survey of King’s Lynn (Norfolk, UK). J. Bryol. 2008, 30, 12–22. [Google Scholar] [CrossRef]
- Kürschner, H.; Frey, W. Life strategies in bryophytes—A prime example for the evolution of functional types. Nova Hedwig. 2013, 96, 83–116. [Google Scholar] [CrossRef]
- Torseth, K.; Aas, W.; Breivik, K.; Fjaeraa, A.M.; Fiebig, M.; Hjellbrekke, A.G.; Myhre, C.L.; Solberg, S.; Yttri, K.E. Introduction to the European Monitoring and Evaluation Programme (EMEP) and observed atmospheric composition change during 1972–2009. Atmos. Chem. Phys. 2012, 12, 5447–5481. [Google Scholar] [CrossRef]
- Harmens, H.; Norris, D.A.; Koerber, G.R.; Buse, A.; Steinnes, E.; Ruhling, A. Temporal trends (1990–2000) in the concentration of cadmium, lead and mercury in mosses across Europe. Environ. Pollut. 2008, 151, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Chen, Q.; Liu, C.; Fang, Y.M. Using moss to assess airborne heavy metal pollution in Taizhou, China. Int. J. Environ. Res. Pub. Health 2017, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Wang, D.Y.; He, M.; Zhang, C. Monitoring of atmospheric heavy metal deposition in Chongqing, China-based on moss bag technique. Environ. Monit. Assess. 2009, 148, 1–9. [Google Scholar] [CrossRef]
- Cao, T.; Wang, M.; An, L.; Yu, Y.H.; Lou, Y.X.; Guo, S.L.; Zuo, B.R.; Liu, Y.; Wu, J.M.; Cao, Y.; et al. Air quality for metals and sulfur in Shanghai, China, determined with moss bags. Environ. Pollut. 2009, 157, 1270–1278. [Google Scholar] [CrossRef]
- Krommer, V.; Zechmeister, H.G.; Roder, I.; Scharf, S.; Hanus-Illnar, A. Monitoring atmospheric pollutants in the biosphere reserve Wienerwald by a combined approach of biomonitoring methods and technical measurements. Chemosphere 2007, 67, 1956–1966. [Google Scholar] [CrossRef]
- Gao, Q. Flora Bryophytarum Sinicorum; Science Press: Beijing, China, 1996; Volume 2. [Google Scholar]
- Li, X. Flora Bryophytarum Sinicorum; Science Press: Beijing, China, 2000; Volume 3. [Google Scholar]
- Li, X. Flora Bryophytarum Sinicorum; Science Press: Beijing, China, 2006; Volume 4. [Google Scholar]
- Wu, P.; Jia, Y. Flora Bryophytarum Sinicorum; Science Press: Beijing, China, 2011; Volume 5. [Google Scholar]
- Wu, P. Flora Bryophytarum Sinicorum; Science Press: Beijing, China, 2002; Volume 6. [Google Scholar]
- Hu, R.; Wang, Y. Flora Bryophytarum Sinicorum; Science Press: Beijing, China, 2005; Volume 7. [Google Scholar]
- Wu, P.; Jia, Y. Flora Bryophytarum Sinicorum; Science Press: Beijing, China, 2004; Volume 8. [Google Scholar]
- Institutum Botanicum Kunmingense Academiae Sinicae Edita. Flora Yunnanica; Science Press: Beijing, China, 2000; Volume 17. [Google Scholar]
- Jiang, Y.; Fan, M.; Hu, R.; Zhao, J.; Wu, Y. Mosses are better than leaves of vascular plants in monitoring atmospheric heavy metal pollution in urban areas. Int. J. Environ. Res. Public Health 2018, 15, 1105. [Google Scholar] [CrossRef]
- le Blancs, F.; de Sloover, J. Relation between industrialization and the distribution and growth of epiphytic lichens and mosses in Montreal. Can. J. Bot. 1970, 48, 1485–1496. [Google Scholar] [CrossRef]
