The Role of Bacterial Colonization of the Suture Thread in Early Identification and Targeted Antibiotic Treatment of Surgical Site Infections: A Prospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Variables and Definitions
2.3. Statistical Analysis
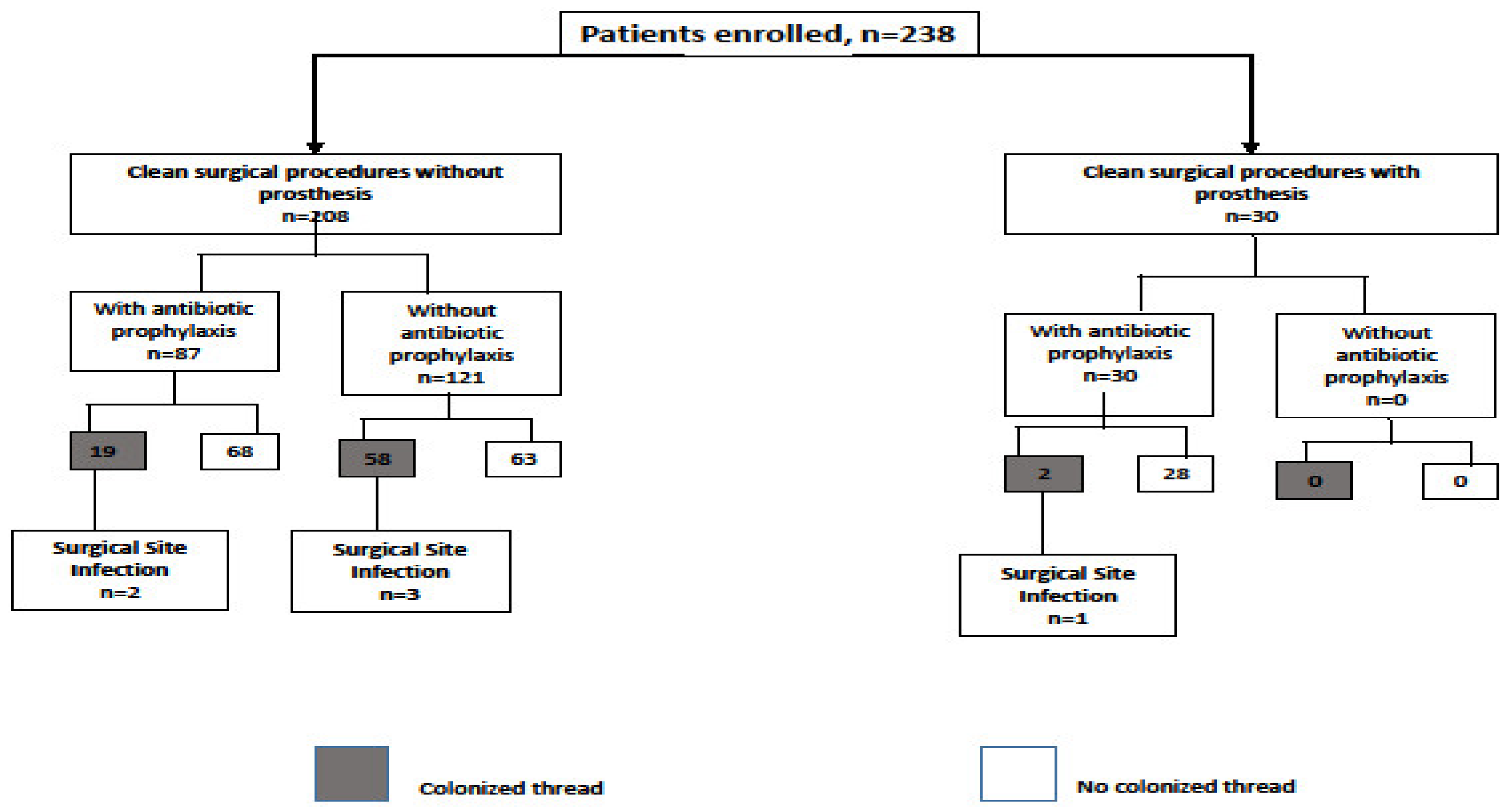
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- European Centre for Disease Prevention and Control—ECDC. Healthcare-associated infections: Surgical site infections. In Annual Epidemiological Report for 2017; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Walczak, S.; Davila, M.; Velanovich, V. Prophylactic antibiotic bundle compliance and surgical site infections: An artificial neural network analysis. Patient Saf. Surg. 2019, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Surgical Site Infection (SSI) Event; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017; pp. 1–31. [Google Scholar]
- Hubner, M.; Diana, M.; Zanetti, G.; Eisenring, M.C.; Demartines, N.; Troillet, N. Surgical site infections in colon surgery: The patient, the hospital and the surgeon. Arch. Surg. 2011, 146, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, B.; D’Agostino, F.; Signoriello, G.; Guida, A. Sorveglianza Delle Infezioni Del sito Chirurgico in Campania. ISBN 978-88-31204-06-4. Available online: http://www.regione.campania.it/assets/documents/rapporto-infezioni-sito-chirurgico-2017-9788831204064.pdf (accessed on 17 June 2020).
