Electroconvulsive Therapy in Super Refractory Status Epilepticus: Case Series with a Defined Protocol
Abstract
1. Introduction
2. Method
2.1. Study Design and Participants
2.2. Procedure
2.3. Intervention: ECT Protocol
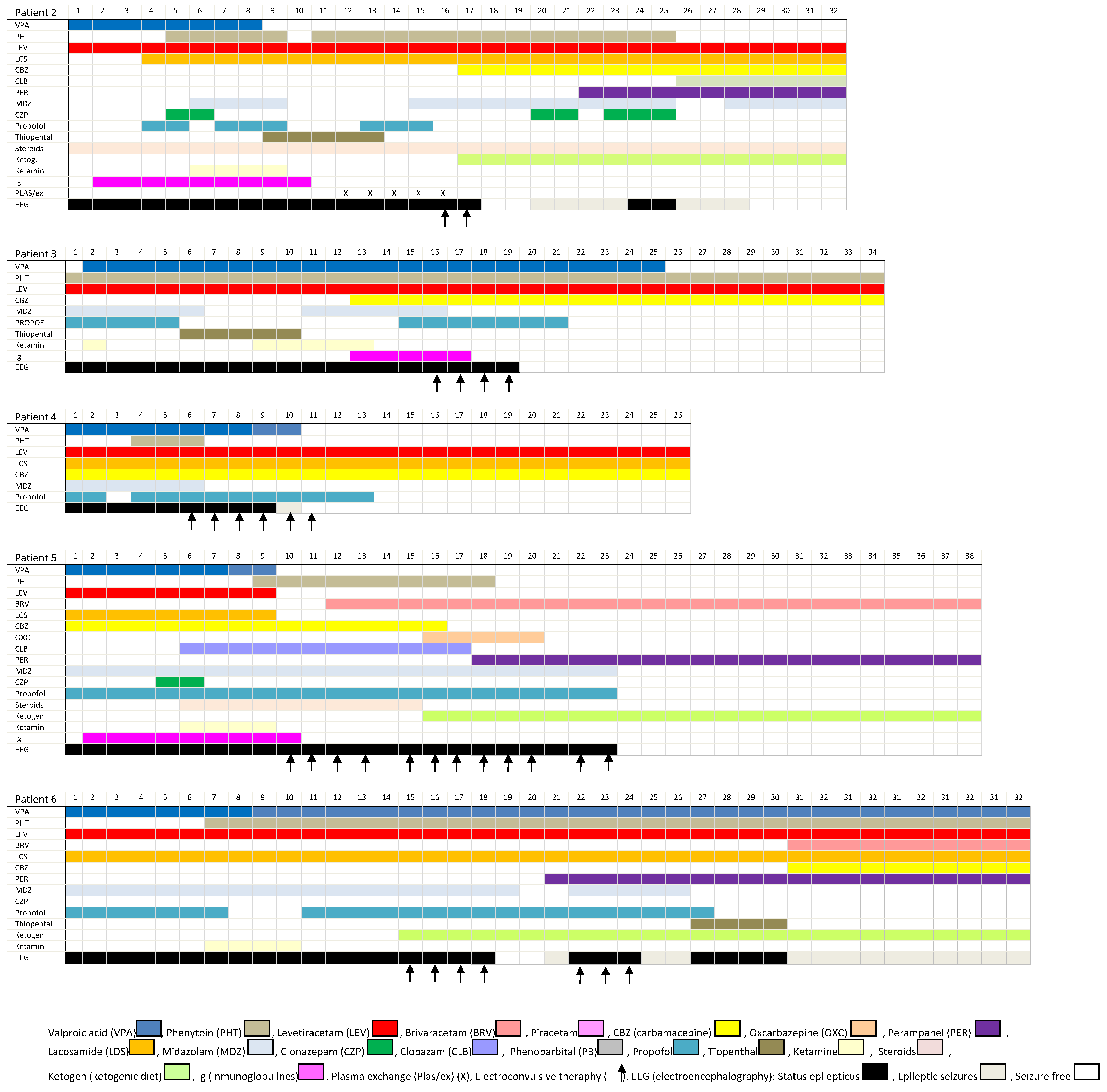
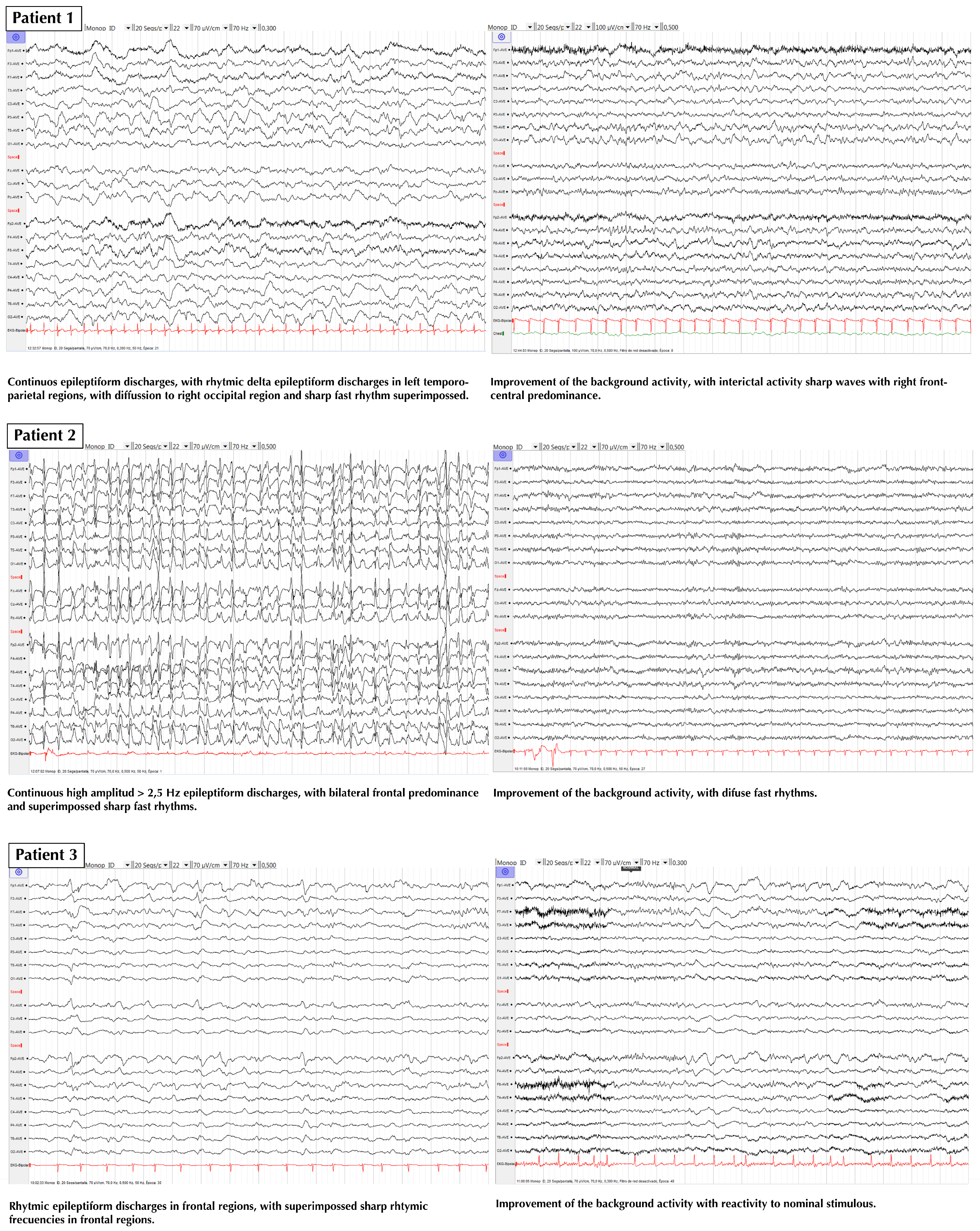
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.O.; Scheffer, I.E.; Shinnar, S.; Shorvon, S.; Lowenstein, D.H. A definition and classification of status epilepticus—Report of the ILAE task force on classification of status epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.J.; Gaspard, N.; van Baalen, A.; Nabbout, R.; Demeret, S.; Loddenkemper, T.; Navarro, V.; Specchio, N.; Lagae, L.; Rossetti, A.O.; et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia 2018, 59, 739–744. [Google Scholar] [CrossRef]
- Samanta, D.; Garrity, L.; Arya, R. Refractory and super-refractory status epilecticus. Indian Pediatr. 2020, 57, 29–53. [Google Scholar] [CrossRef]
- Leitinger, M.; Trinka, E.; Giovannini, G.; Zimmermann, G.; Florea, C.; Rochracher, A.; Kalss, G.; Neuray, C.; Kreidenhuber, R.; Höfler, J.; et al. Epidemiology of status epilepticus in adults: A population-based study on incidence, causes and outcomes. Epilepsia 2019, 60, 53–62. [Google Scholar] [CrossRef]
- Betjemann, J.P.; Josephson, S.A.; Lowenstein, D.H.; Burke, J.F. Trends in status epilepticus-related hospitalizations and mortality: Redefined in US practice over time. JAMA Neurol. 2015, 72, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Dham, B.S.; Hunter, K.; Rincon, F. The epidemiology of status epilepticus in the United States. Neurocrit. Care 2014, 20, 476–483. [Google Scholar] [CrossRef]
- Bhalla, D.; Tchalla, A.E.; Mignard, C.; Marin, B.; Mignard, D.; Jallon, P.; Preux, P.-M. First-ever population-based study on status epilepticus in French Island of La Reunion (France)—Incidence and fatality. Seizure 2014, 23, 769–773. [Google Scholar] [CrossRef]
- Logroscino, G.; Hesdorffer, D.C.; Cascino, G.; Hauser, W.A.; Coeytauz, A.; Galobardes, B.; Morabia, A.; Jallon, P. Mortality after a first episode of status epilepticus in the United States and Europe. Epilepsia 2005, 46 (Suppl. 11), 46–48. [Google Scholar] [CrossRef]
