Synergistic Effect of WTC-Particulate Matter and Lysophosphatidic Acid Exposure and the Role of RAGE: In-Vitro and Translational Assessment
Abstract
1. Introduction
2. Methods
2.1. Cell Lines
2.2. WTC-PM and LPA Preparation
2.3. Exposures
2.4. Immunoblots
2.5. Cytokine/Chemokine Assessment
2.6. NF-κB Assay
2.7. Study Design, Serum Analytes and Metabolomics
2.8. Integration
2.9. Statistical Analysis
2.10. Ethics Approval and Consent to Participate
3. Results
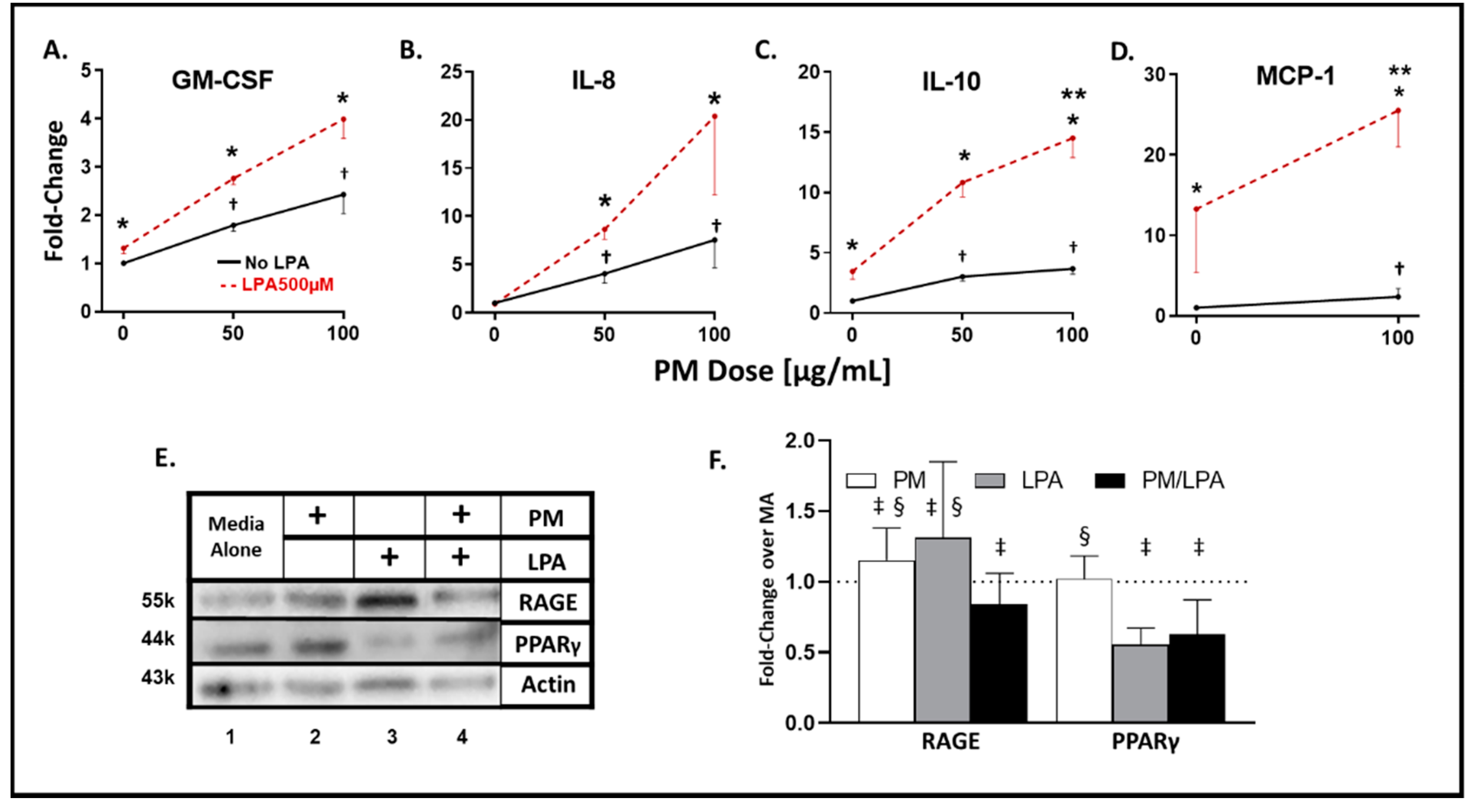
3.1. WTC-PM and LPA Exposure Induce Analyte Elaboration in THP-1-Derived Macrophages
3.2. Synergistic Response to WTC-PM/LPA Co-Exposure in Human-THP-1-Derived Macrophages
3.3. WTC-PM and LPA Induce RAGE/PPARγ Protein Production in Human-THP-1-Derived Macrophages
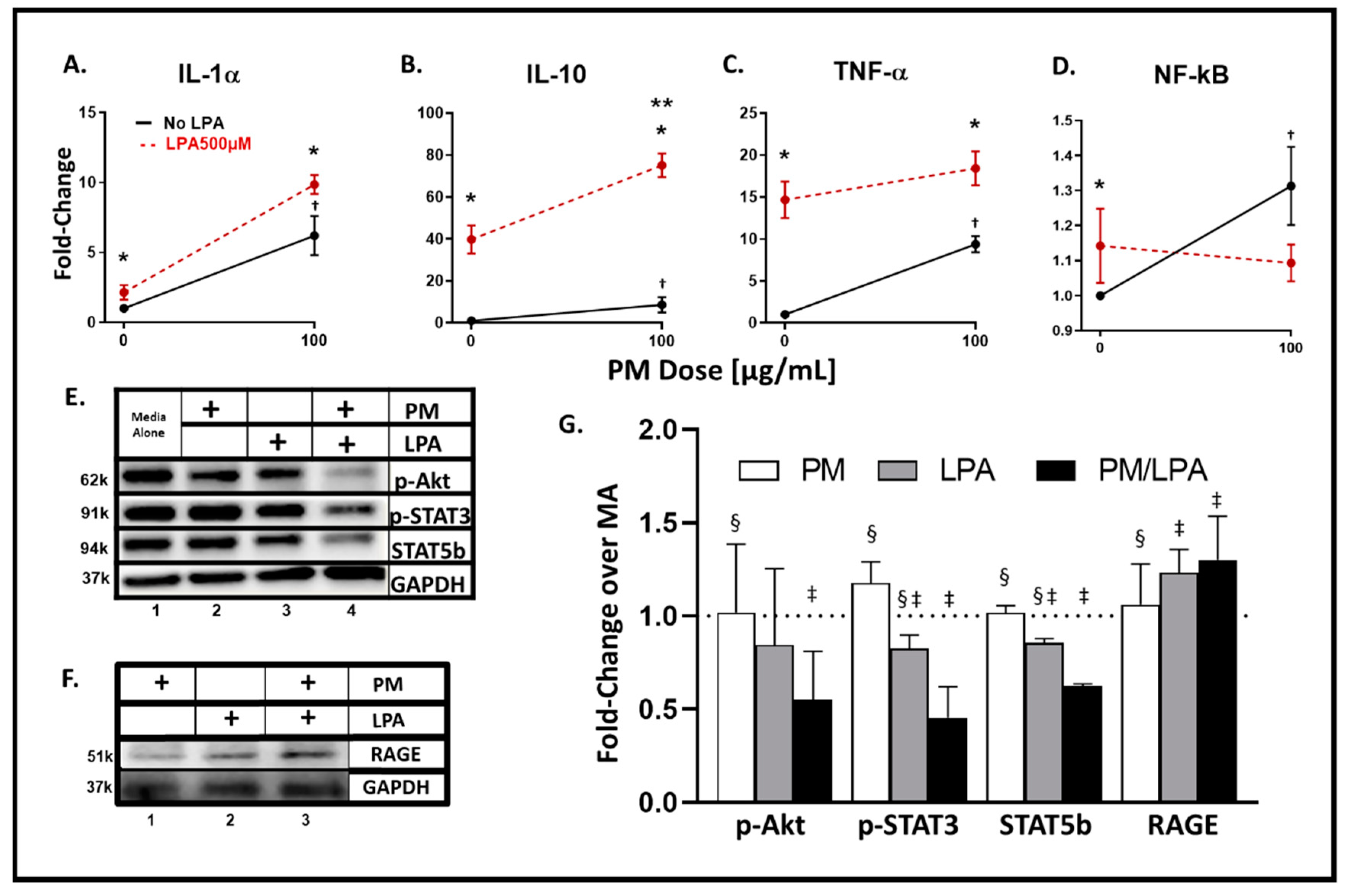
3.4. WTC-PM and LPA Exposures of RAW264.7 Cells Yielded Analyte Elaboration
3.5. Synergistic Response to WTC-PM/LPA Co-Exposure in RAW264.7 Cells
3.6. WTC-PM and LPA Induce RAGE Protein Production in Murine RAW264.7 Cells
3.7. MultiOMIC (Metabolome and Chemome) Integrated Biomarker Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end-products |
| Ager | Murine RAGE gene |
| Ager-/- | RAGE-deficient mice |
| Akt | Protein kinase B |
| AP-1 | Activator protein 1 |
| BCAA | Branched-chain amino acids |
| COPD | Chronic obstructive pulmonary disease |
| EGF | Epidermal growth factor |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular-signal-regulated kinase |
| FCS | Fetal calf serum |
| FDNY | Fire Department of New York |
| FEV1 | Forced expiratory volume |
| FEV1%predicted < LLN | Percent of predicted forced expiratory volume less than the lower limit of normal |
| FGF | fibroblast growth factor |
| Flt | Fms-like tyrosine kinase |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GM-CSF | Granulocyte/macrophage-colony-stimulating factor |
| GPC | Glycero-phosphatidylcholines |
| HDL | High-density lipoprotein |
| IFN | Interferon |
| IgG | Immunoglobulin G |
| IL | Interleukin |
| IP | IFNγ-induced protein |
| JNK | c-Jun N-terminal kinase |
| KC | Chemokine kigand 1 (murine) |
| LDL | Low-density lipoprotein |
| LPA | Lysophosphatidic acid |
| MA | Media alone |
| MCP | Monocyte chemoattractant protein |
| MDC | Macrophage-derived chemokine |
| MetSyn | Metabolic syndrome |
| MIP | Macrophage inflammatory protein |
| NF-κB | Nuclear factor kappa light-chain-enhancer of activated B cells |
| OAD | Obstructive airway disease |
| p-Akt | Phosphorylated protein kinase |
| PBS | Phosphate-buffered saline |
| PDGF | Platelet-derived growth factor |
| PLA2 | Phospholipase 2 |
| PM | Particulate matter |
| PMA | Phorbol-12-myristate-13-acetate |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| p-STAT | Phosphorylated signal transducer and activator of transcription |
| RAGE | Receptor for advanced glycation end-products (membrane-bound) |
| RANTES | Regulated upon activation, normal T cell expressed, and secreted |
| sCD40L | Soluble cluster of differentiation-40 ligand |
| sIL-2Rα | Secreted interleukin-2 receptor alpha |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SP-1 | Specificity protein 1 |
| sRAGE | Soluble RAGE |
| STAT | Signal transducer and activator of transcription |
| TGF | Tumor growth factor |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| VEGF | Vascular endothelial growth factor |
| WTC-HP | WTC Health Program |
| WTC-LI | World Trade Center—lung injury |
| WTC-PM | World Trade Center—particulate matter |
References
- Sint, T.; Donohue, J.F.; Ghio, A.J. Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease. Inhal. Toxicol. 2008, 20, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.H.; van Eeden, S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2009, 4, 233–243. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A., 3rd; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. Short term fluctuations in air pollution and hospital admissions of the elderly for respiratory disease. Thorax 1995, 50, 531–538. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Shanley, R.P.; Hayes, R.B.; Cromar, K.R.; Ito, K.; Gordon, T.; Ahn, J. Particulate Air Pollution and Clinical Cardiovascular Disease Risk Factors. Epidemiology (Cambridge, Mass.) 2016, 27, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Leone, N.; Courbon, D.; Thomas, F.; Bean, K.; Jego, B.; Leynaert, B.; Guize, L.; Zureik, M. Lung function impairment and metabolic syndrome: The critical role of abdominal obesity. Am. J. Respir. Crit. Care Med. 2009, 179, 509–516. [Google Scholar] [CrossRef]
