Industrial Air Pollution and Respiratory Health Status among Residents in an Industrial Area in Central Italy
Abstract
1. Introduction
2. Materials and Methods
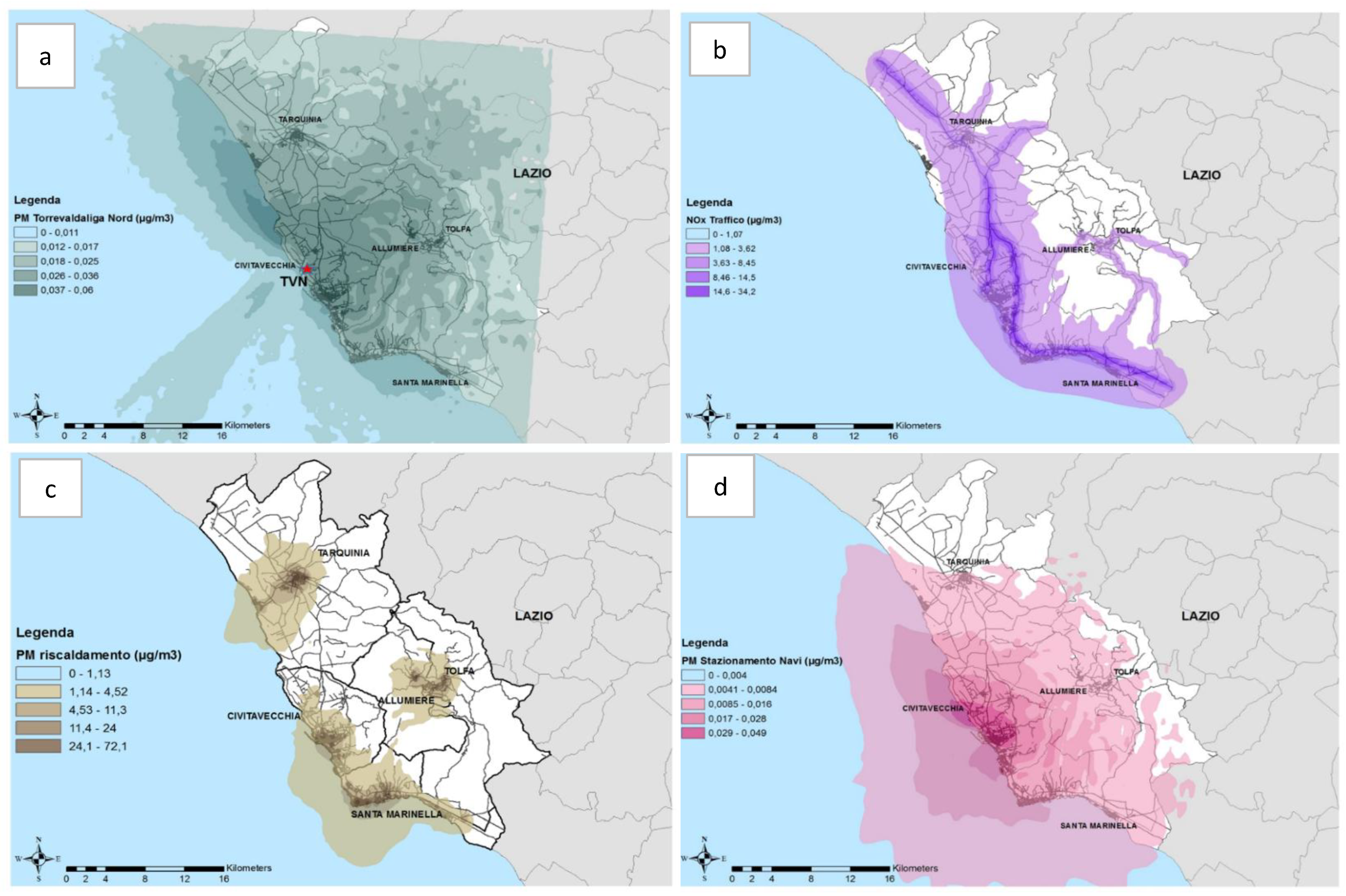
2.1. Industrial Sources
2.2. Study Population
2.3. Exposure Assessment
2.4. Clinical Examination and Lung Function Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent Advances in Particulate Matter and Nanoparticle Toxicology: A Review of the In Vivo and In Vitro Studies. BioMed Res. Int. 2013, 2013, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef]
- Abramson, M.J.; Matheson, M.; Wharton, C.; Sim, M. Prevalence of respiratory symptoms related to chronic obstructive pulmonary disease and asthma among middle aged and older adults. Respirology 2002, 7, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Dorans, K.; Gold, D.R.; Schwartz, J.; Koutrakis, P.; Washko, G.R.; O’Connor, G.T.; Mittleman, M.A. Long-Term Exposure to Traffic Emissions and Fine Particulate Matter and Lung Function Decline in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2015, 191, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Z.; Lau, A.K.H.; Lin, C.; Chuang, Y.C.; Chan, J.; Jiang, W.K.; Tam, T.; Yeoh, E.-K.; Chan, T.-C.; et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: A longitudinal, cohort study. Lancet Planet. Health 2018, 2, e114–e125. [Google Scholar] [CrossRef]
- Nuvolone, D.; Della Maggiore, R.; Maio, S.; Fresco, R.; Baldacci, S.; Carrozzi, L.; Pistelli, F.; Viegi, G. Geographical information system and environmental epidemiology: A cross-sectional spatial analysis of the effects of traffic-related air pollution on population respiratory health. Environ. Health 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Fan, L.; Zhou, Y.; Liu, Y.; Ma, J.; Yang, S.; Wang, B.; Xiao, L.; Ye, Z.; Shi, T.; et al. Personal exposure to PM2.5-bound polycyclic aromatic hydrocarbons and lung function alteration: Results of a panel study in China. Sci. Total Environ. 2019, 684, 458–465. [Google Scholar] [CrossRef]
- Rapiti, E.; Turi, E.; Forastiere, F.; Borgia, P.; Comba, P.; Perucci, C.A.; Axelson, O. A mortality cohort study of seamen in Italy. Am. J. Ind. Med. 1992, 21, 863–872. [Google Scholar] [CrossRef]
- Forastiere, F.; Pupp, N.; Magliola, E.; Valesini, S.; Tidei, F.; A Perucci, C. Respiratory cancer mortality among workers employed in thermoelectric power plants. Scand. J. Work. Environ. Health 1989, 15, 383–386. [Google Scholar] [CrossRef]