- Gao, C.; Cao, T. A preliminary study on indication of bryophytes to air pollution (including acid rain) in southest Chian. Chin. J. Appl. Ecol. 1992, 3, 81–90. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution-control—A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Xu, Z.; Ni, S.; Tuo, X.; Zhang, C. Calculation of heavy metals toxicity coefficient in the evaluation of potential ecological risk index. Environ. Sci. Technol. 2008, 28, 112–115. [Google Scholar]
- Carballeira, A.; Couto, J.A.; Fernandez, J.A. Estimation of background levels of various elements in terrestrial mosses from Galicia (NW Spain). Water Air Soil Pollut. 2002, 133, 235–252. [Google Scholar] [CrossRef]
- Fernández, J.A.; Carballeira, A. A comparison of indigenous mosses and topsoils for use in monitoring atmospheric heavy metal deposition in Galicia (northwest Spain). Environ. Pollut. 2001, 114, 431–441. [Google Scholar] [CrossRef]
- Steinnes, E. A critical-evaluation of the use of naturally growing moss to monitor the deposition of atmospheric metals. Sci. Total Environ. 1995, 160/161, 243–249. [Google Scholar] [CrossRef]
- Bargagli, R.; Monaci, F.; Borghini, F.; Bravi, F.; Agnorelli, C. Mosses and lichens as biomonitors of trace metals. A comparison study on Hypnum cupressiforme and Parmelia caperata in a former mining district in Italy. Environ. Pollut. 2002, 116, 279–287. [Google Scholar] [CrossRef]
- Mao, L.; Li, Y.; Liu, C.; Fang, Y. Predication of potential distribution of Haplocladium microphyllum in China based on MaxEnt model. Chin. J. Ecol. 2017, 36, 54–60. [Google Scholar]
- Yan, Y.; Zhang, Q.; Wang, G.G.; Fang, Y.M. Atmospheric deposition of heavy metals in Wuxi, China: Estimation based on native moss analysis. Environ. Monit. Assess. 2016, 188, 360. [Google Scholar] [CrossRef]
- Melaku, S.; Morris, V.; Raghavan, D.; Hosten, C. Seasonal variation of heavy metals in ambient air and precipitation at a single site in Washington, DC. Environ. Pollut. 2008, 155, 88–98. [Google Scholar] [CrossRef]
- Gong, M.; Wu, L.; Bi, X.Y.; Ren, L.M.; Wang, L.; Ma, Z.D.; Bao, Z.Y.; Li, Z.G. Assessing heavy-metal contamination and sources by GIS-based approach and multivariate analysis of urban-rural topsoils in Wuhan, central China. Environ. Geochem. Health 2010, 32, 59–72. [Google Scholar] [CrossRef]
- Zhang, C.T.; Yang, Y.; Li, W.D.; Zhang, C.R.; Zhang, R.X.; Mei, Y.; Liao, X.S.; Liu, Y.Y. Spatial distribution and ecological risk assessment of trace metals in urban soils in Wuhan, central China. Environ. Monit. Assess. 2015, 187, 556. [Google Scholar] [CrossRef]
- Galuszka, A. The chemistry of soils, rocks and plant bioindicators in three ecosystems of the Holy Cross Mountains, Poland. Environ. Monit. Assess. 2005, 110, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Blagnytė, R.; Paliulis, D. Research into heavy metals pollution of atmosphere applying moss as bioindicator: A literature review. Environ. Res. Eng. Manag. 2010, 4, 26–33. [Google Scholar]
