Dental Anomalies in Rare, Genetic Ciliopathic Disorder—A Case Report and Review of Literature
Abstract
1. Introduction
2. Material and Methods
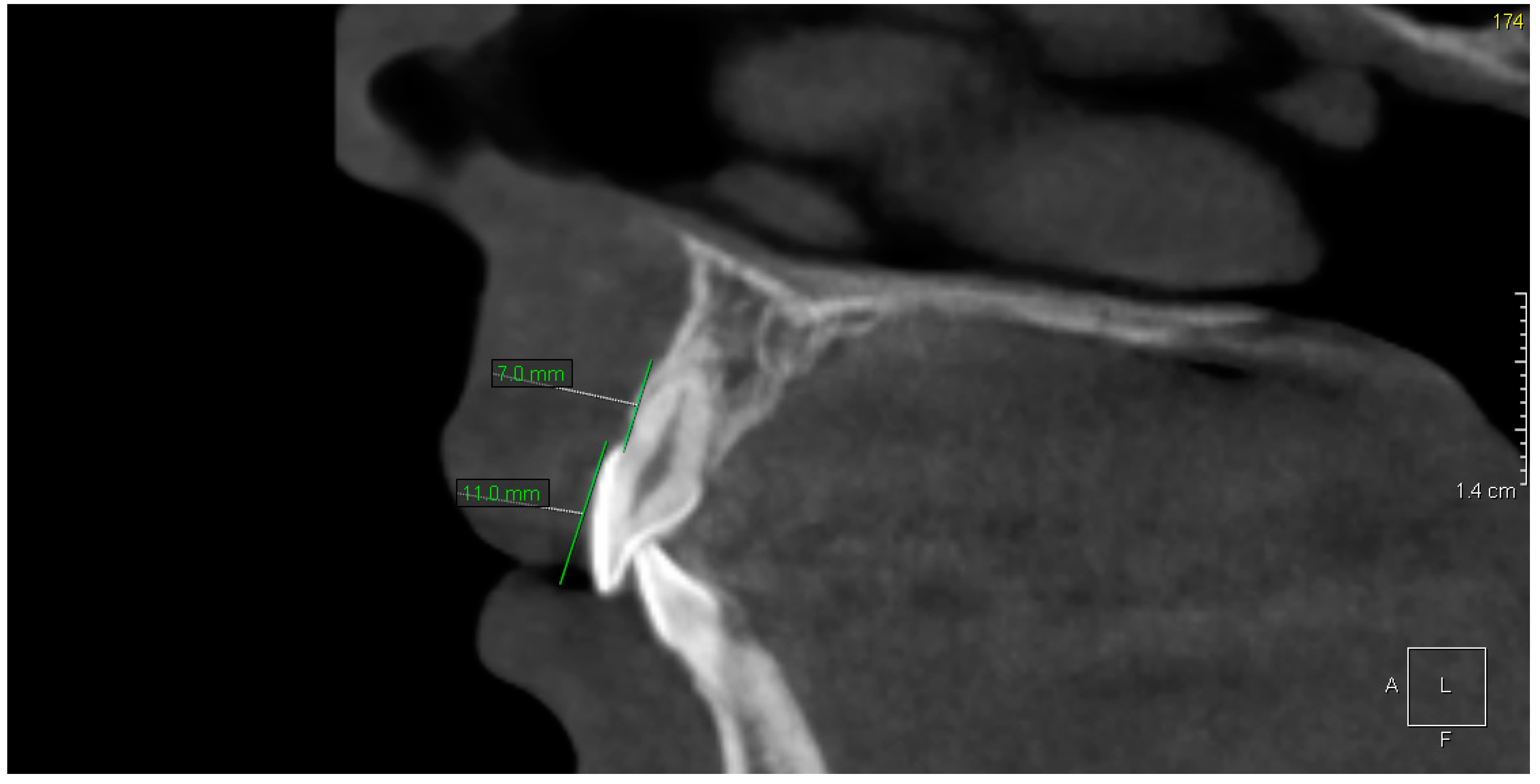
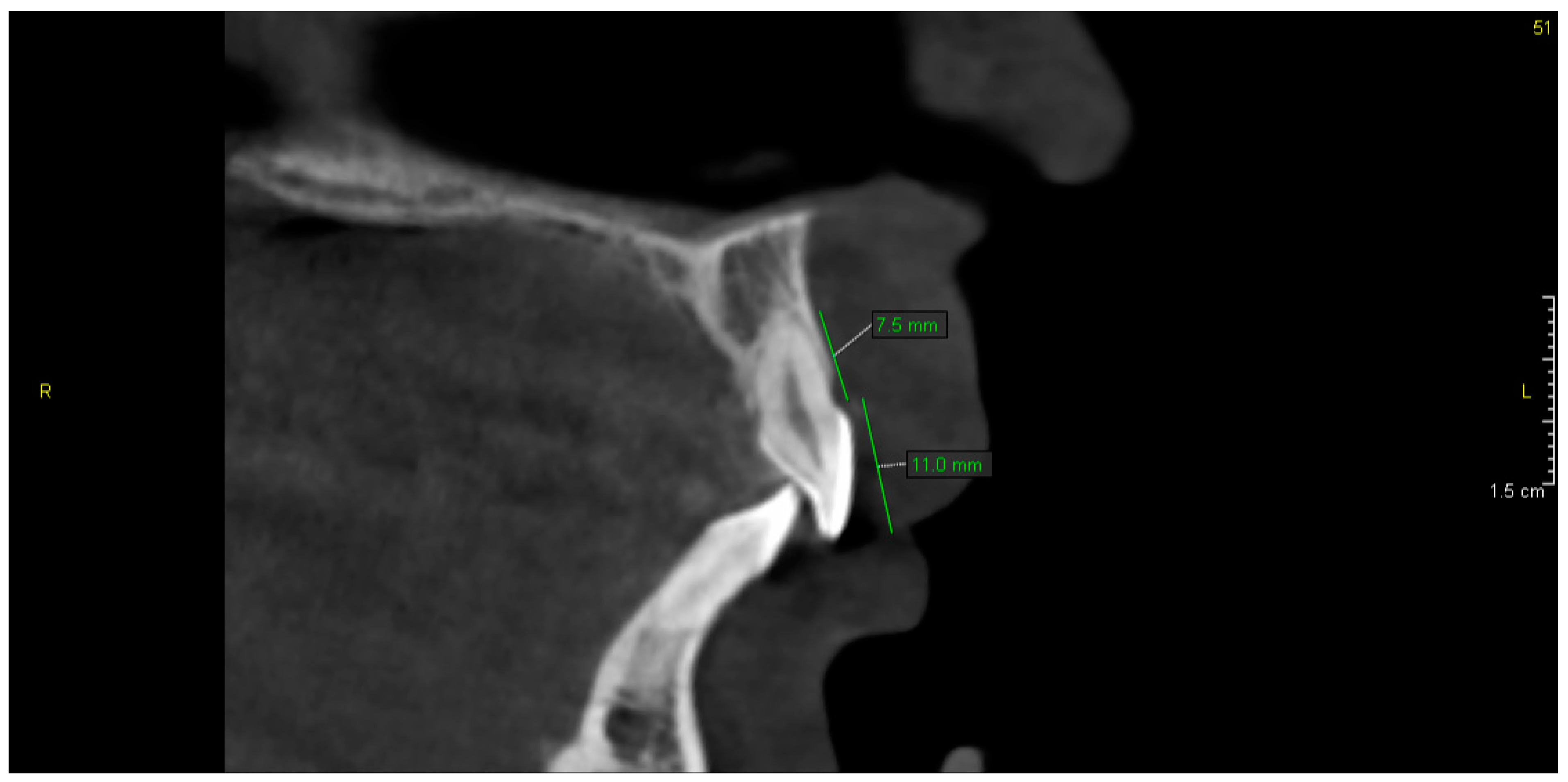
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Damseh, N.; Quercia, N.; Rumman, N.; Dell, S.D.; Kim, R.H. Primary ciliary dyskinesia: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kuehni, C.E.; Frischer, T.; Strippoli, M.-P.F.; Maurer, E.; Bush, A.; Nielsen, K.G.; Escribano, A.; Lucas, J.S.A.; Yiallouros, P.; Omran, H.; et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur. Respir. J. 2010, 36, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Davis, S.D.; Polineni, D.; Manion, M.; Rosenfeld, M.; Dell, S.D.; Chilvers, M.A.; Ferkol, T.W.; Zariwala, M.A.; Sagel, S.D.; et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 197, e24–e39. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, E.D.; Ararat, E. Oral and dental findings in Bardet–Biedl syndrome: A case report. Niger. J. Clin. Pract. 2019, 22, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Panny, A.; Glurich, I.; Haws, R.M.; Acharya, A. Oral and Craniofacial Anomalies of Bardet-Biedl Syndrome: Dental Management in the Context of a Rare Disease. J. Dent. Res. 2017, 96, 1361–1369. [Google Scholar] [CrossRef]
- Miklaszewska, M.; Zachwieja, K.; Herman-Sucharska, I.; Drożdż, D.; Fijak-Moskal, J.; Gergont, A.; Kowalska-Duplaga, K.; Cieszkowska, M.; Pacia-Mędrek, B.; Pietrzyk, J.A. Familial case of oral-facial-digital syndrome type 1 (OFD 1). Przegl. Lek. 2014, 71, 110–114. (In Polish) [Google Scholar]
- Kalaskar, R.; Kalaskar, A.R. Oral manifestations of Ellis-van Creveld syndrome. Contemp. Clin. Dent. 2012, 3, 55–59. [Google Scholar] [CrossRef]
- Shaik, S.; Raviraj, J.; Dirasantchu, S.; Venkata, S.S. Ellis–van Creveld syndrome with unusual oral and dental findings: A rare clinical entity. Dent. Res. J. 2016, 13, 193–197. [Google Scholar] [CrossRef]