- Borchardt, R.A.; Tzizik, D. Update on surgical site infections: The new CDC guidelines. JAAPA 2018, 31, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Li, J.; Kong, Q.; Wang, C.; Ye, N.; Xia, G. Risk factors for surgical site infection in a teaching hospital: A prospective study of 1138 patients. Patient Prefer. Adherence 2015, 9, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Malone, D.L.; Genuit, T.; Tracy, K.M.; Gannon, C.; Napolitano, L.M. Surgical site Infections: Reanalysis of Risk Factors. J. Surg. Res 2002, 103, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm 2013, 70, 195–283. [Google Scholar] [CrossRef] [PubMed]
- Iovino, F.; Auriemma, P.P.; Dani, L.; Donnarumma, G.; Barbarisi, A.; Mallardo, V.; Calò, F.; Coppola, N. Suture thread check test for detection of surgical site contamination: A prospective study. J. Surg. Res. 2017, 220, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sign Guidelines. Antibiotic prophilaxys in surgery. In Healthcare Improvement Scotland; Updated April 2014; Scottish Intercollegiate Guidelines Network: Edinburgh, UK, 2008. [Google Scholar]
- European Centre for Disease Prevention and Control. Systematic Review and Evidence-Based Guidance on Perioperative Antibiotic Prophylaxis; ECDC: Stockholm, Sweden, 2013. [Google Scholar]
- Faulkner, B.C.; Gear, A.J.; Hellewell, T.B.; Mazzarese, P.M.; Watkins, F.H.; Edlich, R.F. Biomechanical performance of a Braided Adsorbable suture. J. Long Term Eff. Med. Implant. 1996, 6, 169–179. [Google Scholar]
- Drake, D.B.; Rodeheaver, P.F.; Edlich, R.F.; Rodeheaver, G.T. Experimental Sutudies in Swine for measurement of suture extrusion. J. Long Term Eff. Med. Implant. 2004, 14, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.E.; Jacobson, M.J.; Cofield, R.H.; Sperling, J.W.; Sanchez-Sotelo, J.; Osmon, D.R.; McDowell, A.; Patrick, S.; Steckelberg, J.M.; Mandrekar, J.N.; et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 2009, 47, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70 (Suppl. S2), 3–10. [Google Scholar] [CrossRef]
- Mawalla, B.; Mshana, S.E.; Chalya, P.L.; Imirzalioglu, C.; Mahalu, W. Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surg. 2011, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Henry-Stanley, M.J.; Hess, D.J.; Barnes, A.M.; Dunny, G.M.; Wells, C.L. Bacterial contamination of surgical suture resembles a biofilm. Surg. Infect. (Larchmt.) 2010, 11, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Classen, D.C.; Evans, R.S.; Pestotnik, S.L.; Horn, S.D.; Menlove, R.L.; Burke, J.P. The timing of prophylactic administration of antibiotics and the risk of surgical- wound infection. N. Engl. J. Med. 1992, 326, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Borade, S.V.; Syed, O. Single dose antibiotic prophylaxis for prevention of surgical site infection in elective surgery. Int. Surg. J. 2018, 5, 27–33. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- Onorato, L.; Macera, M.; Calò, F.; Monari, C.; Russo, F.; Iovene, M.; Signoriello, G.; Annibale, R.; Pace, M.C.; Aurilio, C.; et al. The effect of an Antimicrobial Stewardship Programme in two Intensive Care Units of a teaching hospital: An Interrupted Time Series Analysis. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, P.J.; Rohailla, S.; Taggart, L.R.; Lightfoot, D.; Havey, T.; Daneman, N.; Lowe, C.; Muller, M.P. Antimicrobial Stewardship and Intensive Care Unit Mortality: A Systematic Review. Clin. Infect. Dis. 2019, 68, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, V.A.; van Werkhoven, C.H.; Baño, J.R.; Bielicki, J.; Harbarth, S.; Hulscher, M.; Huttner, B.; Islam, J.; Little, P.; Pulcini, C.; et al. Optimizing design of research to evaluate antibiotic stewardship interventions: Consensus recommendations of a multinational working group. Clin. Microbiol. Infect. 2020, 26, 41–50. [Google Scholar] [CrossRef] [PubMed]
