Daily and Seasonal Variation in Light Exposure among the Old Order Amish
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Light and Activity Measures
2.3. Data Analysis
3. Results
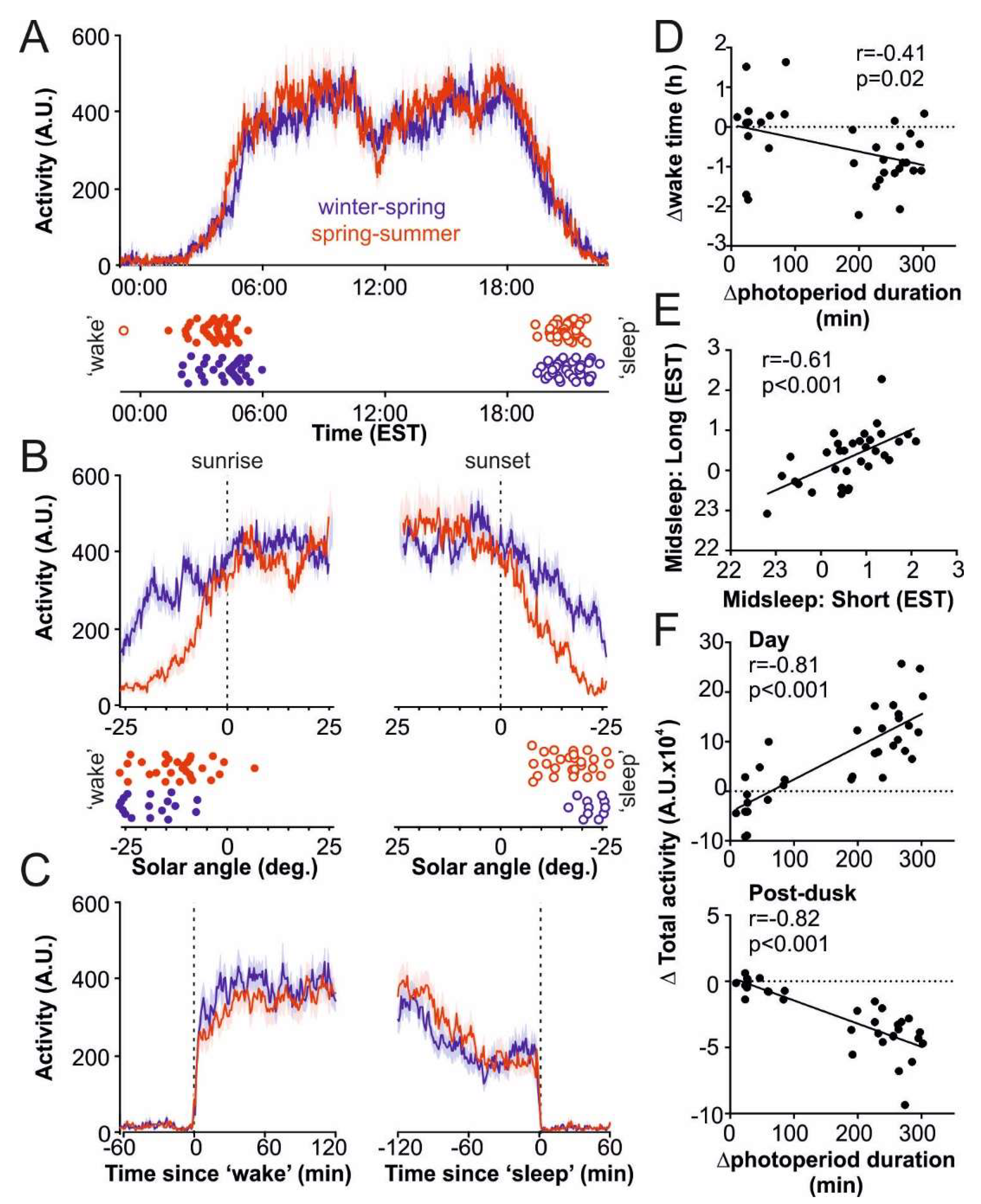
3.1. Daily Activity Patterns among the OOA
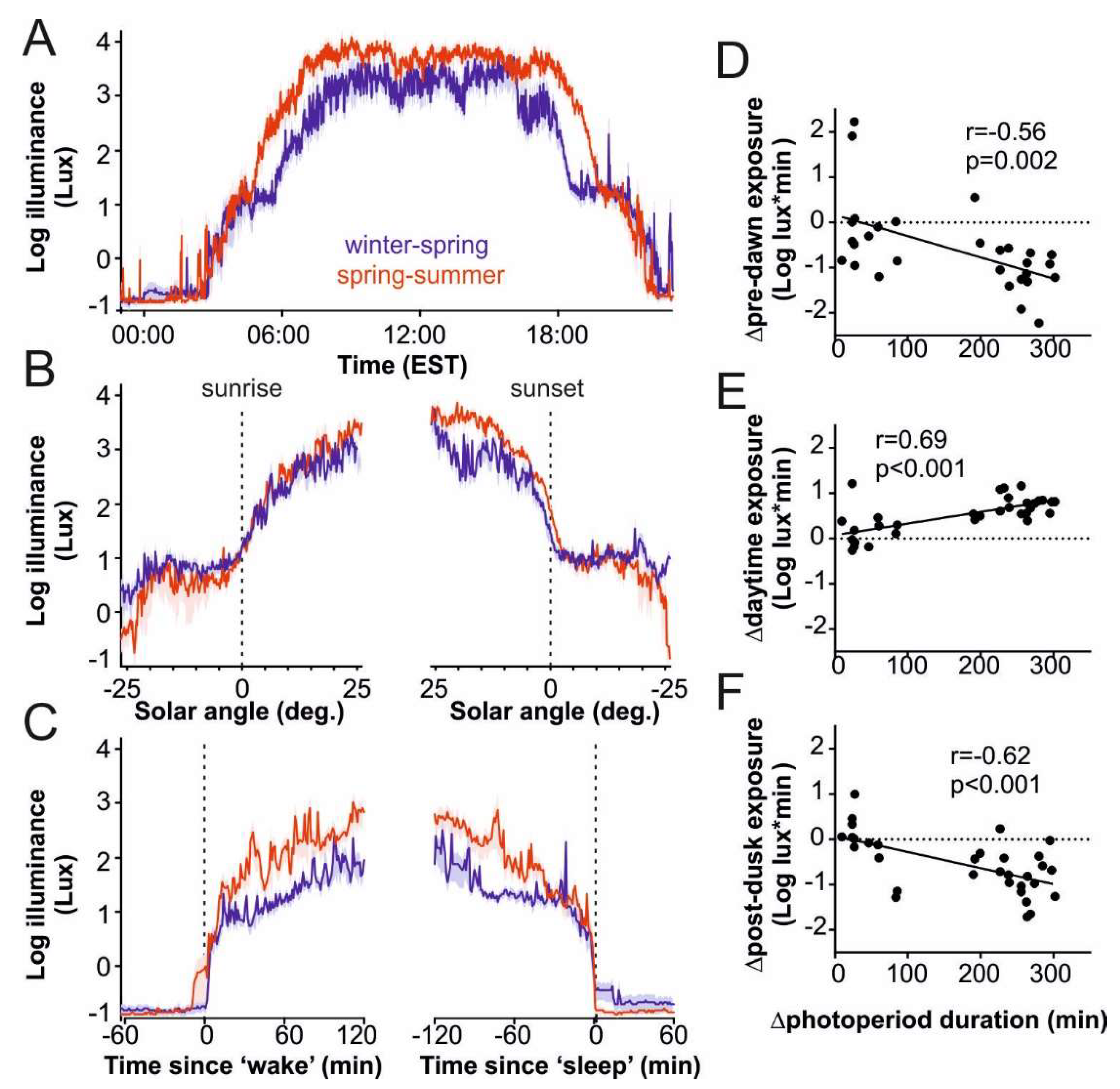
3.2. Seasonal Changes in Light Exposure
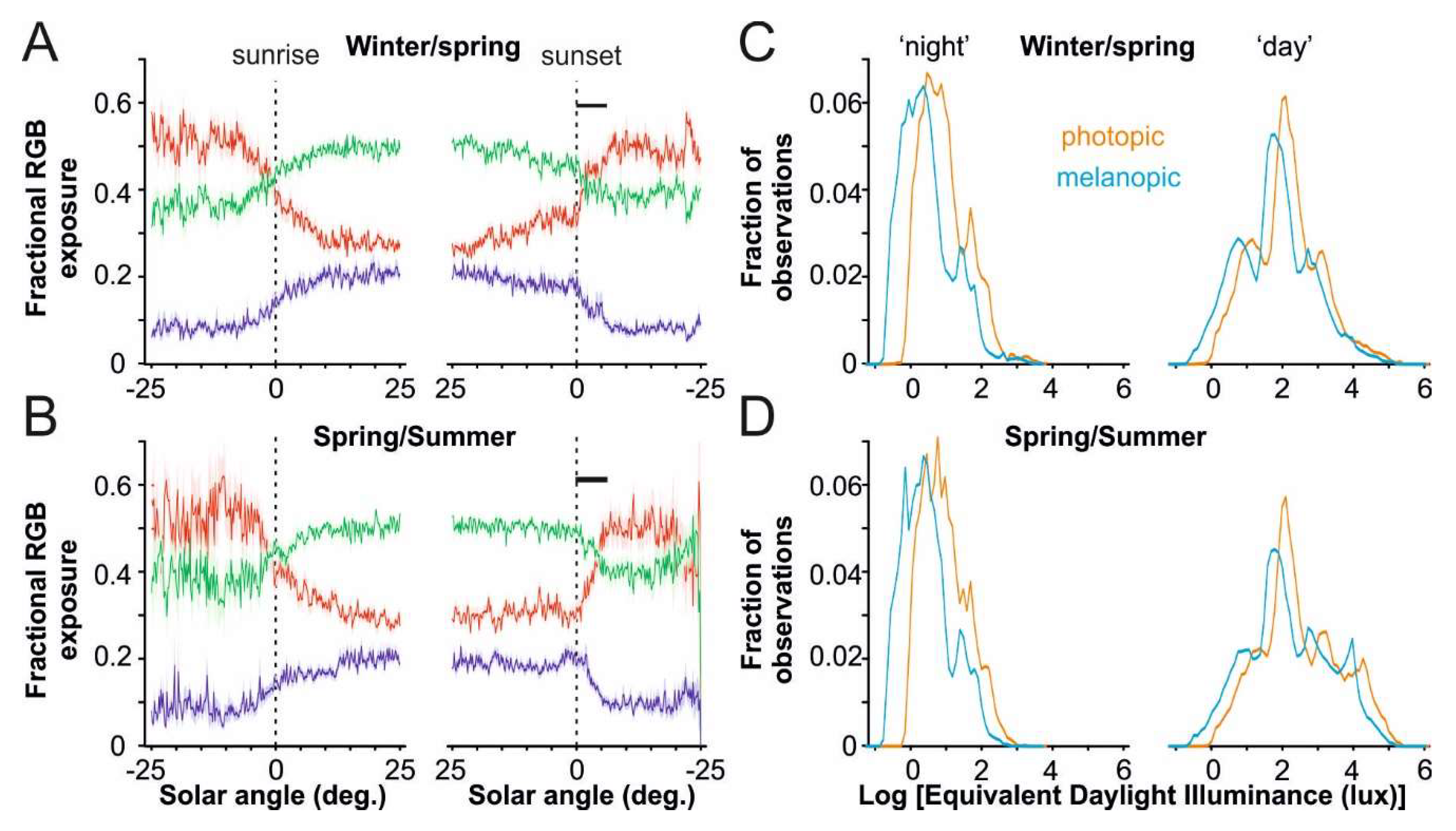
3.3. Spectral Composition of Light
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenthal, N.E.; Sack, D.A.; Gillin, J.C.; Lewy, A.J.; Goodwin, F.K.; Davenport, Y.; Mueller, P.S.; Newsome, D.A.; Wehr, T.A. Seasonal affective disorder: A description of the syndrome and preliminary findings with light therapy. Arch. Gen. Psychiatry 1984, 41, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sohn, C.H.; Lam, R.W. Update on the biology of seasonal affective disorder. CNS Spectr. 2005, 10, 635–646, quiz 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wehr, T.A. Seasonal Affective Disorder: A historical overview. In Seasonal Affective Disorders and Phototherapy; Rosenthal, N.E., Ed.; Guilford Press: New York, NY, USA, 1989; pp. 11–32. [Google Scholar]
- Golden, R.N.; Gaynes, B.N.; Ekstrom, R.D.; Hamer, R.M.; Jacobsen, F.M.; Suppes, T.; Wisner, K.L.; Nemeroff, C.B. The efficacy of light therapy in the treatment of mood disorders: A review and meta-analysis of the evidence. Am. J. Psychiatry 2005, 162, 656–662. [Google Scholar] [CrossRef] [PubMed]
- APA Work Group on Major Depressive Disorder. Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd ed.; American Psychiatric Association: Washington DC, USA, 2010. [Google Scholar]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef]
- Kanikowska, D.; Sato, M.; Witowski, J. Contribution of daily and seasonal biorhythms to obesity in humans. Int J. Biometeorol 2015, 59, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wehr, T.A.; Duncan, W.C., Jr.; Sher, L.; Aeschbach, D.; Schwartz, P.J.; Turner, E.H.; Postolache, T.T.; Rosenthal, N.E. A circadian signal of change of season in patients with seasonal affective disorder. Arch. Gen. Psychiatry 2001, 58, 1108–1114. [Google Scholar] [CrossRef]
- Lewy, A.J.; Lefler, B.J.; Emens, J.S.; Bauer, V.K. The circadian basis of winter depression. Proc. Natl. Acad. Sci. USA 2006, 103, 7414–7419. [Google Scholar] [CrossRef]