- Crosignani, P.; Forastiere, F.; Petrelli, G.; Merler, E.; Chellini, E.; Pupp, N.; Donelli, S.; Magarotto, G.; Rotondo, E.; Perucci, C.; et al. Malignant mesothelioma in thermoelectric power plant workers in Italy. Am. J. Ind. Med. 1995, 27, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, F.; Corbo, G.M.; Pistelli, R.; Michelozzi, P.; Agabiti, N.; Brancato, G.; Ciappi, G.; Perucci, C.A. Bronchial Responsiveness in Children Living in Areas with Different Air Pollution Levels. Arch. Environ. Health Int. J. 1994, 49, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fano, V.; Michelozzi, P.; Ancona, C.; Capon, A.; Forastiere, F.; Perucci, C. Occupational and environmental exposures and lung cancer in an industrialised area in Italy. Occup. Environ. Med. 2004, 61, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Bauleo, L.; Bucci, S.; Antonucci, C.; Sozzi, R.; Davoli, M.; Forastiere, F.; Ancona, C. Long-term exposure to air pollutants from multiple sources and mortality in an industrial area: A cohort study. Occup. Environ. Med. 2018, 76, 48–57. [Google Scholar] [CrossRef]
- Ancona, C.; Bauleo, L.; Biscotti, G.; Bocca, B.; Caimi, S.; Cruciani, F.; Di Lorenzo, S.; Petrolati, M.; Pino, A.; Piras, G.; et al. ABC Study Group. A survey on lifestyle and level of biomarkers of environmental exposure in residents in Civitavecchia (Italy). Ann. Ist. Super. Sanita 2016, 52, 488–494. [Google Scholar]
- Galassi, C.; Forastiere, F.; Biggeri, A.; Gabellini, C.; De Sario, M.; Ciccone, G.; Biocca, M.; Bisanti, L.; Collaborativo, G. SIDRIA second phase: Objectives, study design and methods. Epidemiol. Prev. 2005, 29, 9–13. [Google Scholar]
- Ozgen, S.; Caserini, S.; Galante, S.; Giugliano, M.; Angelino, E.; Marongiu, A.; Hugony, F.; Migliavacca, G.; Morreale, C. Emission factors from small scale appliances burning wood and pellets. Atmos. Environ. 2014, 94, 144–153. [Google Scholar] [CrossRef]
- Miller, M.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Paci, E.; Pigini, D.; Bauleo, L.; Ancona, C.; Forastiere, F.; Tranfo, G. Urinary Cotinine Concentration and Self-Reported Smoking Status in 1075 Subjects Living in Central Italy. Int. J. Environ. Res. Public Health 2018, 15, 804. [Google Scholar] [CrossRef]
- Zou, G.; Donner, A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat. Methods Med. Res. 2011, 22, 661–670. [Google Scholar] [CrossRef]
- Bauleo, L.; Ruggieri, F.; Bucci, S.; Pino, A.; Antonucci, C.; Bocca, B.; Caimi, S.; Alimonti, A.; Davoli, M.; Forastiere, F.; et al. Exposure assessment to air pollutants: Dispersion models versus human biomonitoring. Epidemiol. Prev. 2019, 43, 260–269. [Google Scholar] [PubMed]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Künzli, N.; Pérez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R. An Official American Thoracic Society Public Policy Statement: Novel Risk Factors and the Global Burden of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Chapman, R.S.; Hu, W.; Wei, F.; Korn, L.R.; Zhang, J. Using air pollution based community clusters to explore air pollution health