- Kantanen, A.M.; Reinikainen, M.; Parviainen, I.; Roukonen, E.; Ala-Peijari, M.; Bäcklund, T.; Koskenkari, J.; Laitio, R.; Kälviäinen, R. Incidence and mortality of super-refractory status epilepticus in adults. Epilepsy Behav. 2015, 49, 131–134. [Google Scholar] [CrossRef]
- Beg, J.M.; Anderson, T.D.; Francis, K.; Meckley, L.M.; Fitzhenry, D.; Foster, T.; Sukhtankar, S.; Kanes, S.J.; Moura, L.M.V.R. Burden of illness for super-refractory status epilepticus patients. J. Med. Econ. 2017, 20, 45–53. [Google Scholar] [CrossRef]
- Lai, A.; Outin, H.D.; Jabot, J.; Mégarbane, B.; Gaudry, S.; Coudroy, R.; Louis, G.; Schneider, F.; Barbarot, N.; Roch, A.; et al. Functional outcome of prolonged refractory status epilepticus. Crit. Care 2015, 19, 199. [Google Scholar] [CrossRef]
- Kantanen, A.-M.; Reinikainen, M.; Parviainen, I.; Kälviäinen, R. Long-term outcome of refractory status epilepticus in adults: A retrospective population-based study. Epilepsy Res. 2017, 133, 13–21. [Google Scholar] [CrossRef]
- Tan, R.Y.; Nelligam, A.; Shorvon, S.D. The uncommon causes of status epilepticus: A systematic review. Epilepsy Res. 2010, 91, 111–122. [Google Scholar] [CrossRef]
- Mayer, S.A.; Claassen, J.; Lokin, J.; Mendelsohn, F.; Dennis, L.J.; Fitzsimmons, B.F. Refractory status epilepticus: Frequency, risk factors, and impact on outcome. Arch. Neurol. 2002, 59, 205–210. [Google Scholar] [CrossRef]
- Chen, J.W.; Wasterlain, C.G. Status epilepticus: Pathophysiology and management in adults. Lancet Neurol. 2006, 5, 246–256. [Google Scholar] [CrossRef]
- Naylor, D.E. Glutamate and GABA in the balance: Convergent pathways sustain seizures during status epilepticus. Epilepsia 2010, 51 (Suppl. 3), 106–109. [Google Scholar] [CrossRef]
- Shorvon, S.; Ferlisi, M. The treatment of super-refractory status epilepticus: A critical review of available therapies and a clinical treatment protocol. Brain 2011, 134, 2802–2818. [Google Scholar] [CrossRef]
- Rai, S.; Drislane, F.W. Treatment of refractory and super-refractory status epilepticus. Neurotherapeutics 2018, 15, 679–712. [Google Scholar] [CrossRef] [PubMed]