- Sack, R.L.; Lewy, A.J.; White, D.M.; Singer, C.M.; Fireman, M.J.; Vandiver, R. Morning vs evening light treatment for winter depression. Evidence that the therapeutic effects of light are mediated by circadian phase shifts. Arch. Gen. Psychiatry 1990, 47, 343–351. [Google Scholar] [CrossRef]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef]
- Paul, S.; Brown, T. Direct effects of the light environment on daily neuroendocrine control. J. Endocrinol. 2019. [Google Scholar] [CrossRef]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.W.; Berson, D.M. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Kankipati, L.; Strang, C.E.; Peterson, B.B.; Dacey, D.; Gamlin, P.D. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J. Comp. Neurol. 2014, 522, 2231–2248. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M. Using light to tell the time of day: Sensory coding in the mammalian circadian visual network. J. Exp. Biol 2016, 219, 1779–1792. [Google Scholar] [CrossRef]
- Pilorz, V.; Tam, S.K.; Hughes, S.; Pothecary, C.A.; Jagannath, A.; Hankins, M.W.; Bannerman, D.M.; Lightman, S.L.; Vyazovskiy, V.V.; Nolan, P.M.; et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016, 14, e1002482. [Google Scholar] [CrossRef] [PubMed]
- Roecklein, K.A.; Rohan, K.J.; Duncan, W.C.; Rollag, M.D.; Rosenthal, N.E.; Lipsky, R.H.; Provencio, I. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J. Affect. Disord 2009, 114, 279–285. [Google Scholar] [CrossRef][Green Version]
- LeGates, T.A.; Altimus, C.M.; Wang, H.; Lee, H.K.; Yang, S.; Zhao, H.; Kirkwood, A.; Weber, E.T.; Hattar, S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012, 491, 594–598. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Gordijn, M.C.; Cajochen, C. Can light make us bright? Effects of light on cognition and sleep. Prog. Brain Res. 2011, 190, 119–133. [Google Scholar] [PubMed]
- Meesters, Y.; Dekker, V.; Schlangen, L.J.; Bos, E.H.; Ruiter, M.J. Low-intensity blue-enriched white light (750 lux) and standard bright light (10,000 lux) are equally effective in treating SAD. A randomized controlled study. BMC Psychiatry 2011, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, M.C.; ’t Mannetje, D.; Meesters, Y. The effects of blue-enriched light treatment compared to standard light treatment in Seasonal Affective Disorder. J. Affect. Disord. 