- Goswami, S. Weyers acrofacial dysostosis (Curry-Hall Syndrome): Report of a rare case. Arch. Med. Health Sci. 2018, 6, 143–146. [Google Scholar] [CrossRef]
- Gurjar, V.; Gurjar, M.; Pattanshetti, C.; Sankeshwari, B. Lingual Frenectomy in Joubert Syndrome. J. Contemp. Dent. Pract. 2017, 18, 728–731. [Google Scholar] [CrossRef]
- Walczak-Sztulpa, J.; Eggenschwiler, J.; Osborn, D.; Brown, D.A.; Emma, F.; Klingenberg, C.; Hennekam, R.C.; Torre, J.; Garshasbi, M.; Tzschach, A.; et al. Cranioectodermal Dysplasia, Sensenbrenner Syndrome, Is a Ciliopathy Caused by Mutations in the IFT122 Gene. Am. J. Hum. Genet. 2010, 11, 949–956. [Google Scholar] [CrossRef]
- Hampl, M.; Cela, P.; Szabo-Rogers, H.L.; Kunova Bosakova, M.; Dosedelova, H.; Krejci, P.; Buchtova, M. Role of Primary Cilia in Odontogenesis. J. Dent. Res. 2017, 96, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Merrett, S.J.; Durning, P. Kartagener’s syndrome: Unusual dental morphology. Int. J. Paediatr. Dent. 2005, 15, 220–223. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral health surveys: Basic methods, 4th ed.; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Clarkson, J.; O’Mullane, D. A modified DDE Index for use in epidemiological studies of enamel defects. J. Dent. Res. 1989, 68, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Bei, M. Molecular genetics of tooth development. Curr. Opin. Genet. Dev. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Sakano, M.; Otsu, K.; Fujiwara, N.; Fukumoto, S.; Yamada, A.; Harada, H. Cell dynamics in cervical loop epithelium during transition from crown to root: Implications for Hertwig’s epithelial root sheath formation. J. Periodonal Res. 2013, 48, 262–267. [Google Scholar] [CrossRef]