- Kwon, S.; Crowley, G.; Caraher, E.J.; Haider, S.H.; Lam, R.; Veerappan, A.; Yang, L.; Liu, M.; Zeig-Owens, R.; Schwartz, T.M.; et al. Validation of Predictive Metabolic Syndrome Biomarkers of World Trade Center Lung Injury: A 16-Year Longitudinal Study. Chest 2019, 156, 486–493. [Google Scholar] [CrossRef]
- Naveed, B.; Weiden, M.D.; Kwon, S.; Gracely, E.J.; Comfort, A.L.; Ferrier, N.; Kasturiarachchi, K.J.; Cohen, H.W.; Aldrich, T.K.; Rom, W.N.; et al. Metabolic syndrome biomarkers predict lung function impairment: A nested case-control study. Am. J. Respir. Crit. Care Med. 2012, 185, 392–399. [Google Scholar] [CrossRef]
- Kwon, S.; Echevarria, G.C.; Cho, S.; Tsukiji, J.; Rom, W.N.; Prezant, D.J.; Schmidt, A.; Weiden, M.D.; Nolan, A. Soluble Rage, Mmp-9 And Crp Are Predictive Of Particulate Matter Induced Lung Disease In Wtc Exposed Firefighters. Am. J. Resp. Crit. Care Med. 2014, 189, A3168. [Google Scholar]
- Weiden, M.D.; Kwon, S.; Caraher, E.; Berger, K.I.; Reibman, J.; Rom, W.N.; Prezant, D.J.; Nolan, A. Biomarkers of World Trade Center Particulate Matter Exposure: Physiology of Distal Airway and Blood Biomarkers that Predict FEV(1) Decline. Semin. Respir. Crit. Care Med. 2015, 36, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F. The metabolic syndrome as a risk factor for lung function decline. Am. J. Respir. Crit. Care Med. 2012, 185, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Balmes, J.R. Can we predict who will develop chronic sequelae of acute inhalational injury? Chest 2012, 142, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Antao, V.C. The World Trade Center disaster: A tragic source of medical advancement. Eur. Respir. J. 2013, 41, 999–1001. [Google Scholar] [CrossRef]
- Caraher, E.J.; Kwon, S.; Haider, S.H.; Crowley, G.; Lee, A.; Ebrahim, M.; Zhang, L.; Chen, L.C.; Gordon, T.; Liu, M.; et al. Receptor for advanced glycation end-products and World Trade Center particulate induced lung function loss: A case-cohort study and murine model of acute particulate exposure. PLoS ONE 2017, 12, e0184331. [Google Scholar] [CrossRef]
- Zhao, Y.; Natarajan, V. Lysophosphatidic acid (LPA) and its receptors: Role in airway inflammation and remodeling. Biochim. Biophys. Acta 2013, 1831, 86–92. [Google Scholar] [CrossRef]
- Haider, S.H.; Veerappan, A.; Crowley, G.; Ostrofsky, D.; Mikhail, M.; Lam, R.; Wang, Y.; Sunseri, M.; Kwon, S.; Prezant, D.J.; et al. MultiOMICs of WTC-Particulate Induced Persistent Airway Hyperreactivity: Role of Receptor for Advanced Glycation End Products. Am. J. Respir. Cell Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The RAGE axis: A fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res. 2010, 106, 842–853. [Google Scholar] [CrossRef]
- Koyama, H.; Yamamoto, H.; Nishizawa, Y. Endogenous Secretory RAGE as a Novel Biomarker for Metabolic Syndrome and Cardiovascular Diseases. Biomark. Insights 2007, 2, 331–339. [Google Scholar] [CrossRef]
- Hancock, D.B.; Eijgelsheim, M.; Wilk, J.B.; Gharib, S.A.; Loehr, L.R.; Marciante, K.D.; Franceschini, N.; van Durme, Y.M.; Chen, T.H.; Barr, R.G.; et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat. Genet. 2010, 42, 45–52. [Google Scholar] [CrossRef]
- Repapi, E.; Sayers, I.; Wain, L.V.; Burton, P.R.; Johnson, T.; Obeidat, M.; Zhao, J.H.; Ramasamy, A.; Zhai, G.; Vitart, V.; et al. Genome-wide association study identifies five loci associated with lung function. Nat. Genet. 2010, 42, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Beucher, J.; Boelle, P.Y.; Busson, P.F.; Muselet-Charlier, C.; Clement, A.; Corvol, H.; French, C.F.M.G.S.I. AGER -429T/C is associated with an increased lung disease severity in cystic fibrosis. PLoS ONE 2012, 7, e41913. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Henry, A.P.; Hodge, E.; Kheirallah, A.K.; Billington, C.K.; Rimington, T.L.; Bhaker, S.K.; Obeidat, M.; Melen, E.; Merid, S.K.; et al. The Ser82 RAGE Variant Affects Lung Function and Serum RAGE in Smokers and sRAGE Production In Vitro. PLoS ONE 2016, 11, e0164041. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Guh, J.Y.; Chen, H.C.; Hung, W.C.; Lai, Y.H.; Chuang, L.Y. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J. Cell Biochem. 2001, 81, 102–113. [Google Scholar] [CrossRef]