- Harmens, H.; Ilyin, I.; Mills, G.; Aboal, J.R.; Alber, R.; Blum, O.; Coskun, M.; De Temmerman, L.; Fernandez, J.A.; Figueira, R.; et al. Country-specific correlations across Europe between modelled atmospheric cadmium and lead deposition and concentrations in mosses. Environ. Pollut. 2012, 166, 1–9. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, P.; Fang, Y.M. Monitoring airborne heavy metal using mosses in the city of Xuzhou, China. B Environ. Contam. Tox. 2016, 96, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister, H.G.; Hohenwallner, D. A comparison of biomonitoring methods for the estimation of atmospheric pollutants in an industrial town in Austria. Environ. Monit. Assess. 2006, 117, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Boch, S.; Allan, E.; Humbert, J.Y.; Kurtogullari, Y.; Lessard-Therrien, M.; Muller, J.; Prati, D.; Rieder, N.S.; Arlettaz, R.; Fischer, M. Direct and indirect effects of land use on bryophytes in grasslands. Sci. Total Environ. 2018, 644, 60–67. [Google Scholar] [CrossRef]

| No. | Species | Genus | Family | Coverage (%) | Frequency (%) | Important Value |
|---|---|---|---|---|---|---|
| S1 | Haplocladium angustifolium | Haplocladium | Thuidiaceae | 15.56 | 77 | 0.140 |
| S2 | Entodon obtusatus | Entodon | Entodontaceae | 7.75 | 41 | 0.071 |
| S3 | Fabronia curvirostris | Fabronia | Fabroniaceae | 4.45 | 15 | 0.035 |
| S4 | Claopodium aciculums | Claopodium | Thuidiaceae | 3.52 | 21 | 0.034 |
| S5 | Plagiomnium cuspidatum | Plagiomnium | Mniaceae | 4.31 | 13 | 0.033 |
| S6 | Weissia exserta | Weissia | Pottiaceae | 1.25 | 26 | 0.025 |
| S7 | Schwetschkeopsis fabronia | Schwetschkeopsis | Fabroniaceae | 3.10 | 10 | 0.024 |
| S8 | Sematophyllum phoeniceum | Sematophyllum | Sematophyllaceae | 2.11 | 15 | 0.022 |
| S9 | Fissidens adelphinus | Fissidens | Fissidentaceae | 1.82 | 15 | 0.021 |
| S10 | Bryum coronatum | Bryum | Bryaceae | 1.44 | 18 | 0.020 |
| S11 | Bryum dichotomum | Bryum | Bryaceae | 1.59 | 15 | 0.019 |
| S12 | Physcomitrium sphaericum | Physcomitrium | Funariaceae | 1.58 | 15 | 0.019 |
| S13 | Taxiphyllum taxirameum | Taxiphyllum | Hypnaceae | 0.86 | 21 | 0.019 |
| S14 | Bryum argenteum | Bryum | Bryaceae | 0.75 | 21 | 0.018 |
| S15 | Hyophila stenophylla | Hyophila | Pottiaceae | 1.69 | 13 | 0.018 |
| S16 | Barbula subcontorta | Barbula | Pottiaceae | 0.87 | 18 | 0.017 |
| S17 | Weissia microstoma | Weissia | Pottiaceae | 0.85 | 18 | 0.017 |
| S18 | Claopodium rugulosifolium | Claopodium | Thuidiaceae | 1.15 | 15 | 0.017 |
| S19 | Taxiphyllum subarcuatum | Taxiphyllum | Hypnaceae | 1.09 | 15 | 0.017 |
| S20 | Taxiphyllum cusoidifolium | Taxiphyllum | Hypnaceae | 1.68 | 10 | 0.016 |
| S21 | Brachythecium amnicolum | Brachythecium | Brachytheciaceae | 1.62 | 10 | 0.016 |
| S22 | Fissidens bryoides | Fissidens | Fissidentaceae | 0.83 | 15 | 0.015 |
| S23 | Brachythecium plumosum | Brachythecium | Brachytheciaceae | 1.94 | 5 | 0.014 |
| S24 | Venturiella sinensis | Venturiella | Erpodiaceae | 1.46 | 8 | 0.013 |
| S25 | Brotherella nictans | Brotherella | Sematophyllaceae | 1.29 | 8 | 0.012 |
| S26 | Weissia controversa | Weissia | Pottiaceae | 0.62 | 13 | 0.012 |
| S27 | Entodon plicatus | Entodon | Entodontaceae | 1.13 | 8 | 0.012 |
| S28 | Entodon scariosus | Entodon | Entodontaceae | 1.06 | 8 | 0.011 |
| S29 | Brotherella fauriei | Brotherella | Sematophyllaceae | 1.05 | 8 | 0.011 |