- Bae, C.H.; Chu, J.Y.; Cho, E.S. New population of odontoblasts responsible for tooth root formation. Gene Expr. Patterns 2013, 13, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Fleischmannova, J.; Matalova, E.; Tucker, A.S.; Sharpe, P.T. Mouse models of tooth abnormalities. Eur. J. Oral Sci. 2008, 116, 1–10. [Google Scholar] [CrossRef]
- Thesleff, I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 2003, 116, 1647–1648. [Google Scholar] [CrossRef]
- Gholkar, A.A.; Senese, S.; Lo, Y.C.; Capri, J.; Deardorff, W.J.; Dharmarajan, H.; Contreras, E.; Hodara, E.; Whitelegge, J.P.; Jackson, P.K.; et al. Tctex1d2 associates with short-rib polydactyly syndrome proteins and is required for ciliogenesis. Cell Cycle 2015, 14, 1116–1125. [Google Scholar] [CrossRef]
- Fliegauf, M.; Benzing, T.; Omran, H. When cilia go bad: Cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007, 8, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Haycraft, C.J.; Serra, R. Cilia involvement in patterning and maintenance of the skeleton. Curr. Top Dev. Biol. 2008, 85, 303–332. [Google Scholar] [CrossRef] [PubMed]
- Thivichon-Prince, B.; Couble, M.L.; Giamarchi, A.; Delmas, P.; Franco, B.; Romio, L.; Struys, T.; Lambrichts, I.; Ressnikoff, D.; Magloire, H.; et al. Primary cilia of odontoblasts: Possible role in molar morphogenesis. J. Dent. Res. 2009, 88, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Magloire, H.; Couble, M.L.; Thivichon-Prince, B.; Maurin, J.C.; Bleicher, F. Odontoblast: A mechano-sensory cell. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312, 416–424. [Google Scholar] [CrossRef]
- Pawlaczyk-Kamieńska, T.; Borysewicz-Lewicka, M.; Śniatała, R.; Batura-Gabryel, H. Clinical evaluation of the dental hard tissues in an adult population with cystic fibrosis. Pol. Arch. Int. Med. 2019, 129, 725–727. [Google Scholar] [CrossRef]