- Oczypok, E.A.; Perkins, T.N.; Oury, T.D. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr. Respir. Rev. 2017, 23, 40–49. [Google Scholar] [CrossRef]
- Tobon-Velasco, J.C.; Cuevas, E.; Torres-Ramos, M.A. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets 2014, 13, 1615–1626. [Google Scholar] [CrossRef]
- Wu, L.; Ma, L.; Nicholson, L.F.; Black, P.N. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir. Med. 2011, 105, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Celli, B.R.; Owen, C.A. COPD as an endothelial disorder: Endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series). Pulm. Circ. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, M.B.; Ullah, M.A.; Gan, W.J.; Wark, P.A.; Chung, K.F.; Hughes, J.M.; Armour, C.L.; Phipps, S. RAGE: A new frontier in chronic airways disease. Br. J. Pharmacol. 2012, 167, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, M.B.; Wood, L.G.; Tooze, M.; Simpson, J.L.; McDonald, V.M.; Gibson, P.G.; Wark, P.A. Soluble RAGE is deficient in neutrophilic asthma and COPD. Eur. Respir. J. 2012, 39, 721–729. [Google Scholar] [CrossRef]
- Tsukiji, J.; Cho, S.J.; Echevarria, G.C.; Kwon, S.; Joseph, P.; Schenck, E.J.; Naveed, B.; Prezant, D.J.; Rom, W.N.; Schmidt, A.M.; et al. Lysophosphatidic acid and apolipoprotein A1 predict increased risk of developing World Trade Center-lung injury: A nested case-control study. Biomarkers 2014, 19, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, W.H.; van Meeteren, L.A.; Giepmans, B.N. The ins and outs of lysophosphatidic acid signaling. BioEssays News Rev. Mol. Cell. Dev. Biol. 2004, 26, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.E.; Herr, D.R.; Chun, J. Lysophosphatidic acid (LPA) receptors: Signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010, 91, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Murph, M.; Mills, G.B. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev. Mol. Med. 2007, 9, 1–18. [Google Scholar] [CrossRef]
- Smyth, S.S.; Cheng, H.Y.; Miriyala, S.; Panchatcharam, M.; Morris, A.J. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim. Biophys. Acta 2008, 1781, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, N.V. Toxicity of the AGEs generated from the Maillard reaction: On the relationship of food-AGEs and biological-AGEs. Mol. Nutr. Food Res. 2006, 50, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; AlAbed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef]
- Aoki, J.; Inoue, A.; Okudaira, S. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta 2008, 1781, 513–518. [Google Scholar] [CrossRef]
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591. [Google Scholar] [CrossRef]
- Georas, S.N. Lysophosphatidic acid and autotaxin: Emerging roles in innate and adaptive immunity. Immunol. Res. 2009, 45, 229. [Google Scholar] [CrossRef]
- Pamuklar, Z.; Federico, L.; Liu, S.; Umezu-Goto, M.; Dong, A.; Panchatcharam, M.; Fulkerson, Z.; Berdyshev, E.; Natarajan, V.; Fang, X.; et al. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J. Biol. Chem. 2009, 284, 7385–7394. [Google Scholar] [CrossRef] [PubMed]
- Brindley, D.N.; Pilquil, C. Lipid phosphate phosphatases and signaling. J. Lipid Res. 2009, 50, S225–S230. [Google Scholar] [CrossRef]
- Rodriguez-Roisin, R.; Drakulovic, M.; Rodriguez, D.A.; Roca, J.; Barbera, J.A.; Wagner, P.D. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J. Appl. Physiol. 2009, 106, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Liebow, A.A. Pulmonary emphysema with special reference to vascular changes. Am. Rev. Respir. Dis. 1959, 80, 67–93. [Google Scholar] [PubMed]
- Caplan-Shaw, C.E.; Yee, H.; Rogers, L.; Abraham, J.L.; Parsia, S.S.; Naidich, D.P.; Borczuk, A.; Moreira, A.; Shiau, M.C.; Ko, J.P.; et al. Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas, and fumes. J. Occup. Environ. Med. 2011, 53, 981–991. [Google Scholar] [CrossRef] [PubMed]
- King, M.S.; Eisenberg, R.; Newman, J.H.; Tolle, J.J.; Harrell, F.E., Jr.; Nian, H.; Ninan, M.; Lambright, E.S.; Sheller, J.R.; Johnson, J.E.; et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N. Engl. J. Med. 2011, 365, 222–230. [Google Scholar] [CrossRef]
- Noguchi, K.; Herr, D.; Mutoh, T.; Chun, J. Lysophosphatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol. 2009, 9, 15–23. [Google Scholar] [CrossRef]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef]
- Chun, J.; Hla, T.; Lynch, K.R.; Spiegel, S.; Moolenaar, W.H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2010, 62, 579–587. [Google Scholar] [CrossRef]