| S30 | Oxystegus cuspidatus | Oxystegus | Pottiaceae | 1.62 | 3 | 0.011 |
| S31 | Eurhynchium laxirete | Eurhynchium | Brachytheciaceae | 0.99 | 8 | 0.011 |
| S32 | Fabronia matsumurae | Fabronia | Fabroniaceae | 1.08 | 5 | 0.010 |
| S33 | Frullania parvistipula | Frullania | Frullaniaceae | 1.39 | 3 | 0.010 |
| Material | Metal | As | Cd | Co | Cr | Cu | Mn | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moss | Maximum | 19.5 | 1.68 | 69.3 | 72.5 | 77.7 | 704 | 50.8 | 47.0 | 96.3 | 458.0 |
| Minimum | 1.33 | 0.276 | 1.27 | 5.39 | 9.31 | 71.5 | 5.00 | 8.03 | 10.0 | 48.7 | |
| Mean | 8.21 | 0.776 | 14.4 | 33.8 | 36.7 | 348 | 19.0 | 25.11 | 38.9 | 214 | |
| SD | 4.78 | 0.32 | 16.6 | 16.8 | 17.1 | 178 | 12.0 | 11.6 | 21.7 | 105 | |
| CV (%) | 58.3 | 41.3 | 115 | 49.8 | 46.4 | 51.0 | 63.5 | 46.1 | 55.8 | 48.9 | |
| Soil | Maximum | 34.3 | 0.737 | 22.7 | 144 | 80.5 | 952 | 51.8 | 67.5 | 219 | 170.5 |
| Minimum | 13.3 | 0.11 | 6.47 | 55.5 | 24.9 | 163 | 15.4 | 13.8 | 41.3 | 41.6 | |
| Mean | 21.1 | 0.353 | 15.2 | 83.6 | 38.8 | 693 | 31.8 | 38.1 | 127 | 82.5 | |
| SD | 5.24 | 0.185 | 3.69 | 20.6 | 12.7 | 190 | 8.64 | 16.2 | 45.7 | 34.9 | |
| CV (%) | 24.8 | 52.2 | 24.3 | 24.6 | 32.8 | 27.5 | 27.2 | 42.6 | 35.8 | 42.3 | |
| Difference (P) | 0.000 | 0.000 | 0.016 | 0.000 | 0.293 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | |
| Pearson’s correlation | 0.458 * | 0.416 * | 0.303 | 0.387 | 0.479 * | 0.268 | 0.176 | 0.414 * | 0.512 ** | 0.272 | |
| As | Cd | Co | Cr | Cu | Mn | Ni | Pb | V | Zn | IAP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | 0.29 | ||||||||||
| Co | 0.76 ** | 0.12 | |||||||||
| Cr | 0.72 ** | −0.01 | 0.77 ** | ||||||||
| Cu | 0.60 ** | 0.33 | 0.61 ** | 0.62 ** | |||||||
| Mn | 0.48 * | −0.04 | 0.86 ** | 0.70 ** | 0.49 * | ||||||
| Ni | 0.35 | 0.07 | 0.79 ** | 0.60 ** | 0.44 * | 0.90 ** | |||||
| Pb | 0.48 * | 0.54 * | 0.43 * | 0.27 | 0.52 * | 0.34 | 0.34 | ||||
| V | 0.75 ** | −0.08 | 0.75 ** | 0.89 ** | 0.44 * | 0.68 ** | 0.54 * | 0.24 | |||
| Zn | 0.06 | 0.44 * | 0.09 | 0.08 | 0.44 * | 0.10 | 0.15 | 0.47 * | −0.15 | ||
| IAP | −0.54 * | 0.03 | −0.5 * | −0.58 ** | −0.39 | −0.47 * | −0.28 | −0.27 | −0.59 ** | −0.01 | |
| RI | 0.86 ** | 0.49 | 0.87 ** | 0.71 ** | 0.64 ** | 0.69 ** | 0.65 ** | 0.60 ** | 0.67 ** | 0.25 | −0.43 * |
| Sample Site | RI | Category | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Co | Cr | Cu | Mn | Ni | Pb | V | Zn | |||
| N1 | 95.71 | 164.65 | 8.66 | 4.46 | 13.02 | 1.35 | 11.21 | 12.11 | 3.94 | 8.88 | 324.00 | III |
| N2 | 116.06 | 94.71 | 26.87 | 11.14 | 12.91 | 3.14 | 21.48 | 12.37 | 10.35 | 3.49 | 312.52 | III |
| N3 | 105.52 | 145.64 | 22.80 | 13.77 | 22.62 | 2.88 | 21.58 | 11.39 | 10.85 | 4.31 | 361.34 | III |
| N4 | 72.43 | 140.58 | 39.26 | 9.16 | 15.92 | 2.75 | 19.68 | 9.88 | 8.55 | 3.74 | 321.94 | III |
| N5 | 97.34 | 122.97 | 44.68 | 15.97 | 40.85 | 4.62 | 25.57 | 27.64 | 10.75 | 7.49 | 397.88 | III |
| N6 | 97.52 | 138.31 | 55.02 | 14.08 | 19.53 | 3.35 | 22.31 | 14.72 | 9.08 | 6.95 | 380.87 | III |
| N7 | 181.39 | 192.91 | 99.72 | 20.38 | 31.98 | 5.00 | 37.64 | 23.65 | 15.26 | 6.39 | 614.31 | IV |
