Epidemiological and Microbiological Aspects of the Peritonsillar Abscess
Abstract
1. Introduction:
2. Materials and Methods
2.1. Group of Patients
2.2. Methods Used
2.3. Statistical Methods
3. Results
4. Discussion
4.1. Gender and Age Spectrum in the PTA Patient Group
4.2. The Spectrum of Microbial Agents
4.3. The Spectrum of Microbial Agents based on Gender, Age and Seasons
4.4. The Spectrum of Microbial Agents and PTA Therapy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sowerby, L.J.; Hussain, Z.; Husein, M. The epidemiology, antibiotic resistance and post-discharge course of peritonsillar abscesses in London, Ontario. J. Otolaryngol. Head Neck Surg. 2013, 42, 154–159. [Google Scholar] [CrossRef]
- Wikstén, J.; Blomgren, K.; Eriksson, T. Variations in treatment of peritonsillar abscess in four Nordic countries. Acta Otolaryngol. 2014, 134, 813–817. [Google Scholar] [CrossRef]
- Kanesada, K.; Mogi, G. Bilateral peritonsillar abscesses. Auris Nasus Larynx. 1981, 8, 35–39. [Google Scholar] [CrossRef]
- Papacharalampous, G.X.; Vlastarakos, P.V.; Kotsis, G. Bilateral Peritonsillar Abscesses: A Case Presentation and Review of the Current Literature with regard to the Controversies in Diagnosis and Treatment. Case Rep. Med. 2011, 20, 254–259. [Google Scholar] [CrossRef]
- Hanna, B.C.; McMullan, R.; Gallagher, G. The epidemiology of peritonsillar abscess disease in Northern Ireland. J. Infect. 2006, 52, 247–253. [Google Scholar] [CrossRef]
- Segal, N.; El-Saied, S.; Puterman, M. Peritonsillar abscess in children in the southern district of Israel. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1148–1150. [Google Scholar] [CrossRef]
- Acharya, A.; Gurung, R.; Khanal, B. Bacteriology and antibiotic susceptibility pattern of peritonsillar abscess. JNMA J. Nepal. Med. Assoc. 2010, 49, 139–142. [Google Scholar]
- Albertz, N.; Nazar, G. Peritonsillar abscess: Treatment with immediate tonsillectomy—10 years of experience. Acta Otolaryngol. 2012, 132, 1102–1107. [Google Scholar] [CrossRef]
- Goldenberg, D.; Golz, A.; Joachims, H.Z. Retropharyngeal abscess: A clinical review. J. Laryngol. Otol. 1997, 111, 546–550. [Google Scholar] [CrossRef]
- Snow, N.; Lucas, A.E.; Grau, M. Purulent mediastinal abscess secondary to Ludwig’s angina. Arch. Otolaryngol. 1983, 109, 53–55. [Google Scholar] [CrossRef]
- Parhiscar, A.; Har-El, G. Deep neck abscess: A retrospective review of 210 cases. Ann. Otol. Rhinol. Laryngol. 2001, 110, 1051–1054. [Google Scholar] [CrossRef]
- McClay, J.E.; Murray, A.D.; Booth, T. Intravenous antibiotic therapy for deep neck abscesses defined by computed tomography. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 1207–1212. [Google Scholar] [CrossRef]