- Poblete, R.; Sung, G. Status epilecticus and beyond: A clinical review of status epilepticus and an update on current management strategies in super-refractory status eplilepticus. Korean J. Crit. Care Med. 2017, 32, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Bayrlee, A.; Ganeshalingam, N.; Kurczewski, L.; Brophy, G.M. Treatment of super-refractory status eplilepticus. Curr. Neurol. Neurosci. Rep. 2015, 15, 66. [Google Scholar] [CrossRef]
- Ferlisi, M.; Shorvon, S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain 2012, 135, 2314–2328. [Google Scholar] [CrossRef]
- San Juan, D.; Dávila-Rodríguez, D.O.; Jiménez, C.R.; Gonzalez, M.S.; Carranza, S.M.; Hernández-Mendoza, J.R.; Anschel, D.J. Neuromodulation techniques or status epilecticus: A review. Brain Stimul. 2019, 12, 835–844. [Google Scholar] [CrossRef]
- Tronka, E.; Brigo, F. Neurostimulation in the treatment of refractory and super-refractory status epilepticus. Epilepsy Behav. 2019, 101, 106551. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Matuszczak, M.; Teitelbaum, J.; Gillman, L.M.; Kazina, C.J. Electroconvulsive therapy for refractory status epilepticus: A systematic review. Seizure 2016, 35, 23–32. [Google Scholar] [CrossRef]
- Lambrecq, V.; Villéga, F.; Marchal, C.; Michel, V.; Guehl, D.; Rotge, J.Y.; Burbaud, P. Refractory status epilepticus: Electroconvulsive therapy as a possible therapeutic strategy. Seizure 2012, 21, 661–664. [Google Scholar] [CrossRef]
- Pinchotti, D.M.; Abbott, C.; Quinn, D.K. Targeted electroconvulsive therapy for super refractory status epilepticus: A case report and literature review. Psychosomatics 2018, 59, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Přikryl, R.; Ustohal, L.; Kucerová, H.P.; Cesková, E. Effect of electroconvulsive therapy on cortical excitability in a patient with long-term remission of schizophrenia: A transcranial magnetic stimulation study. JECT 2011, 27, e9–e11. [Google Scholar] [CrossRef]
- Sackeim, H.A. Convulsant and anticonvulsant properties of electroconvulsive therapy: Towards a focal form of brain stimulation. Clin. Neurosci. Res. 2004, 4, 39–57. [Google Scholar] [CrossRef]