- McIntyre, T.M.; Pontsler, A.V.; Silva, A.R.; St Hilaire, A.; Xu, Y.; Hinshaw, J.C.; Zimmerman, G.A.; Hama, K.; Aoki, J.; Arai, H.; et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. USA 2003, 100, 131–136. [Google Scholar] [CrossRef]
- Stapleton, C.M.; Mashek, D.G.; Wang, S.; Nagle, C.A.; Cline, G.W.; Thuillier, P.; Leesnitzer, L.M.; Li, L.O.; Stimmel, J.B.; Shulman, G.I.; et al. Lysophosphatidic acid activates peroxisome proliferator activated receptor-gamma in CHO cells that over-express glycerol 3-phosphate acyltransferase-1. PLoS ONE 2011, 6, e18932. [Google Scholar] [CrossRef] [PubMed]
- Bodine, B.G.; Bennion, B.G.; Leatham, E.; Jimenez, F.R.; Wright, A.J.; Jergensen, Z.R.; Erickson, C.J.; Jones, C.M.; Johnson, J.P.; Knapp, S.M.; et al. Conditionally induced RAGE expression by proximal airway epithelial cells in transgenic mice causes lung inflammation. Respir. Res. 2014, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.; Herr, C.; Niederstrasser, J.; Beisswenger, C.; Bals, R. Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke. PLoS ONE 2017, 12, e0180092. [Google Scholar] [CrossRef] [PubMed]
- Weiden, M.D.; Naveed, B.; Kwon, S.; Segal, L.N.; Cho, S.J.; Tsukiji, J.; Kulkarni, R.; Comfort, A.L.; Kasturiarachchi, K.J.; Prophete, C.; et al. Comparison of WTC dust size on macrophage inflammatory cytokine release in vivo and in vitro. PLoS ONE 2012, 7, e40016. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Dailey, L.A.; Soukup, J.M.; Grambow, S.C.; Devlin, R.B.; Huang, Y.C. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ. Health Perspect. 2005, 113, 1032–1038. [Google Scholar] [CrossRef]
- Theus, S.A.; Cave, M.D.; Eisenach, K.D. Activated THP-1 cells: An attractive model for the assessment of intracellular growth rates of Mycobacterium tuberculosis isolates. Infect. Immun. 2004, 72, 1169–1173. [Google Scholar] [CrossRef]
- Smith, L.S.; Gharib, S.A.; Frevert, C.W.; Martin, T.R. Effects of Age on the Synergistic Interactions between Lipopolysaccharide and Mechanical Ventilation in Mice. Am. J. Resp. Cell Mol. 2010, 43, 475–486. [Google Scholar] [CrossRef]
- McGee, J.K.; Chen, L.C.; Cohen, M.D.; Chee, G.R.; Prophete, C.M.; Haykal-Coates, N.; Wasson, S.J.; Conner, T.L.; Costa, D.L.; Gavett, S.H. Chemical analysis of World Trade Center fine particulate matter for use in toxicologic assessment. Environ. Health Perspect. 2003, 111, 972–980. [Google Scholar] [CrossRef]
- Michalczyk, A.; Budkowska, M.; Dolegowska, B.; Chlubek, D.; Safranow, K. Lysophosphatidic acid plasma concentrations in healthy subjects: Circadian rhythm and associations with demographic, anthropometric and biochemical parameters. Lipids Health Dis. 2017, 16, 140. [Google Scholar] [CrossRef]
- Mathew, D.; Kremer, K.N.; Strauch, P.; Tigyi, G.; Pelanda, R.; Torres, R.M. LPA5 Is an Inhibitory Receptor That Suppresses CD8 T-Cell Cytotoxic Function via Disruption of Early TCR Signaling. Front. Immunol. 2019, 10, 1159. [Google Scholar] [CrossRef]
- Gavett, S.H.; Haykal-Coates, N.; Highfill, J.W.; Ledbetter, A.D.; Chen, L.C.; Cohen, M.D.; Harkema, J.R.; Wagner, J.G.; Costa, D.L. World Trade Center fine particulate matter causes respiratory tract hyperresponsiveness in mice. Environ. Health Perspect. 2003, 111, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.A.; Parsey, M.; Hoshino, Y.; Hoshino, S.; Nolan, A.; Yee, H.; Tse, D.B.; Weiden, M.D. CD40 contributes to lethality in acute sepsis: In vivo role for CD40 in innate immunity. Infect. Immun. 2003, 71, 3521–3528. [Google Scholar] [CrossRef]
- Kobayashi, H.; Nolan, A.; Naveed, B.; Hoshino, Y.; Segal, L.N.; Fujita, Y.; Rom, W.N.; Weiden, M.D. Neutrophils activate alveolar macrophages by producing caspase-6-mediated cleavage of IL-1 receptor-associated kinase-M. J. Immunol. 2011, 186, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Nolan, A.; Weiden, M.; Kelly, A.; Hoshino, Y.; Hoshino, S.; Mehta, N.; Gold, J.A. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am. J. Respir. Crit. Care Med. 2008, 177, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Nolan, A.; Weiden, M.D.; Hoshino, Y.; Gold, J.A. Cd40 but not CD154 knockout mice have reduced inflammatory response in polymicrobial sepsis: A potential role for Escherichia coli heat shock protein 70 in CD40-mediated inflammation in vivo. Shock 2004, 22, 538–542. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ, U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2018.