effects in children. Environ. Int. 2004, 30, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Zemp, E.; Elsasser, S.; Schindler, C.; Künzli, N.; Perruchoud, A.P.; Domenighetti, G.; Medici, T.; Ackermann-Liebrich, U.; Leuenberger, P.; Monn, C.; et al. Long-Term Ambient Air Pollution and Respiratory Symptoms in Adults (SAPALDIA Study). Am. J. Respir. Crit. Care Med. 1999, 159, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Schikowski, T.; Adam, M.; Buschka, A.; Carsin, A.-E.; Jacquemin, B.; Marcon, A.; Sanchez, M.; Vierkötter, A.; Al-Kanaani, Z.; et al. Cross-sectional associations between air pollution and chronic bronchitis: An ESCAPE meta-analysis across five cohorts. Thorax 2014, 69, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Bentayeb, M.; Helmer, C.; Raherison, C.; Dartigues, J.F.; Tessier, J.-F.; Annesi-Maesano, I. Bronchitis-like symptoms and proximity air pollution in French elderly. Respir. Med. 2010, 104, 880–888. [Google Scholar] [CrossRef]
- Schindler, C.; Künzli, N.; Bongard, J.-P.; Leuenberger, P.; Karrer, W.; Rapp, R.; Monn, C.; Ackermann-Liebrich, U. The Swiss Study on Air Pollution and Lung Diseases In Adults Investigators Short-Term Variation in Air Pollution and in Average Lung Function Among Never-Smokers. Am. J. Respir. Crit. Care Med. 2001, 163, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Zhao, Z.; Mukherjee, B. Construction of environmental risk score beyond standard linear models using machine learning methods: Application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ. Health 2017, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Deng, F.; Hao, Y.; Shima, M.; Wang, X.; Zheng, C.; Wei, H.; Lv, H.; Lu, X.; Huang, J.; et al. Chemical constituents of fine particulate air pollution and pulmonary function in healthy adults: The Healthy Volunteer Natural Relocation study. J. Hazard. Mater. 2013, 260, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.; Kauri, L.; Cakmak, S.; Mahmud, M.; Weichenthal, S.; Van Ryswyk, K.; Kumarathasan, P.; Thomson, E.M.; Vincent, R.; Broad, G.; et al. Acute changes in lung function associated with proximity to a steel plant: A randomized study. Environ. Int. 2013, 55, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Olesen, H.R. Toward the Establishment of a Common Framework for Model Evaluation. In Air Pollution Modeling and Its Application XI; Springer Science and Business Media LLC: Berlin, Germany, 1996; pp. 519–528. [Google Scholar]
- Anfossi, D.; Tinarelli, G.; Nibart, M.; Olry, C.; Commanay, J.; Castelli, S.T. A new Lagrangian particle model for the simulation of dense gas dispersion. Atmos. Environ. 2010, 44, 753–762. [Google Scholar] [CrossRef]