| N8 | 136.44 | 144.77 | 90.12 | 16.76 | 34.55 | 4.11 | 33.41 | 14.28 | 14.24 | 7.53 | 496.22 | III |
| N9 | 130.95 | 130.99 | 183.10 | 18.43 | 16.70 | 7.13 | 41.19 | 12.12 | 16.93 | 4.02 | 561.54 | III |
| N10 | 197.26 | 147.21 | 312.77 | 27.11 | 22.80 | 8.43 | 64.44 | 24.58 | 23.39 | 3.89 | 831.87 | IV |
| N11 | 138.23 | 212.27 | 191.49 | 10.90 | 24.54 | 5.32 | 76.41 | 25.33 | 6.40 | 6.68 | 697.55 | IV |
| N12 | 58.58 | 75.17 | 111.48 | 11.76 | 23.28 | 7.29 | 85.29 | 12.03 | 7.88 | 6.79 | 399.55 | III |
| N13 | 97.43 | 94.36 | 146.25 | 26.06 | 30.83 | 8.74 | 102.34 | 10.90 | 16.61 | 11.81 | 545.33 | III |
| N14 | 95.98 | 87.38 | 90.07 | 17.50 | 23.96 | 8.20 | 73.60 | 9.69 | 13.35 | 3.87 | 423.59 | III |
| N15 | 62.47 | 91.22 | 17.70 | 18.01 | 9.81 | 2.66 | 34.92 | 9.77 | 14.62 | 2.80 | 263.98 | II |
| N16 | 39.44 | 118.60 | 24.08 | 8.07 | 12.11 | 4.53 | 36.88 | 12.44 | 11.46 | 2.95 | 270.56 | II |
| N17 | 26.83 | 143.90 | 13.91 | 4.84 | 12.52 | 3.01 | 25.60 | 21.28 | 3.66 | 12.63 | 268.17 | II |
| N18 | 39.50 | 88.43 | 23.05 | 9.18 | 11.73 | 4.09 | 31.63 | 9.44 | 7.28 | 3.23 | 227.57 | II |
| N19 | 39.76 | 118.95 | 17.16 | 7.57 | 9.86 | 2.59 | 26.42 | 14.37 | 4.78 | 5.94 | 247.41 | II |
| N20 | 37.69 | 156.63 | 29.95 | 7.66 | 15.74 | 6.00 | 55.90 | 20.94 | 5.94 | 4.32 | 340.77 | III |
| N21 | 76.28 | 292.33 | 23.07 | 13.01 | 30.07 | 3.71 | 31.56 | 27.88 | 5.46 | 11.04 | 514.39 | III |
| N22 | 28.86 | 57.21 | 12.57 | 12.28 | 15.60 | 2.02 | 17.33 | 5.67 | 3.31 | 4.42 | 159.26 | II |
| N23 | 43.40 | 242.09 | 17.55 | 7.14 | 15.30 | 2.64 | 28.30 | 6.55 | 4.48 | 6.09 | 373.54 | III |
| N24 | 13.48 | 48.14 | 5.73 | 2.01 | 4.89 | 0.89 | 10.09 | 4.76 | 2.43 | 1.34 | 93.77 | I |
| N25 | 47.14 | 132.91 | 19.93 | 9.07 | 11.99 | 3.56 | 21.18 | 18.54 | 5.43 | 7.18 | 276.93 | II |
| Average | 84.52 | 135.39 | 66.96 | 12.80 | 19.63 | 4.35 | 38.95 | 14.74 | 9.62 | 5.86 | 392.83 | III |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhang, X.; Hu, R.; Zhao, J.; Fan, M.; Shaaban, M.; Wu, Y. Urban Atmospheric Environment Quality Assessment by Naturally Growing Bryophytes in Central China. Int. J. Environ. Res. Public Health 2020, 17, 4537. https://doi.org/10.3390/ijerph17124537
Jiang Y, Zhang X, Hu R, Zhao J, Fan M, Shaaban M, Wu Y. Urban Atmospheric Environment Quality Assessment by Naturally Growing Bryophytes in Central China. International Journal of Environmental Research and Public Health. 2020; 17(12):4537. https://doi.org/10.3390/ijerph17124537
Chicago/Turabian StyleJiang, Yanbin, Xifeng Zhang, Ronggui Hu, Jinsong Zhao, Miao Fan, Muhammad Shaaban, and Yupeng Wu. 2020. "Urban Atmospheric Environment Quality Assessment by Naturally Growing Bryophytes in Central China" International Journal of Environmental Research and Public Health 17, no. 12: 4537. https://doi.org/10.3390/ijerph17124537
APA StyleJiang, Y., Zhang, X., Hu, R., Zhao, J., Fan, M., Shaaban, M., & Wu, Y. (2020). Urban Atmospheric Environment Quality Assessment by Naturally Growing Bryophytes in Central China. International Journal of Environmental Research and Public Health, 17(12), 4537. https://doi.org/10.3390/ijerph17124537