- Atar, M.; Körperich, E.J. Systemic disorders and their influence on the development of dental hard tissues: A literature review. J. Dent. 2010, 38, 296–306. [Google Scholar] [CrossRef]
- Tervonen, S.A.; Stratmann, U.; Mokrys, K.; Reichart, P.A. Regional odontodysplasia: A review of the literature and report of four cases. Clin. Oral Investig. 2004, 8, 45–51. [Google Scholar] [CrossRef]
- Minicucci, E.M.; Lopes, L.F.; Crocci, A.J. Dental abnormalities in children after chemotherapy treatment for acute lymphoid leukemia. Leuk. Res. 2003, 27, 45–50. [Google Scholar] [CrossRef]
- Pinzon, M.L.; Gong, S.G. Arrested root formation of 4 s premolars: Report of a patient. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 652–656. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlaczyk-Kamieńska, T.; Winiarska, H.; Kulczyk, T.; Cofta, S. Dental Anomalies in Rare, Genetic Ciliopathic Disorder—A Case Report and Review of Literature. Int. J. Environ. Res. Public Health 2020, 17, 4337. https://doi.org/10.3390/ijerph17124337
Pawlaczyk-Kamieńska T, Winiarska H, Kulczyk T, Cofta S. Dental Anomalies in Rare, Genetic Ciliopathic Disorder—A Case Report and Review of Literature. International Journal of Environmental Research and Public Health. 2020; 17(12):4337. https://doi.org/10.3390/ijerph17124337
Chicago/Turabian StylePawlaczyk-Kamieńska, Tamara, Hanna Winiarska, Tomasz Kulczyk, and Szczepan Cofta. 2020. "Dental Anomalies in Rare, Genetic Ciliopathic Disorder—A Case Report and Review of Literature" International Journal of Environmental Research and Public Health 17, no. 12: 4337. https://doi.org/10.3390/ijerph17124337
APA StylePawlaczyk-Kamieńska, T., Winiarska, H., Kulczyk, T., & Cofta, S. (2020). Dental Anomalies in Rare, Genetic Ciliopathic Disorder—A Case Report and Review of Literature. International Journal of Environmental Research and Public Health, 17(12), 4337. https://doi.org/10.3390/ijerph17124337