- Klug, T.E. Peritonsillar abscess: Clinical aspects of microbiology, risk factors, and the association with parapharyngeal abscess. Dan. Med. J. 2017, 64, 354–359. [Google Scholar]
- Sichel, J.Y.; Dano, I.; Hocwald, E. Nonsurgical management of parapharyngeal space infections: A prospective study. Laryngoscope 2002, 112, 906–910. [Google Scholar] [CrossRef]
- Toews, A.; Rocha, A.G. Oropharyngeal sepsis with endothoracic spread. Can. J. Surg. 1980, 23, 265–268. [Google Scholar]
- Mora, R.; Jankowska, B.; Catrambone, U. Descending necrotizing mediastinitis: Ten years’ experience. Ear Nose Throat J. 2004, 83, 776–780. [Google Scholar] [CrossRef]
- Maharaj, D.; Rajah, V.; Hemsley, S. Management of peritonsillar abscess. J. Laryngol. Otol. 1991, 105, 743–745. [Google Scholar] [CrossRef]
- Wolf, M.; Kronenberg, J.; Kessler, A. Peritonsillar abscess in children and its indication for tonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 1988, 16, 113–117. [Google Scholar] [CrossRef]
- Broekema, N.M.; Van, T.T.; Monson, T.A.; Marshall, S.A.; Warshauer, D.M. Comparison of cefoxitin and oxacillin disk diffusion methods for detection of mecA-mediated resistance in Staphylococcus aureus in a large-scale study. J. Clin. Microbiol. 2009, 47, 217–219. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Risberg, S.; Engfeldt, P.; Hugosson, S. Incidence of peritonsillar abscess and relationship toage and gender: Retrospective study. Scand. J. Infect. Dis. 2008, 40, 726–792. [Google Scholar] [CrossRef]
- Krtickova, J.; Haviger, J.; Ryskova, L. Peritonsillar complications of acute inflammations of palatine tonsils (a retrospective study). Otorinolaryng. Phoniat. 2016, 65, 24–29. [Google Scholar]
- Marom, T.; Cinamon, U.; Itskoviz, D. Changing trends of peritonsillar abscess. Am. J. Otolaryngol. 2010, 31, 162–167. [Google Scholar] [CrossRef]
- Klug, T.E.; Henriksen, J.J.; Fuursted, K. Significant pathogens in peritonsillar abscesses. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 619–627. [Google Scholar] [CrossRef][Green Version]
- Gavriel, H.; Lazarovitch, T.; Pomortsev, A. Variations in the microbiology of peritonsillar abscess. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 27–31. [Google Scholar] [CrossRef]
- Herzon, F.S.; Aldridge, J.H. Peritonsillar abscess: Needle aspiration. Otolaryngol. Head Neck Surg. 1981, 89, 910–911. [Google Scholar] [CrossRef]
- Brook, I.; Frazier, E.H.; Thompson, D.H. Aerobic and anaerobic microbiology of peritonsillar abscess. Laryngoscope 1991, 101, 289–292. [Google Scholar] [CrossRef]
- Wolf, M.; Even-Chen, I.; Kronenberg, J. Peritonsillar abscess: Repeated needle aspiration versus incision and drainage. Ann. Otol. Rhinol. Laryngol. 1994, 103, 554–557. [Google Scholar] [CrossRef]