2012, 136, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Meesters, Y.; Winthorst, W.H.; Duijzer, W.B.; Hommes, V. The effects of low-intensity narrow-band blue-light treatment compared to bright white-light treatment in sub-syndromal seasonal affective disorder. BMC Psychiatry 2016, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Knutsson, A.; Kempe, A. Shift work and diabetes--a systematic review. Chronobiol. Int. 2014, 31, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, W.; Wei, F.; Ying, M.; Wei, W.; Xie, X. Night-shift work increases morbidity of breast cancer and all-cause mortality: A meta-analysis of 16 prospective cohort studies. SleepMed 2015, 16, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, A.; Zhu, Y.; Liang, Z.; Wu, J.; Li, S.; Meng, S.; Zheng, X.; Xie, L. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget 2015, 6, 25046–25060. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Yu, H.; Bai, Y.; Zheng, X.; Xie, L. Does night-shift work increase the risk of prostate cancer? a systematic review and meta-analysis. OncoTargets Ther. 2015, 8, 2817–2826. [Google Scholar]
- Woo, J.M.; Postolache, T.T. The impact of work environment on mood disorders and suicide: Evidence and implications. Int. J. Disabil. Hum. Dev. 2008, 7, 185–200. [Google Scholar] [CrossRef]
- Zee, P.C. Circadian Clocks: Implication for Health and Disease. Sleep Med. Clin. 2015, 10, xiii. [Google Scholar] [CrossRef]
- Wyse, C.A.; Biello, S.M.; Gill, J.M. The bright-nights and dim-days of the urban photoperiod: Implications for circadian rhythmicity, metabolism and obesity. Ann. Med. 2014, 46, 253–263. [Google Scholar] [CrossRef]
- Falchi, F.; Cinzano, P.; Elvidge, C.D.; Keith, D.M.; Haim, A. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manag. 2011, 92, 2714–2722. [Google Scholar] [CrossRef]
- Cajochen, C.; Frey, S.; Anders, D.; Spati, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef]
- Cheung, I.N.; Zee, P.C.; Shalman, D.; Malkani, R.G.; Kang, J.; Reid, K.J. Morning and Evening Blue-Enriched Light Exposure Alters Metabolic Function in Normal Weight Adults. PLoS ONE 2016, 11, e0155601. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Kurumatani, N. Bedroom Light Exposure at Night and the Incidence of Depressive Symptoms: A Longitudinal Study of the HEIJO-KYO Cohort. Am. J. Epidemiol. 