- Mirás-Veiga, A.; Conejo-Moreno, D.; Gómez-Menéndez, A.I.; Muñoz-Siscart, I.; del-Olmo-Fernández, M.; Gómez-Sánchez, E.; García-González, M.; Gómez-Sáez, F. Effectiveness of electroconvulsive therapy for refractory status epilepticus in febrile infection-related epilepsy syndrome. Neuropediatrics 2017, 48, 45–48. [Google Scholar] [CrossRef]
- Ray, A.K. Treatment of refractory status epilepticus with electroconvulsive therapy: Need for future clinical studies. Int. J. Epilepsy 2017, 4, 98–103. [Google Scholar] [CrossRef]
- Trinka, E.; Leitinger, M. Which EEG patterns in coma are non-convulsive status epilepticus? Epilepsy Behav. 2015, 49, 203–222. [Google Scholar] [CrossRef]
- Beniczky, S.; Hirsch, L.J.; Kaplan, P.W.; Pressler, R.; Bauer, G.; Aurlien, H.; Brøgger, J.C.; Trinka, E. Unified EEG terminology and criteria for non-convulsive status epilepticus. Epilepsia 2013, 54, 28–29. [Google Scholar] [CrossRef]
- Leitinger, M.; Beniczky, S.; Rohracher, A.; Gardella, E.; Kalss, G.; Qerama, E.; Höfler, J.; Lindberg-Larsen, A.H.; Kuchukhidze, G.; Dobesberger, J.; et al. Salzburg Consensus Criteria for Non-Convulsive Status Epilepticus—Approach to clinical application. Epilepsy Behav. 2015, 49, 158–163. [Google Scholar] [CrossRef]
- Hirsch, L.J.; LaRoche, S.M.; Gaspard, N.; Gerard, E.; Svoronos, A.; Herman, S.T.; Mani, R.; Arif, H.; Jette, N.; Minazad, Y.; et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J. Clin. Neurophysiol. 2013, 30, 1–27. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X. Potential mechanisms and clinical applications of mild hypothermia and electroconvulsive therapy on refractory status epilepticus. Expert Rev. Neurother. 2015, 15, 135–144. [Google Scholar] [CrossRef]
- Shin, H.W.; O’Donovan, C.A.; Boggs, J.G.; Grefe, A.; Harper, A.; Bell, W.L.; McCall, W.V.; Rosenquist, P. Successful ECT treatment for medically refractory non-convulsive status epilepticus in pediatric patient. Seizure 2011, 20, 433–436. [Google Scholar] [CrossRef]
- Kellner, C.H.; Fink, M. Electroconvulsive therapy in the treatment of intractable status epilepticus. Epilepsy Behav. 2009, 16, 189–190. [Google Scholar] [CrossRef]