- Nolan, A.; Naveed, B.; Comfort, A.L.; Ferrier, N.; Hall, C.B.; Kwon, S.; Kasturiarachchi, K.J.; Cohen, H.W.; Zeig-Owens, R.; Glaser, M.S.; et al. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest 2012, 142, 412–418. [Google Scholar] [CrossRef]
- Weiden, M.D.; Naveed, B.; Kwon, S.; Cho, S.J.; Comfort, A.L.; Prezant, D.J.; Rom, W.N.; Nolan, A. Cardiovascular biomarkers predict susceptibility to lung injury in World Trade Center dust-exposed firefighters. Eur. Respir. J. 2013, 41, 1023–1030. [Google Scholar] [CrossRef]
- Cho, S.; Echevarria, G.C.; Kwon, S.; Naveed, B.; Schenck, E.; Tsukiji, J.; Rom, W.N.; Prezant, D.J.; Nolan, A. One Airway: Biomarkers Of Protection From Upper And Lower Airway Injury After World Trade Center Exposure. Respir. Med. 2018, 189, 162–170. [Google Scholar] [CrossRef]
- Banauch, G.I.; Dhala, A.; Prezant, D.J. Pulmonary disease in rescue workers at the World Trade Center site. Curr. Opin. Pulm. Med. 2005, 11, 160–168. [Google Scholar] [CrossRef]
- Prezant, D.J.; Weiden, M.; Banauch, G.I.; McGuinness, G.; Rom, W.N.; Aldrich, T.K.; Kelly, K.J. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N. Engl. J. Med. 2002, 347, 806–815. [Google Scholar] [CrossRef]
- Weiden, M.D.; Ferrier, N.; Nolan, A.; Rom, W.N.; Comfort, A.; Gustave, J.; Zeig-Owens, R.; Zheng, S.; Goldring, R.M.; Berger, K.I.; et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest 2010, 137, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Crowley, G.; Kwon, S.; Haider, S.H.; Caraher, E.J.; Lam, R.; St-Jules, D.E.; Liu, M.; Prezant, D.J.; Nolan, A. Metabolomics of World Trade Center-Lung Injury: A machine learning approach. BMJ Open Respir. Res. 2018, 5, e000274. [Google Scholar] [CrossRef] [PubMed]
- Crowley, G.; Kwon, S.; Ostrofsky, D.F.; Clementi, E.A.; Haider, S.H.; Caraher, E.J.; Lam, R.; St-Jules, D.E.; Liu, M.; Prezant, D.J.; et al. Assessing the Protective Metabolome Using Machine Learning in World Trade Center Particulate Exposed Firefighters at Risk for Lung Injury. Sci. Rep. 2019, 9, 11939. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Crowley, G.; Mikhail, M.; Lam, R.; Clementi, E.; Zeig-Owens, R.; Schwartz, T.M.; Liu, M.; Prezant, D.J.; Nolan, A. Metabolic Syndrome Biomarkers of World Trade Center Airway Hyperreactivity: A 16-Year Prospective Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 1486. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Reichmann, F.; Meinitzer, A.; Mayerhofer, R.; Jain, P.; Hassan, A.M.; Frohlich, E.E.; Wagner, K.; Painsipp, E.; Rinner, B.; et al. Synergistic effects of NOD1 or NOD2 and TLR4 activation on mouse sickness behavior in relation to immune and brain activity markers. Brain Behav. Immun. 2014, 44, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Gunja, N.J.; Athanasiou, K.A. Additive and synergistic effects of bFGF and hypoxia on leporine meniscus cell-seeded PLLA scaffolds. J. Tissue Eng. Regen. Med. 2010, 4, 115–122. [Google Scholar] [CrossRef]
- Haider, S.H.; Kwon, S.; Lam, R.; Lee, A.K.; Caraher, E.J.; Crowley, G.; Zhang, L.; Schwartz, T.M.; Zeig-Owens, R.; Liu, M.; et al. Predictive Biomarkers of Gastroesophageal Reflux Disease and Barrett’s Esophagus in World Trade Center Exposed Firefighters: A 15 Year Longitudinal Study. Sci. Rep. 2018, 8, 3106. [Google Scholar] [CrossRef]
- Zhang, L.; Haider, S.H.; Crowley, G.; Lam, R.; Kwon, S.; Chen, L.C.; Schmidt, A.M.; Prezant, D.J.; Nolan, A. World Trade Center Particulates And Lysophosphatdic Acid: Co-Exposure Induces Inflammatory Mediators. Am. J. Respir. Crit. Care Med. 2017, 195, A3911. [Google Scholar]
- Caraher, E.J.; Kwon, S.; Lee, A.K.; Chen, L.-C.; Gordon, T.; Prezant, D.J.; Rom, W.N.; Weiden, M.D.; Nolan, A. Additive and Synergistic Effects of LPA in World Trade Center Particulate Matter-Induced Inflammation. Am. J. Respir. Crit. Care Med. 2015, 195, 1. [Google Scholar]
- Crowley, G.; Kwon, S.; Haider, S.; Caraher, E.J.; Lam, R.; Liu, M.; Prezant, D.J.; Nolan, A. Metabolite and Biomarker Predictors of World Trade Center-Lung Injury: An Integrated Multiplatform Machine Learning Approach. Am. J. Resp. Crit. Care 2018, 197, A2588. [Google Scholar]
- Huang, W.; Wang, L.; Li, J.P.; Liu, M.C.; Xu, H.B.; Liu, S.C.; Chen, J.; Zhang, Y.; Morishita, M.; Bard, R.L.; et al. Short-Term Blood Pressure Responses to Ambient Fine Particulate Matter Exposures at the Extremes of Global Air Pollution Concentrations. Am. J. Hypertens. 2018, 31, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Eze, I.C.; Schaffner, E.; Foraster, M.; Imboden, M.; von Eckardstein, A.; Gerbase, M.W.; Rothe, T.; Rochat, T.; Kunzli, N.; Schindler, C.; et al. Long-Term Exposure to Ambient Air Pollution and Metabolic Syndrome in Adults. PLoS ONE 2015, 10, e0130337. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Cakmak, S.; Turner, M.C.; Brook, J.R.; Crouse, D.L.; Peters, P.A.; van Donkelaar, A.; Villeneuve, P.J.; Brion, O.; Jerrett, M.; et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 2013, 36, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Traves, S.L.; Culpitt, S.V.; Russell, R.E.; Barnes, P.J.; Donnelly, L.E. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax 2002, 57, 590–595. [Google Scholar] [CrossRef]