| N° of Patients | 238 |
| Age, median (range) | 58 (19–88) |
| Males, n (%) | 112 (47.1) |
| Type of procedure, n (%) | |
| 208 (87.4) 30 (12.6) |
| ASA score, median (range) | 2 (1–3) |
| BMI, median (range) | 26 (24–29) |
| Patients with antibiotic prophylaxis, n (%) | 117 (49.2) |
| Patients with cancer, n (%) | 174 (73.1) |
| Characteristics | Thread Colonization N = 79 (33.2%) | No Thread Colonization N = 159 (66.8%) |
|---|---|---|
| Age median, (range) | 57 (21–83) | 58 (19–88) |
| Males, n (%) | 31 (39.2) | 81 (50.9) |
| Type of procedure, n (%) | ||
| 77 (97.5) 2 (2.5) | 131 (82.4) 28 (17.6) |
| ASA, median | 2 | 2 |
| BMI, median (range) | 26 (24–28) | 25 (24–29) |
| Patients with antibiotic prophylaxis, n (%) | 21 (26.6) | 96 (60.4) |
| Patients with cancer, n (%) | 62 (78.5) | 112 (70.4) |
| SSI, n (%) | 6 (7.6) | 0 |
| Patients with SSI | N1 | N2 | N3 | N4 | N5 | N6 |
|---|---|---|---|---|---|---|
| Age | 71 | 57 | 72 | 58 | 63 | 45 |
| Sex | F | F | M | M | M | M |
| Operation | Clean (quadrantectomy with lymph node dissection) | Clean (mastectomy with lymph node dissection) | Clean with prosthesis (inguinal hernia repair) | Clean (cutaneous melanoma radicalization) | Clean (sentinel lymph node biopsy) | Clean (sentinel lymph node biopsy) |
| Prosthesis | No | No | Yes | No | No | No |
| Cancer | Yes | Yes | No | Yes | Yes | Yes |
| ASA | 2 | 2 | 1 | 2 | 3 | 2 |
| Antibiotic prophylaxis | No | No | Yes | Yes | Yes | No |
| Duration of surgery | 75 * | <75 * | 50 * | 50 * | <75 * | <75 * |
| Antibiotic therapy after procedure | No | No | No | No | No | No |
| Skin swab | Negative | Negative | Negative | Negative | Negative | Negative |
| Bacteria identified on the colonized thread | Staphylococcus epidermidis/ Enterococcus faecalis | Kocuria rosea/ Staphylococcus hominis | Enterococcus faecalis | Staphylococcus aureus | Staphylococcus aureus | Staphylococcus lugdunensis |
| Bacteria responsible for SSI | Enterococcus faecalis | Kocuria rosea | Enterococcus faecalis | Staphylococcus aureus | Staphylococcus aureus | Staphylococcus lugdunensis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iovino, F.; Calò, F.; Orabona, C.; Pizza, A.; Fisone, F.; Caputo, P.; Fusco, A.; Macera, M.; Coppola, N. The Role of Bacterial Colonization of the Suture Thread in Early Identification and Targeted Antibiotic Treatment of Surgical Site Infections: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 4416. https://doi.org/10.3390/ijerph17124416
Iovino F, Calò F, Orabona C, Pizza A, Fisone F, Caputo P, Fusco A, Macera M, Coppola N. The Role of Bacterial Colonization of the Suture Thread in Early Identification and Targeted Antibiotic Treatment of Surgical Site Infections: A Prospective Cohort Study. International Journal of Environmental Research and Public Health. 2020; 17(12):4416. https://doi.org/10.3390/ijerph17124416
Chicago/Turabian StyleIovino, Francesco, Federica Calò, Consiglia Orabona, Alessandra Pizza, Francesca Fisone, Pina Caputo, Alessandra Fusco, Margherita Macera, and Nicola Coppola. 2020. "The Role of Bacterial Colonization of the Suture Thread in Early Identification and Targeted Antibiotic Treatment of Surgical Site Infections: A Prospective Cohort Study" International Journal of Environmental Research and Public Health 17, no. 12: 4416. https://doi.org/10.3390/ijerph17124416
APA StyleIovino, F., Calò, F., Orabona, C., Pizza, A., Fisone, F., Caputo, P., Fusco, A., Macera, M., & Coppola, N. (2020). The Role of Bacterial Colonization of the Suture Thread in Early Identification and Targeted Antibiotic Treatment of Surgical Site Infections: A Prospective Cohort Study. International Journal of Environmental Research and Public Health, 17(12), 4416. https://doi.org/10.3390/ijerph17124416