- Prior, A.; Montgomery, P.; Mitchelmore, I. The microbiology and antibiotic treatment of peritonsillar abscesses. Clin. Otolaryngol. Allied. Sci. 1995, 20, 219–223. [Google Scholar] [CrossRef]
- Love, R.L.; Allison, R.; Chambers, S.T. Peritonsillar infection in Christchurch 2006–2008: Epidemiology and microbiology. N. Z. Med. J. 2011, 124, 16–23. [Google Scholar]
- Stjernquist-Desatnik, A.; Holst, E. Tonsillar microbial flora: Comparison of recurrent tonsillitis and normal tonsils. Acta Otolaryngol. 1999, 119, 102–106. [Google Scholar]
- Stjernquist-Desatnik, A.; Prellner, K.; Schalen, C. High recovery of Haemophilus influenzae and group a streptococci in recurrent tonsillar infection or hypertrophy as compared with normal tonsils. J. Laryngol. Otol. 1991, 105, 439–441. [Google Scholar] [CrossRef]
- Brook, I.; Foote, P.A. Comparison of the microbiology of recurrent tonsillitis between children and adults. Laryngoscope 1986, 96, 1385–1388. [Google Scholar] [CrossRef]
- Timon, C.I.; McAllister, V.A.; Walsh, M. Changes in tonsillar bacteriology of recurrent acute tonsillitis: 1980 vs. 1989. Respir. Med. 1990, 84, 395–400. [Google Scholar] [CrossRef]
- Brook, I.; Yocum, P.; Foote, P.A. Changes in the core tonsillar bacteriology of recurrent tonsillitis: 1977–1993. Clin. Infect. Dis. 1995, 21, 171–176. [Google Scholar] [CrossRef]
- Gavriel, H.; Golna, Y.; Lazarovitch, T. Bacteriology of peritonsillar abscess in patiens over 40 years—A neglected age group. Eur. Arch. Otorinoloaryngol. 2015, 272, 981–984. [Google Scholar] [CrossRef]
- Herzon, F.S.; Harris, P. Peritonsillar abscess: Incidence, current management practices, and a proposal for treatment guidelines. Laryngoscope 1995, 105, 1–17. [Google Scholar] [CrossRef]
- Sousa Menezes, A.; Ribeiro, D.C.; Guimarães, J.R.; Lima, A.F.; Dias, L. Management of pediatric peritonsillar and deep neck infections- cross- sectional retrospective analysis. World J. Otorhinolaryngol. Head Neck Surg. 2019, 5, 207–214. [Google Scholar] [CrossRef]
- Walker, L.W.; Montoya, L.; Chochua, S.; Beall, B.; Green, M. Increase in Invasive Group A Streptococcal Disease and Emergence of Mucoid Strains in a Pediatric Population: February-June 2017. Open Forum. Infect. Dis. 2019, 6, 275. [Google Scholar] [CrossRef]
- Weinberg, E.; Brodsky, L.; Stanievich, J. Needle aspiration of peritonsillar abscess in children. Arch. Otolaryngol. Head Neck Surg. 1993, 119, 169–172. [Google Scholar]
- Khayr, W.; Taepke, J. Management of peritonsillar abscess: Needle aspiration versus incision and drainage versus tonsillectomy. Am. J. Ther. 2005, 12, 344–350. [Google Scholar] [CrossRef]
- Spires, J.R.; Owens, J.J.; Woodson, G.E. Treatment of peritonsillar abscess. A prospective study of aspiration vs. incision and drainage. Arch. Otolaryngol. Head Neck Surg. 1987, 113, 984–986. [Google Scholar] [CrossRef]