2018, 187, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Rybnikova, N.A.; Haim, A.; Portnov, B.A. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int. J. Obes. 2016, 40, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Ikada, Y.; Kurumatani, N. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol. Int. 2014, 31, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kraybill, D.B. The Amish of Lancaster County; Stackpole Books: Mechanicsburg, PA, USA, 2008. [Google Scholar]
- Raheja, U.K.; Stephens, S.H.; Mitchell, B.D.; Rohan, K.J.; Vaswani, D.; Balis, T.G.; Nijjar, G.V.; Sleemi, A.; Pollin, T.I.; Ryan, K.; et al. Seasonality of mood and behavior in the Old Order Amish. J. Affect. Disord. 2013, 147, 112–117. [Google Scholar] [CrossRef][Green Version]
- Rosen, L.N.; Targum, S.D.; Terman, M.; Bryant, M.J.; Hoffman, H.; Kasper, S.F.; Hamovit, J.R.; Docherty, J.P.; Welch, B.; Rosenthal, N.E. Prevalence of seasonal affective disorder at four latitudes. Psychiatry Res. 1990, 31, 131–144. [Google Scholar] [CrossRef]
- Hsueh, W.C.; Mitchell, B.D.; Aburomia, R.; Pollin, T.; Sakul, H.; Gelder Ehm, M.; Michelsen, B.K.; Wagner, M.J.; St Jean, P.L.; Knowler, W.C.; et al. Diabetes in the Old Order Amish: Characterization and heritability analysis of the Amish Family Diabetes Study. Diabetes Care 2000, 23, 595–601. [Google Scholar] [CrossRef]
- Zhang, M.; Ryan, K.A.; Wickwire, E.; Postolache, T.T.; Xu, H.; Daue, M.; Snitker, S.; Pollin, T.I.; Shuldiner, A.R.; Mitchell, B.D. Self-Reported Sleep Duration and Pattern in Old Order Amish and Non-Amish Adults. J. Clin. Sleep Med. 2019, 15, 1321–1328. [Google Scholar] [CrossRef]
- Price, L.; Khazova, M.; O’Hagan, J. Performance assessment of commercial circadian personal exposure devices. Light. Res. Technol. 2012, 44, 17–26. [Google Scholar] [CrossRef]
- Reda, I.; Andreas, A. Solar position algorithm for solar radiation application. In National Renewable Energy Laboratory (NREL) Technical Report; NREL/TP-560-34302; National Renewable Energy Laboratory: Golden, CO, USA, 2003. [Google Scholar]
- Al Enezi, J.; Revell, V.; Brown, T.; Wynne, J.; Schlangen, L.; Lucas, R. A "Melanopic" Spectral Efficiency Function Predicts the Sensitivity of Melanopsin Photoreceptors to Polychromatic Lights. J. Biol. Rhythm. 2011, 26, 314–323. [Google Scholar] [CrossRef]