- Di Stefano, A.; Coccini, T.; Roda, E.; Signorini, C.; Balbi, B.; Brunetti, G.; Ceriana, P. Blood MCP-1 levels are increased in chronic obstructive pulmonary disease patients with prevalent emphysema. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1691–1700. [Google Scholar] [CrossRef]
- Basta, G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 2008, 196, 9–21. [Google Scholar] [CrossRef]
- Fritz, G. RAGE: A single receptor fits multiple ligands. Trends Biochem. Sci. 2011, 36, 625–632. [Google Scholar] [CrossRef]
- Simeonova, P.P.; Luster, M.I. Iron and reactive oxygen species in the asbestos-induced tumor necrosis factor-alpha response from alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1995, 12, 676–683. [Google Scholar] [CrossRef]
- Funahashi, S.; Okazaki, Y.; Ito, D.; Asakawa, A.; Nagai, H.; Tajima, M.; Toyokuni, S. Asbestos and multi-walled carbon nanotubes generate distinct oxidative responses in inflammatory cells. J. Clin. Biochem. Nutr. 2015, 56, 111–117. [Google Scholar] [CrossRef]
- Hart, G.A.; Hesterberg, T.W. In vitro toxicity of respirable-size particles of diatomaceous earth and crystalline silica compared with asbestos and titanium dioxide. J. Occup. Environ. Med. 1998, 40, 29–42. [Google Scholar] [CrossRef]
- Huang, S.X.; Jaurand, M.C.; Kamp, D.W.; Whysner, J.; Hei, T.K. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 179–245. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, T.W.; Chase, G.; Axten, C.; Miller, W.C.; Musselman, R.P.; Kamstrup, O.; Hadley, J.; Morscheidt, C.; Bernstein, D.M.; Thevenaz, P. Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following inhalation. Toxicol. Appl. Pharmacol. 1998, 151, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Simeonova, P.P.; Toriumi, W.; Kommineni, C.; Erkan, M.; Munson, A.E.; Rom, W.N.; Luster, M.I. Molecular regulation of IL-6 activation by asbestos in lung epithelial cells: Role of reactive oxygen species. J. Immunol. 1997, 159, 3921–3928. [Google Scholar] [PubMed]
- Satpathy, S.R.; Jala, V.R.; Bodduluri, S.R.; Krishnan, E.; Hegde, B.; Hoyle, G.W.; Fraig, M.; Luster, A.D.; Haribabu, B. Crystalline silica-induced leukotriene B4-dependent inflammation promotes lung tumour growth. Nat. Commun. 2015, 6, 7064. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Brown, D.M.; Watt, N.; Wilson, M.; Donaldson, K.; Ritchie, H.; MacNee, W. Ultrafine Particle-Mediated Activation of Macrophages: Intracellular Calcium Signaling and Oxidative Stress. Inhal. Toxicol. 2000, 12 (Suppl. 3), 345–351. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, G.; Andujar, P.; Pairon, J.C.; Billon-Galland, M.A.; Dion, C.; Dumortier, P.; Brochard, P.; Sobaszek, A.; Bartsch, P.; Paris, C.; et al. Quantification of short and long asbestos fibers to assess asbestos exposure: A review of fiber size toxicity. Environ. Health 2014, 13, 59. [Google Scholar] [CrossRef]
- Msiska, Z.; Pacurari, M.; Mishra, A.; Leonard, S.S.; Castranova, V.; Vallyathan, V. DNA double-strand breaks by asbestos, silica, and titanium dioxide: Possible biomarker of carcinogenic potential? Am. J. Respir. Cell Mol. Biol. 2010, 43, 210–219. [Google Scholar] [CrossRef]
- Chen, Q.; Marsh, J.; Ames, B.; Mossman, B. Detection of 8-oxo-2’-deoxyguanosine, a marker of oxidative DNA damage, in culture medium from human mesothelial cells exposed to crocidolite asbestos. Carcinogenesis 1996, 17, 2525–2527. [Google Scholar] [CrossRef]
- An, D.; Hao, F.; Zhang, F.Q.; Kong, W.; Chun, J.; Xu, X.M.; Cui, M.Z. CD14 is a key mediator of both lysophosphatidic acid and lipopolysaccharide induction of foam cell formation. J. Biol. Chem. 2017, 292, 14391–14400. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Deng, X.; Liu, Y.; Yang, X.; Wu, Q.; Yu, C. Lysophosphatidic acid directly induces macrophage-derived foam cell formation by blocking the expression of SRBI. Biochem. Biophys. Res. Commun. 2017, 491, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Rai, V. Lysophosphatidic acid converts monocytes into macrophages in both mice and humans. Blood 2017, 129, 1177–1183. [Google Scholar] [CrossRef]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Bradford, E.; Jacobson, S.; Varasteh, J.; Comellas, A.P.; Woodruff, P.; O’Neal, W.; DeMeo, D.L.; Li, X.; Kim, V.; Cho, M.; et al. The value of blood cytokines and chemokines in assessing COPD. Respir. Res. 2017, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, C.L.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. The impact of metabolism on DNA methylation. Hum. Mol. Genet 2005, 14, R139–R147. [Google Scholar] [CrossRef] [PubMed]
- Zaina, S.; Lindholm, M.W.; Lund, G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: More than just hyperhomocysteinemia? J. Nutr. 2005, 135, 5–8. [Google Scholar] [CrossRef]
- Lund, G.; Andersson, L.; Lauria, M.; Lindholm, M.; Fraga, M.F.; Villar-Garea, A.; Ballestar, E.; Esteller, M.; Zaina, S. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J. Biol. Chem. 2004, 279, 29147–29154. [Google Scholar] [CrossRef]