- Visvanathan, V.; Nix, P. National UK survey of antibiotics prescribed for acute tonsillitis and peritonsillar abscess. J. Laryngol. Otol. 2010, 124, 420–423. [Google Scholar] [CrossRef]
- Ryan, S.; Papanikolaou, V.; Keogh, I. Appraisal of the perihospital management and evolving microbiology of peritonsillar ascess disease. ENT 2014, 10, 15–20. [Google Scholar]
- Mazur, E.; Czerwińska, E.; Korona-Głowniak, I.M. Epidemiology, clinical history and microbiology of peritonsillar abscess. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 549–554. [Google Scholar] [CrossRef]
- Jokipii, A.M.; Jokipii, L.; Sipila, P. Semiquantitative culture results and pathogenic significance of obligate anaerobes in peritonsillar abscesses. J. Clin. Microbiol. 1988, 26, 957–961. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Oreskovic, N.M.; Fu, X.; Shebl, F.M.; Mancini, C.M.; Maniates, J.M.; Walensky, R.P. High-cost, high-need patients: The impact of reported penicillin allergy. Am. J. Manag. Care 2020, 26, 154–161. [Google Scholar]
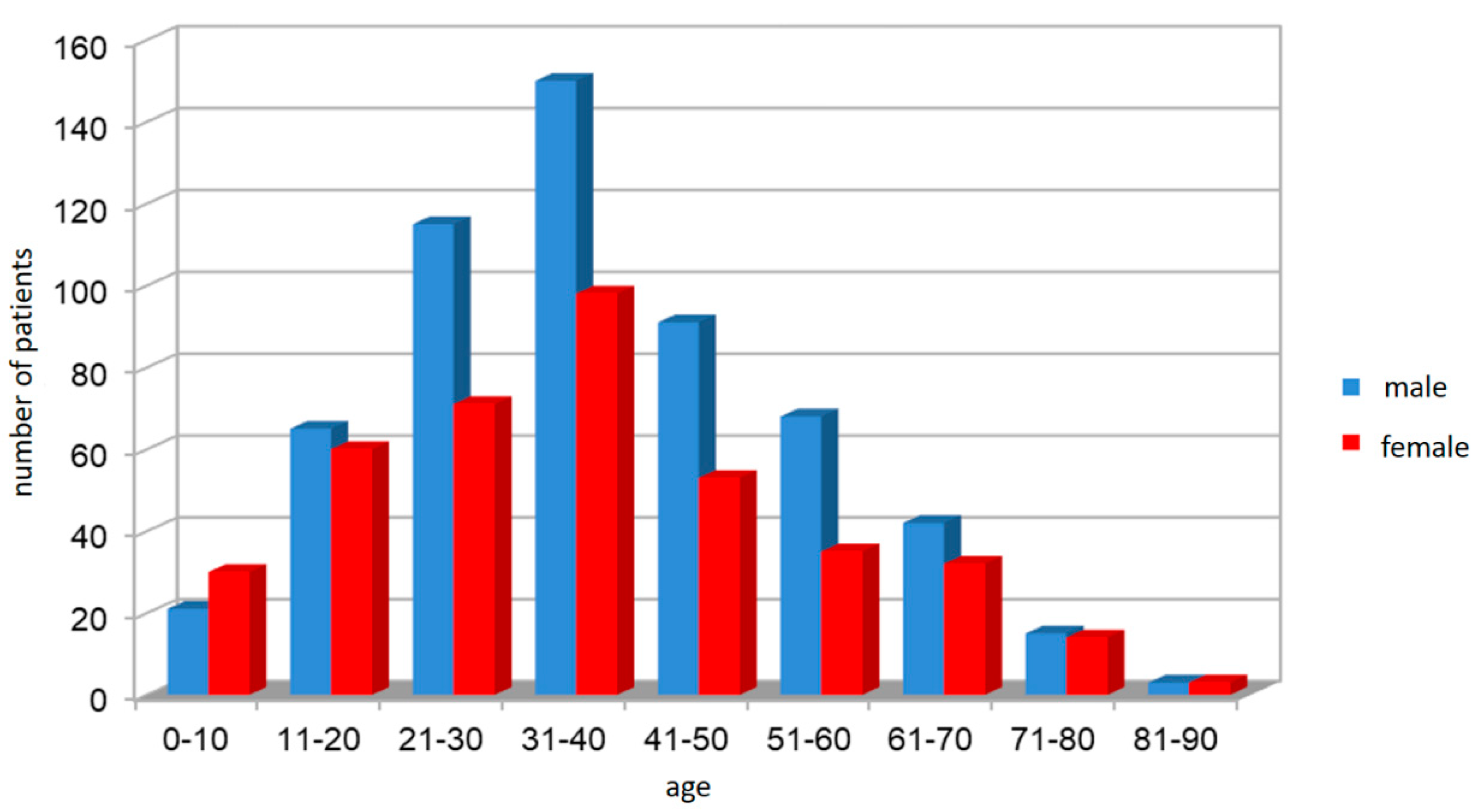
| Age Category | Male (n/%) | Female (n/%) | Total (n/%) | M/W Difference p-Value |
|---|---|---|---|---|
| I. 0–18 | 64/ 45.7 | 76/ 54.3 | 140/ 14.5 | 0.3105 |
| II. 19–50 | 378/ 61.6 | 236/ 38.4 | 614/ 63.6 | <0.0001 |
| III. >50 | 128/ 60.4 | 84/ 39.6 | 212/ 21.9 | 0.0025 |
| Total (n/%) | 570/ 59.0 | 396/ 41.0 | 966/ 100 | <0.0001 |
| Group | Male | Entire Group | Female | Entire Group | p-Value |
|---|---|---|---|---|---|
| Incidence | N | % | N | % | |
| Total (n = 966) | 570 | 59.01% | 396 | 40.99% | <0.0001 |
| Microbial agents | n | Group Male (%) | n | Group Female (%) | |
| Negative cultivation result | 98 | 17.19 | 125 | 31.57 | - |
| Streptococcus pyogenes | 128 | 22.46 | 82 | 20.71 | 0.4551 |
| Streptococcus (other species) | 93 | 16.31 | 47 | 11.87 | 0.1145 |
| Staphylococcus aureus | 63 | 11.05 | 51 | 12.88 | 0.3644 |
| MRSA | 2 | 0.35 | 3 | 0.75 | 0.4286 |
| Escherichia coli | 5 | 0.88 | 2 | 0.50 | 0.4531 |
| Serratia marcescens | 3 | 0.53 | 1 | 0.25 | 0.4771 |
| Pseudomonas aeruginosa | 2 | 0.35 | 0 | 0 | 0.2232 |
| Haemophilus influenzae | 9 | 1.58 | 8 | 2.02 | 0.7039 |
| Klebsiella pneumoniae | 17 | 2.99 | 9 | 2.28 | 0.6699 |
| Enterobacter species | 20 | 3.51 | 9 | 2.28 | 0.2034 |
| Granulicatella species | 1 | 0.18 | 0 | 0 | 0.3893 |
| Gemella morbillorum | 0 | 0 | 1 | 0.25 | 0.2449 |
| Aerobes total | 343 | 60.18 | 213 | 53.79 | <0.0001 |
| Fusobacterium species | 45 | 7.88 | 15 | 3.79 | 0.0078 |
| Prevotella species | 36 | 6.32 | 19 | 4.80 | 0.3404 |
| Veillonella species | 26 | 4.56 | 12 | 3.03 | 0.1634 |
| Peptostreptococcus species | 11 | 1.93 | 4 | 1.01 | 0.2102 |