- Brown, T.M.; Allen, A.E.; al-Enezi, J.; Wynne, J.; Schlangen, L.; Hommes, V.; Lucas, R.J. The melanopic sensitivity function accounts for melanopsin-driven responses in mice under diverse lighting conditions. PLoS ONE 2013, 8, e53583. [Google Scholar] [CrossRef]
- Cao, D.; Barrionuevo, P.A. Estimating photoreceptor excitations from spectral outputs of a personal light exposure measurement device. Chronobiol. Int. 2015, 32, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Schlangen, L. CIE Draft International Standard (DIS 026/E: 2018): CIE System for Metrology of Optical Radiation for ipRGC-Influenced Responses to Light; International Commission on Illumination: Vienna, Austria, 2018. [Google Scholar]
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the human circadian clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Thorne, H.C.; Jones, K.H.; Peters, S.P.; Archer, S.N.; Dijk, D.J. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol. Int. 2009, 26, 854–866. [Google Scholar] [CrossRef]
- Rufiange, M.; Beaulieu, C.; Lachapelle, P.; Dumont, M. Circadian light sensitivity and rate of retinal dark adaptation in indoor and outdoor workers. J. Biol. Rhythms 2007, 22, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P., Jr.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Yetish, G.; Kaplan, H.; Gurven, M.; Wood, B.; Pontzer, H.; Manger, P.R.; Wilson, C.; McGregor, R.; Siegel, J.M. Natural sleep and its seasonal variations in three pre-industrial societies. Curr. Biol. 2015, 25, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.S.; Snitker, S.; Wu, S.H.; Mody, A.; Njajou, O.T.; Perlis, M.L.; Gehrman, P.R.; Shuldiner, A.R.; Hsueh, W.C. Habitual sleep/wake patterns in the Old Order Amish: Heritability and association with non-genetic factors. Sleep 2011, 34, 661–669. [Google Scholar] [CrossRef]
- Byrne, E.M.; Raheja, U.K.; Stephens, S.H.; Heath, A.C.; Madden, P.A.; Vaswani, D.; Nijjar, G.V.; Ryan, K.A.; Youssufi, H.; Gehrman, P.R.; et al. Seasonality shows evidence for polygenic architecture and genetic correlation with schizophrenia and bipolar disorder. J. Clin. Psychiatry 2015, 76, 128–134. [Google Scholar] [CrossRef]
- Zhang, L.; Evans, D.S.; Raheja, U.K.; Stephens, S.H.; Stiller, J.W.; Reeves, G.M.; Johnson, M.; Ryan, K.A.; Weizel, N.; Vaswani, D.; et al. Chronotype and seasonality: Morningness is associated with lower seasonal mood and behavior changes in the Old Order Amish. J. Affect. Disord. 2015, 174, 209–214. [Google Scholar] [CrossRef]