- Castro, R.; Rivera, I.; Struys, E.A.; Jansen, E.E.; Ravasco, P.; Camilo, M.E.; Blom, H.J.; Jakobs, C.; Tavares de Almeida, I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003, 49, 1292–1296. [Google Scholar] [CrossRef]
- Birukova, A.A.; Starosta, V.; Tian, X.; Higginbotham, K.; Koroniak, L.; Berliner, J.A.; Birukov, K.G. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl. Res. 2013, 161, 495–504. [Google Scholar] [CrossRef]
- Hurley, B.P.; McCormick, B.A. Multiple roles of phospholipase A2 during lung infection and inflammation. Infect. Immun. 2008, 76, 2259–2272. [Google Scholar] [CrossRef]
- Wu, T.; Ikezono, T.; Angus, C.W.; Shelhamer, J.H. Tumor necrosis factor-alpha induces the 85-kDa cytosolic phospholipase A2 gene expression in human bronchial epithelial cells. Biochim. Biophys. Acta 1996, 1310, 175–184. [Google Scholar] [CrossRef][Green Version]
- Bhowmick, R.; Clark, S.; Bonventre, J.V.; Leong, J.M.; McCormick, B.A. Cytosolic Phospholipase A2alpha Promotes Pulmonary Inflammation and Systemic Disease during Streptococcus pneumoniae Infection. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- Jemel, I.; Ii, H.; Oslund, R.C.; Payre, C.; Dabert-Gay, A.S.; Douguet, D.; Chargui, K.; Scarzello, S.; Gelb, M.H.; Lambeau, G. Group X secreted phospholipase A2 proenzyme is matured by a furin-like proprotein convertase and releases arachidonic acid inside of human HEK293 cells. J. Biol. Chem. 2011, 286, 36509–36521. [Google Scholar] [CrossRef]
- Pawliczak, R.; Huang, X.L.; Nanavaty, U.B.; Lawrence, M.; Madara, P.; Shelhamer, J.H. Oxidative stress induces arachidonate release from human lung cells through the epithelial growth factor receptor pathway. Am. J. Respir. Cell Mol. Biol. 2002, 27, 722–731. [Google Scholar] [CrossRef]
- Liu, G.; Cheresh, P.; Kamp, D.W. Molecular basis of asbestos-induced lung disease. Annu. Rev. Pathol. 2013, 8, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Jenkins, C.M.; Han, X.; Mancuso, D.J.; Sims, H.F.; Yang, K.; Gross, R.W. The highly selective production of 2-arachidonoyl lysophosphatidylcholine catalyzed by purified calcium-independent phospholipase A2gamma: Identification of a novel enzymatic mediator for the generation of a key branch point intermediate in eicosanoid signaling. J. Biol. Chem. 2005, 280, 26669–26679. [Google Scholar] [CrossRef]
- Rajdl, D.; Racek, J.; Trefil, L.; Stehlik, P.; Dobra, J.; Babuska, V. Effect of Folic Acid, Betaine, Vitamin B(6), and Vitamin B12 on Homocysteine and Dimethylglycine Levels in Middle-Aged Men Drinking White Wine. Nutrients 2016, 8, 34. [Google Scholar] [CrossRef]
- Lioy, P.J.; Weisel, C.P.; Millette, J.R.; Eisenreich, S.; Vallero, D.; Offenberg, J.; Buckley, B.; Turpin, B.; Zhong, M.; Cohen, M.D.; et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ. Health Perspect. 2002, 110, 703–714. [Google Scholar] [CrossRef]
- World Health Organization. 9 out of 10 People Worldwide Breathe Polluted Air, but More Countries are Taking Action. Available online: https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 14 June 2020).
- Peters, U.; Suratt, B.T.; Bates, J.H.T.; Dixon, A.E. Beyond BMI: Obesity and Lung Disease. Chest 2018, 153, 702–709. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Dixon, A.E.; Holguin, F. Diet and Metabolism in the Evolution of Asthma and Obesity. Clin. Chest Med. 2019, 40, 97. [Google Scholar] [CrossRef] [PubMed]


| Analyte | PBS | WTC-PM100μg/mL | LPA | WTC-PM100μg/mL + LPA | p | |
|---|---|---|---|---|---|---|
| Cytokine | G-CSF | 3.44 (3.44–4.29) | 22.58 (18.71–27.21) | 12.28 (12.28–15.1) | 22.01 (21.72–24.79) | *, † |
| GM-CSF | 0.29 (0.25–0.41) | 3.01 (2.67–3.29) | 1.74 (1.43–1.83) | 4.12 (3.61–4.31) | *, †, ‡ | |
| EGF | 6.12 (6.05–6.48) | 6.89 (6.29–6.93) | 6.62 (6–6.73) | 7.51 (7.38–7.58) | ‡ | |
| IFN-γ | 4.87 (4.72–5.03) | 7.20 (6.81–7.20) | 6.10 (5.99–6.34) | 6.65 (6.65–7.01) | * | |
| IFNA2 | 4.74 (4.15–5.29) | 18.78(17.3–20.62) | 14.50 (14.06–15.37) | 19.20 (17.72–21.03) | * | |
| IL-1β | 9.39 (7.85–10.15) | 28.39 (27.54–30.63) | 11.21 (10.08–15.39) | 27.84 (27.57–31.62) | * | |
| IL-1RA | 403.06 (390.72–477.38) | 539.47 (475.3–571.61) | 455.53 (435.86–493.64) | 520.76 (486.96–604.23) | ||
| IL-7 | 1.69 (1.62–2.07) | 6.81 (6.63–7.41) | 4.97 (4.16–5.71) | 5.16 (4.87–6.5) | *, † | |
| IL-8 | 389 (334.96–396.63) | 3251.24 (3039.24–3264) | 1011.74 (886.89–1501.24) | 3550.84 (3496.72–4043.95) | *, †, ‡ | |
| IL-10 | 12.88 (10.38–14.53) | 92.71 (67.79–157.5) | 467.4 (435.4–594.8) | 978.9 (861–985.7) | *, †, ‡ | |
| IL-15 | 7.99 (7.94–7.99) | 8.51 (8.41–8.51) | 8.4 (8.3–8.51) | 8.61 (8.51–8.66) | *, † | |
| TNF-α | 11.05 (10.21–14.11) | 59.75 (54.98–66.54) | 16.59 (14.72–20.36) | 46.2 (45.3–54.14) | * | |
| VEGF | 595.13 (529.48–615.77) | 674.96 (585.87–677.4) | 737.59 (731.89–800.41) | 828.49 (805.12–920.2) | †, ‡ | |
| IL-12(p40) | 6.94 (6.38–7.21) | 11.62 (10.09–12.11) | 9.59 (9.07–9.85) | 10.61 (10.36–10.99) | *, † | |
| IL-12(p70) | 5.23 (5.23–5.34) | 6.00 (5.86–6.12) | 5.78 (5.75–5.78) | 5.78 (5.73–5.89) | *, † | |