| Actinomyces species | 0 | 0 | 1 | 0.25 | 0.2449 |
| Bacteroides species | 0 | 0 | 1 | 0.25 | 0.2449 |
| Anaerobes total | 118 | 20.70 | 52 | 13.13 | <0.0001 |
| Candida albicans | 11 | 1.93 | 6 | 1.51 | 0.2253 |
| Age Incidence | 0–18 | 19–50 | >51 | p-Value | |||
|---|---|---|---|---|---|---|---|
| N | Entire Group (%) | N | Entire Group (%) | N | Entire Group (%) | ||
| Total (n = 966) | 140 | 14.49 | 614 | 63.56 | 212 | 21.95 | <0.0001 |
| Microbial agents | n | Group 0–18 (%) | N | Group 19–50 (%) | N | Group >51 (%) | |
| Negative cultivation result | 59 | 42.14 | 142 | 23.12 | 21 | 9.91 | - |
| Streptococcus pyogenes | 31 | 22.14 | 158 | 25.73 | 21 | 9.91 | 0.0001 |
| Streptococcus (other species) | 12 | 8.57 | 88 | 14.33 | 40 | 18.87 | 0.0267 |
| Staphylococcus aureus | 11 | 7.87 | 81 | 13.19 | 22 | 10.38 | 0.1615 |
| MRSA | 4 | 2.86 | 1 | 0.16 | 0 | 0 | 0.0002 |
| Escherichia coli | 1 | 0.71 | 3 | 0.49 | 3 | 1.42 | 0.3905 |
| Serratia marcescens | 0 | 0 | 2 | 0.33 | 2 | 0.93 | 0.3432 |
| Pseudomonas aeruginosa | 0 | 0 | 0 | 0 | 2 | 0.93 | 0.0283 |
| Haemophilus influenzae | 1 | 0.71 | 12 | 1.95 | 4 | 1.89 | 0.5947 |
| Klebsiella pneumoniae | 0 | 0 | 17 | 2.77 | 9 | 4.25 | 0.0539 |
| Enterobacter species | 2 | 1.43 | 14 | 2.28 | 13 | 6.13 | 0.0090 |
| Granulicatella species | 1 | 0.71 | 0 | 0 | 0 | 0 | 0.0522 |
| Gemella morbillorum | 0 | 0 | 1 | 0.16 | 0 | 0 | 0.7506 |
| Aerobes total | 63 | 45.00 | 377 | 61.40 | 116 | 54.72 | <0.0001 |
| Fusobacterium species | 6 | 4.29 | 34 | 5.54 | 21 | 9.91 | 0.0446 |
| Prevotella species | 5 | 3.57 | 28 | 4.57 | 22 | 10.38 | 0.0035 |
| Veillonella species | 5 | 3.57 | 16 | 2.61 | 17 | 8.02 | 0.0022 |
| Peptostreptococcus species | 2 | 1.43 | 7 | 1.14 | 6 | 2.83 | 0.2275 |
| Actinomyces species | 0 | 0 | 0 | 0 | 1 | 0.47 | 0.1686 |
| Bacteroides species | 0 | 0 | 1 | 0.16 | 0 | 0 | 0.7506 |
| Anaerobes total | 18 | 12.86 | 86 | 14.00 | 67 | 31.60 | <0.0001 |
| Candida albicans | 0 | 0 | 9 | 1.48 | 8 | 3.77 | 0.0204 |
| Seasons | Spring | Summer | Autumn | Winter | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence | N | Entire Group (%) | N | Entire Group (%) | N | Entire Group (%) | N | Entire Group (%) | |
| Total (n = 966) | 246 | 25.47 | 259 | 26.81 | 224 | 23.19 | 237 | 24.53 | 0.4396 |
| Microbial Agents | n | Spring Period (%) | n | Summer Period (%) | n | Autumn Period (%) | n | Winter Period (%) | |
| Negative cultivation result | 61 | 24.80 | 71 | 27.41 | 15 | 6.70 | 75 | 31.65 | - |
| Streptococcus pyogenes | 48 | 19.51 | 46 | 17.76 | 61 | 27.23 | 55 | 23.21 | 0.4012 |
| Streptococcus (other species) | 33 | 13.41 | 32 | 12.36 | 36 | 16.07 | 39 | 16.46 | 0.8282 |
| Staphylococcus aureus | 31 | 12.60 | 28 | 10.81 | 31 | 13.84 | 24 | 10.13 | 0.7549 |