- Kochunov, P.; Fu, M.; Nugent, K.; Wright, S.N.; Du, X.; Muellerklein, F.; Morrissey, M.; Eskandar, G.; Shukla, D.K.; Jahanshad, N.; et al. Heritability of complex white matter diffusion traits assessed in a population isolate. Hum. Brain Mapp. 2016, 37, 525–535. [Google Scholar] [CrossRef][Green Version]
- Allebrandt, K.V.; Teder-Laving, M.; Kantermann, T.; Peters, A.; Campbell, H.; Rudan, I.; Wilson, J.F.; Metspalu, A.; Roenneberg, T. Chronotype and sleep duration: The influence of season of assessment. Chronobiol. Int. 2014, 31, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Santhi, N.; Groeger, J.A.; Archer, S.N.; Gimenez, M.; Schlangen, L.J.; Dijk, D.J. Morning sleep inertia in alertness and performance: Effect of cognitive domain and white light conditions. PLoS ONE 2013, 8, e79688. [Google Scholar] [CrossRef] [PubMed]
- Santhi, N.; Thorne, H.C.; van der Veen, D.R.; Johnsen, S.; Mills, S.L.; Hommes, V.; Schlangen, L.J.; Archer, S.N.; Dijk, D.J. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res. 2012, 53, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Brainard, G.C.; Hanifin, J.P.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [CrossRef]
- Thapan, K.; Arendt, J.; Skene, D.J. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 2001, 535, 261–267. [Google Scholar] [CrossRef]
- Gooley, J.J.; Rajaratnam, S.M.; Brainard, G.C.; Kronauer, R.E.; Czeisler, C.A.; Lockley, S.W. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci. Transl. Med. 2010, 2, 31ra33. [Google Scholar] [CrossRef]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Lang, D.; Götz, T.; Krebs, J.; Cajochen, C. Acute exposure to evening blue-enriched light impacts on human sleep. J. Sleep Res. 2013, 22, 573–580. [Google Scholar] [CrossRef]
- Kantermann, T.; Juda, M.; Merrow, M.; Roenneberg, T. The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr. Biol. 2007, 17, 1996–2000. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Okamoto, N.; Tomioka, K.; Nezu, S.; Ikada, Y.; Kurumatani, N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: A cross-sectional analysis of the HEIJO-KYO study. J. Clin. Endocrinol. Metab. 2013, 98, 337–344. [Google Scholar] [CrossRef]