| Chemokine | Eotaxin | 6.61 (6.35–7.11) | 12.79 (12.74–13.45) | 8.99 (8.88–9.1) | 11.91 (11.91–12.11) | *, †, ‡ |
| IP-10 | 238.36 (228.03–242.36) | 257.46 (253.73–272.04) | 216.56 (210.26–244.94) | 145.72 (136.5–157.56) | *, †, ‡ | |
| MCP-1 | 111.40 (98.25–115.35) | 249.95 (240.64–303.02) | 1399.73 (983.82–1443.44) | 2687.61 (2447.81–2832.52) | *, †, ‡ | |
| MIP-1α | 20.11 (18.01–24.46) | 176.13 (172.82–217.53) | 248.09 (168.89–283.43) | 525.93 (509.38–552.97) | *, †, ‡ | |
| MIP-1β | 130.16 (115.28–144.06) | 752.4 (676.59–814.62) | 187.55 (183.67–204.07) | 587.40 (538.08–592.08) | *, ‡ | |
| Analyte | PBS | WTC-PM100μg/mL | LPA | WTC-PM100μg/mL + LPA | p | |
|---|---|---|---|---|---|---|
| Cytokine | G-CSF | 80.20 (43.71–94.81) | 2728.19 (2609.32–3177.45) | 50.55 (49.12–65.04) | 2669.91 (2411.36–2814.15) | * |
| GM-CSF | 7.96 (3.98–10.21) | 21.69 (18.83–21.69) | 0.00 (0.00–2.4) | 12.45 (10.21–15.72) | * | |
| IFN-g | 0 (0–0) | 4.04 (3.87–4.14) | 2.44 (2.30–2.66) | 3.83 (3.49–5.37) | *, † | |
| IL-1a | 8.07 (4.04–9.30) | 40.23 (34.63–43.20) | 12.42 (11.48–14.69) | 59.04 (58.69–62.51) | *, ‡ | |
| IL-4 | 2.43 (2.41–2.43) | 2.57 (2.52–2.58) | 2.43 (2.43–2.45) | 2.49 (2.46–2.52) | * | |
| IL-5 | 0 (0–0) | 3.95 (2.69–4.11) | 0.50 (0.25–1.07) | 1.22 (0.61–1.53) | * | |
| IL-6 | 1.02 (0.51–1.13) | 23.02 (21.79–30.13) | 0.32 (0.29–0.40) | 5.17 (4.87–5.59) | *, ‡ | |
| IL-7 | 3.27 (3.17–3.36) | 3.84 (3.51–3.95) | 3.10 (3.02–3.19) | 3.44 (3.36–3.47) | ||
| IL-9 | 57.24 (37.37–57.24) | 86.70 (84.98–88.38) | 99.82 (84.38–123.08) | 86.70 (83.26–93.26) | *, † | |
| IL-13 | 10.88 (10.18–10.88) | 15.13 (15.13–17.27) | 12.30 (11.59–13.01) | 16.55 (16.55–16.55) | * | |
| IL-15 | 1.00 (0.00–2.08) | 17.79 (11.86–20.28) | 2.38 (1.46–2.38) | 9.36 (4.68–11.06) | * | |
| IL-17 | 2.13 (2.10–2.17) | 3.60 (3.58–3.99) | 2.67 (2.56–2.78) | 3.03 (2.99–2.03) | *, †, ‡ | |
| TNF-a | 31.50 (29.96–31.64) | 425.42 (410.97–474.19) | 292.78 (273.69–302.52) | 584.33 (538.91–598.24) | *, † | |
| IL-10 | 1.85 (1.70–1.85) | 7.77 (7.53–8.18) | 6.00 (5.20–6.16) | 26.78 (25.48–27.27) | *, ‡ | |
| IL-12(p40) | 10.50 (10.50–10.50) | 0.49 (0.25–0.66) | 0 (0–0.03) | 0 (0–0.14) | ||
| IL-12(p70) | 0.40 (0.40–0.40) | 0.40 (0.40–2.51) | 0.40 (0.40–0.40) | 1.40 (0.70–2.06) | ||
| Chemokine | IP-10 | 66.24 (65.56–69.22) | 90.06 (82.55–110.61) | 58.72 (54.7–59.24) | 46.83 (40.20–47.04) | *, †, ‡ |
| KC | 1.74 (1.66–1.79) | 3.92 (3.62–4.11) | 1.83 (1.65–1.92) | 3.69 (3.58–4.18) | * | |
| MCP-1 | 886.86 (818.32–913.08) | 1824.05 (1764.68–1949.54) | 1810.84 (1749.61–1863.66) | 2101.59 (2077.03–2203.92) | *, †, ‡ | |
| RANTES | 4.72 (4.63–4.74) | 46.72 (43.50–50.28) | 4.50 (4.49–4.64) | 10.72 (10.04–13.95) | *, ‡ | |
| Other | NF-κB | 0.31 (0.31–0.38) | 0.46 (0.40–0.46) | 0.37 (0.35–0.42) | 0.35 (0.35–0.39) | † |
| Analyte (pg/mL) | Controls (n = 15) | WTC-LI (n = 15) |
|---|---|---|
| GM-CSF | 24.94 (15.16–64.70) | 30.05 (22.34–84.34) |
| IL-10 | 14.02 (3.78–27.69) | 10.80 (4.03–32.00) |
| IL-8 | 12.26 (10.46–36.64) | 14.45 (12.88–24.06) |
| MCP-1 a | 398.23 (314.11–493.11) | 589.41 (495.64–1087.35) |
| MIP-1α | 28.11 (17.12–38.38) | 26.32 (16.35–36.83) |
| IL-1α | 8.62 (3.20–28.57) | 4.83 (0.20–36.56) |
| TNF-α | 5.83 (4.35–8.84) | 7.29 (6.38–9.24) |
| LPA | 12.46 (4.73–27.72) | 12.02 (8.70–51.94) |
| RAGE | 80.59 (68.92–86.70) | 77.77 (60.84–100.13) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, R.; Haider, S.H.; Crowley, G.; Caraher, E.J.; Ostrofsky, D.F.; Talusan, A.; Kwon, S.; Prezant, D.J.; Wang, Y.; Liu, M.; et al. Synergistic Effect of WTC-Particulate Matter and Lysophosphatidic Acid Exposure and the Role of RAGE: In-Vitro and Translational Assessment. Int. J. Environ. Res. Public Health 2020, 17, 4318. https://doi.org/10.3390/ijerph17124318
Lam R, Haider SH, Crowley G, Caraher EJ, Ostrofsky DF, Talusan A, Kwon S, Prezant DJ, Wang Y, Liu M, et al. Synergistic Effect of WTC-Particulate Matter and Lysophosphatidic Acid Exposure and the Role of RAGE: In-Vitro and Translational Assessment. International Journal of Environmental Research and Public Health. 2020; 17(12):4318. https://doi.org/10.3390/ijerph17124318
Chicago/Turabian StyleLam, Rachel, Syed H. Haider, George Crowley, Erin J. Caraher, Dean F. Ostrofsky, Angela Talusan, Sophia Kwon, David J. Prezant, Yuyan Wang, Mengling Liu, and et al. 2020. "Synergistic Effect of WTC-Particulate Matter and Lysophosphatidic Acid Exposure and the Role of RAGE: In-Vitro and Translational Assessment" International Journal of Environmental Research and Public Health 17, no. 12: 4318. https://doi.org/10.3390/ijerph17124318
APA StyleLam, R., Haider, S. H., Crowley, G., Caraher, E. J., Ostrofsky, D. F., Talusan, A., Kwon, S., Prezant, D. J., Wang, Y., Liu, M., & Nolan, A. (2020). Synergistic Effect of WTC-Particulate Matter and Lysophosphatidic Acid Exposure and the Role of RAGE: In-Vitro and Translational Assessment. International Journal of Environmental Research and Public Health, 17(12), 4318. https://doi.org/10.3390/ijerph17124318