| MRSA | 0 | 0 | 0 | 0 | 4 | 1.78 | 1 | 0.42 | 0.0349 |
| Escherichia coli | 0 | 0 | 2 | 0.77 | 4 | 1.78 | 1 | 0.42 | 0.1712 |
| Serratia marcescens | 0 | 0 | 2 | 0.77 | 2 | 0.89 | 0 | 0 | 0.2610 |
| Pseudomonas aeruginosa | 0 | 0 | 1 | 0.39 | 1 | 0.45 | 0 | 0 | 0.5722 |
| Haemophilus influenzae | 4 | 1.62 | 5 | 1.93 | 4 | 1.78 | 4 | 1.70 | 0.8803 |
| Klebsiella pneumoniae | 7 | 2.85 | 6 | 2.32 | 10 | 4.45 | 3 | 1.26 | 0.2757 |
| Enterobacter species | 7 | 2.85 | 13 | 5.02 | 6 | 2.68 | 3 | 1.26 | 0.0621 |
| Granulicatella species | 0 | 0 | 0 | 0 | 1 | 0.45 | 0 | 0 | 0.3915 |
| Gemella morbillorum | 0 | 0 | 0 | 0 | 1 | 0.45 | 0 | 0 | 0.3915 |
| Aerobes total | 130 | 52.85 | 135 | 52.12 | 161 | 71.88 | 130 | 54.85 | 0.1900 |
| Fusobacterium species | 18 | 7.32 | 13 | 5.02 | 15 | 6.70 | 15 | 6.33 | 0.8377 |
| Prevotella species | 13 | 5.28 | 19 | 7.34 | 15 | 6.70 | 8 | 3.38 | 0.2012 |
| Veillonella species | 11 | 4.47 | 13 | 5.02 | 11 | 4.91 | 3 | 1.26 | 0.0992 |
| Peptostreptococcus species | 5 | 2.03 | 4 | 1.54 | 3 | 1.36 | 3 | 1.26 | 0.8647 |
| Actinomyces species | 1 | 0.41 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3915 |
| Bacteroides species | 1 | 0.41 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3915 |
| Anaerobes total | 49 | 19.91 | 49 | 18.92 | 44 | 19.64 | 29 | 12.24 | 0.0985 |
| Candida albicans | 6 | 2.44 | 4 | 1.54 | 4 | 1.78 | 3 | 1.26 | 0.7728 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slouka, D.; Hanakova, J.; Kostlivy, T.; Skopek, P.; Kubec, V.; Babuska, V.; Pecen, L.; Topolcan, O.; Kucera, R. Epidemiological and Microbiological Aspects of the Peritonsillar Abscess. Int. J. Environ. Res. Public Health 2020, 17, 4020. https://doi.org/10.3390/ijerph17114020
Slouka D, Hanakova J, Kostlivy T, Skopek P, Kubec V, Babuska V, Pecen L, Topolcan O, Kucera R. Epidemiological and Microbiological Aspects of the Peritonsillar Abscess. International Journal of Environmental Research and Public Health. 2020; 17(11):4020. https://doi.org/10.3390/ijerph17114020
Chicago/Turabian StyleSlouka, David, Jana Hanakova, Tomas Kostlivy, Petr Skopek, Vojtech Kubec, Vaclav Babuska, Ladislav Pecen, Ondřej Topolcan, and Radek Kucera. 2020. "Epidemiological and Microbiological Aspects of the Peritonsillar Abscess" International Journal of Environmental Research and Public Health 17, no. 11: 4020. https://doi.org/10.3390/ijerph17114020
APA StyleSlouka, D., Hanakova, J., Kostlivy, T., Skopek, P., Kubec, V., Babuska, V., Pecen, L., Topolcan, O., & Kucera, R. (2020). Epidemiological and Microbiological Aspects of the Peritonsillar Abscess. International Journal of Environmental Research and Public Health, 17(11), 4020. https://doi.org/10.3390/ijerph17114020