- Roecklein, K.A.; Wong, P.M.; Miller, M.A.; Donofry, S.D.; Kamarck, M.L.; Brainard, G.C. Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neurosci. Biobehav. Rev. 2013, 37, 229–239. [Google Scholar] [CrossRef] [PubMed]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.B.; Jewett, M.E.; Cajochen, C.; Czeisler, C.A. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003, 549, 945–952. [Google Scholar] [CrossRef]
- Bordyugov, G.; Abraham, U.; Granada, A.; Rose, P.; Imkeller, K.; Kramer, A.; Herzel, H. Tuning the phase of circadian entrainment. J. R. Soc. Interface 2015, 12, 20150282. [Google Scholar] [CrossRef]
- Murray, G.; Allen, N.B.; Trinder, J. Seasonality and circadian phase delay: Prospective evidence that winter lowering of mood is associated with a shift towards Eveningness. J. Affect. Disord. 2003, 76, 15–22. [Google Scholar] [CrossRef]
- Natale, V.; Adan, A.; Scapellato, P. Are seasonality of mood and eveningness closely associated? Psychiatry Res. 2005, 136, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, L.; Fabbri, M.; Martoni, M.; Natale, V. Circadian type and mood seasonality in adolescents. Psychiatry Clin. Neurosci. 2012, 66, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.D.; Lee, W.J.; Tolea, M.I.; Shields, K.; Ashktorab, Z.; Magder, L.S.; Ryan, K.A.; Pollin, T.I.; McArdle, P.F.; Shuldiner, A.R.; et al. Living the good life? Mortality and hospital utilization patterns in the Old Order Amish. PLoS ONE 2012, 7, e51560. [Google Scholar] [CrossRef] [PubMed]
| Total Activity (A.U.) | Average Activity (A.U.) | |||||||
|---|---|---|---|---|---|---|---|---|
| Winter–Spring | Spring–Summer | Correlation with Photoperiod | Winter–Spring | Spring–Summer | Correlation with Photoperiod | |||
| Epoch | Mean ± SD | Mean ± SD | r | p | Mean ± SD | Mean ± SD | r | p |
| 24 h total | 398,944 ± 93,574 | 411,465 ± 88,517 | 0.46 | 0.007 | ||||
| Pre-dawn | 37,192 ± 25,091 | 15,033 ± 16,434 | −0.44 | 0.011 | 345 ± 110 | 221 ± 135 | −0.10 | 0.605 |
| Dawn | 10,316 ± 4171 | 9538 ± 6306 | −0.07 | 0.718 | 353 ± 140 | 298 ± 195 | −0.14 | 0.424 |
| Day | 284,043 ± 66,078 | 353,081 ± 89,889 | 0.81 | <0.001 | 417 ± 99 | 413 ± 93 | 0.38 | 0.03 |
| Dusk | 11,869 ± 5587 | 10,543 ± 5338 | −0.02 | 0.925 | 407 ± 193 | 326 ± 157 | −0.11 | 0.531 |
| Post-dusk | 41,965 ± 25,289 | 15,407 ± 10,494 | −0.82 | <0.001 | 256 ± 87 | 187 ± 85 | −0.32 | 0.066 |