- Ahmed, J.; Metrick, M.; Gilbert, A.; Glasson, A.; Singh, T.; Ambrous, W.; Brown, L.; Aykroyd, L.; Bobel, K. Electroconvulsive therapy for super refractory status epilepticus. J. ECT 2018, 34, e5–e9. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-López, B.; Gómez-Menéndez, A.I.; Vázquez-Sánchez, F.; Pérez-Cabo, E.; Isidro-Mesas, F.; Zabalegui-Pérez, A.; Muñoz-Siscart, I.; Lloria-Gil, M.C.; Soto-Cámara, R.; González-Bernal, J.J.; et al. Electroconvulsive Therapy in Super Refractory Status Epilepticus: Case Series with a Defined Protocol. Int. J. Environ. Res. Public Health 2020, 17, 4023. https://doi.org/10.3390/ijerph17114023
García-López B, Gómez-Menéndez AI, Vázquez-Sánchez F, Pérez-Cabo E, Isidro-Mesas F, Zabalegui-Pérez A, Muñoz-Siscart I, Lloria-Gil MC, Soto-Cámara R, González-Bernal JJ, et al. Electroconvulsive Therapy in Super Refractory Status Epilepticus: Case Series with a Defined Protocol. International Journal of Environmental Research and Public Health. 2020; 17(11):4023. https://doi.org/10.3390/ijerph17114023
Chicago/Turabian StyleGarcía-López, Beatriz, Ana Isabel Gómez-Menéndez, Fernando Vázquez-Sánchez, Eva Pérez-Cabo, Francisco Isidro-Mesas, Arturo Zabalegui-Pérez, Ignacio Muñoz-Siscart, María Carmen Lloria-Gil, Raúl Soto-Cámara, Jerónimo J. González-Bernal, and et al. 2020. "Electroconvulsive Therapy in Super Refractory Status Epilepticus: Case Series with a Defined Protocol" International Journal of Environmental Research and Public Health 17, no. 11: 4023. https://doi.org/10.3390/ijerph17114023
APA StyleGarcía-López, B., Gómez-Menéndez, A. I., Vázquez-Sánchez, F., Pérez-Cabo, E., Isidro-Mesas, F., Zabalegui-Pérez, A., Muñoz-Siscart, I., Lloria-Gil, M. C., Soto-Cámara, R., González-Bernal, J. J., González-Santos, J., Aguilar-Parra, J. M., Trigueros, R., López-Liria, R., & Kjær, T. W. (2020). Electroconvulsive Therapy in Super Refractory Status Epilepticus: Case Series with a Defined Protocol. International Journal of Environmental Research and Public Health, 17(11), 4023. https://doi.org/10.3390/ijerph17114023