| 2 h post-wake onset | 43,598 ± 12,840 | 38,358 ± 16,657 | 0.39 | 0.03 | ||||
| 2 h pre-sleep onset | 28,039 ± 9874 | 30,621 ± 11,035 | 0.33 | 0.06 | ||||
| Total Exposure (Log lux*min) | Average Exposure (Log lux) | |||||||
|---|---|---|---|---|---|---|---|---|
| Winter–Spring | Spring–Summer | Correlation with Photoperiod | Winter–Spring | Spring–Summer | Correlation with Photoperiod | |||
| Epoch | Mean ± SD | Mean ± SD | r | p | Mean ± SD | Mean ± SD | r | p |
| 24 h total | 6.01 ± 0.29 | 6.53 ± 0.27 | 0.72 | <0.001 | ||||
| Pre-dawn | 2.93 ± 0.66 | 2.32 ± 0.87 | −0.56 | 0.002 | 1.03 ± 0.5 | 0.63 ± 0.65 | −0.44 | 0.017 |
| Dawn | 2.71 ± 0.38 | 2.28 ± 1.04 | −0.18 | 0.313 | 1.24 ± 0.38 | 0.77 ± 1.05 | −0.12 | 0.266 |
| Day | 6.02 ± 0.3 | 6.53 ± 0.26 | 0.69 | <0.001 | 3.19 ± 0.28 | 3.60 ± 0.25 | 0.59 | <0.001 |
| Dusk | 2.72 ± 0.4 | 2.97 ± 0.38 | 0.16 | 0.372 | 1.26 ± 0.4 | 1.47 ± 0.39 | 0.11 | 0.531 |
| Post-dusk | 3.46 ± 0.48 | 2.93 ± 0.72 | −0.62 | <0.001 | 1.31 ± 0.37 | 1.15 ± 0.48 | −0.37 | 0.035 |
| 2 h post-wake onset | 3.44 ± 0.53 | 3.92 ± 0.73 | 0.42 | 0.016 | ||||
| 2 h pre-sleep onset | 3.52 ± 0.45 | 3.87 ± 0.74 | 0.17 | 0.356 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.E.; Amritwar, A.; Hong, L.E.; Mohyuddin, I.; Brown, T.; Postolache, T.T. Daily and Seasonal Variation in Light Exposure among the Old Order Amish. Int. J. Environ. Res. Public Health 2020, 17, 4460. https://doi.org/10.3390/ijerph17124460
Lee EE, Amritwar A, Hong LE, Mohyuddin I, Brown T, Postolache TT. Daily and Seasonal Variation in Light Exposure among the Old Order Amish. International Journal of Environmental Research and Public Health. 2020; 17(12):4460. https://doi.org/10.3390/ijerph17124460
Chicago/Turabian StyleLee, Ellen E., Ameya Amritwar, L. Elliot Hong, Iqra Mohyuddin, Timothy Brown, and Teodor T. Postolache. 2020. "Daily and Seasonal Variation in Light Exposure among the Old Order Amish" International Journal of Environmental Research and Public Health 17, no. 12: 4460. https://doi.org/10.3390/ijerph17124460
APA StyleLee, E. E., Amritwar, A., Hong, L. E., Mohyuddin, I., Brown, T., & Postolache, T. T. (2020). Daily and Seasonal Variation in Light Exposure among the Old Order Amish. International Journal of Environmental Research and Public Health, 17(12), 4460. https://doi.org/10.3390/ijerph17124460