- Panis, L.I.; Provost, E.B.; Cox, B.; Louwies, T.; Laeremans, M.; Standaert, A.; Dons, E.; Holmstock, L.; Nawrot, T.S.; De Boever, P. Short-term air pollution exposure decreases lung function: A repeated measures study in healthy adults. Environ. Health 2017, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, K.K.; Kostikas, K.; Kontakiotis, T.; Information, P.E.K.F.C. Techniques for assessing small airways function: Possible applications in asthma and COPD. Respir. Med. 2016, 119, e2–e9. [Google Scholar] [CrossRef] [PubMed]
- Berend, N. Contribution of air pollution to COPD and small airway dysfunction. Respirology 2015, 21, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Molnár, P.; Jonson, J.E.; Stockfelt, L. Impact on Population Health of Baltic Shipping Emissions. Int. J. Environ. Res. Public Health 2019, 16, 1954. [Google Scholar] [CrossRef]
- Chambers, L.; Finch, J.; Edwards, K.; Jeanjean, A.; Leigh, R.; Gonem, S. Effects of personal air pollution exposure on asthma symptoms, lung function and airway inflammation. Clin. Exp. Allergy 2018, 48, 798–805. [Google Scholar] [CrossRef]
- Clougherty, J.E. A growing role for gender analysis in air pollution epidemiology. Environ. Health Perspect. 2010, 118, 167–176. [Google Scholar] [CrossRef]
- Doiron, D.; De Hoogh, K.; Probst-Hensch, N.; Fortier, I.; Cai, Y.; De Matteis, S.; Hansell, A. Air pollution, lung function and COPD: Results from the population-based UK Biobank study. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- ISPRA—Istituto Superiore per la Protezione e la Ricerca Ambientale. Available online: https://www.isprambiente.gov.it (accessed on 26 May 2020).
- World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summery of Risk Assessment; World Health Organization: Geneva, Switzerland, 2005; pp. 1–22. [Google Scholar]
- Blanc, P.; Annesi-Maesano, I.; Balmes, J.R.; Cummings, K.J.; Fishwick, D.; Miedinger, D.; Murgia, N.; Naidoo, R.N.; Reynolds, C.J.; Sigsgaard, T.; et al. The Occupational Burden of Nonmalignant Respiratory Diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am. J. Respir. Crit. Care Med. 2019, 199, 1312–1334. [Google Scholar] [CrossRef]
- Wright, L.S.; Phipatanakul, W. Environmental remediation in the treatment of allergy and asthma: Latest updates. Curr. Allergy Asthma Rep. 2014, 14, 419. [Google Scholar] [CrossRef]
- Editorial- Pollution control up in the air. Lancet Respir. Med. 2015, 3, 87. [CrossRef]
| PM10 From Thermoelectric Power Plant | NOx From Urban Traffic | PM10 From Shipping Emissions | PM10 From Biomass Combustion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5th (<0.02) | 5°–95° (0.02–0.04) | >95th (>0.04) | <5th (1.89) | 5°–95° (1.89–16.58) | >95th (>16.58) | <5th (<0.00) | 5°–95° (0.00–0.04) | >95th (>0.04) | <5th (<1.65) | 5°–95° (1.65–33.98) | >95th (>33.98) | ||
| Total sample (%) | 1141 | 49 (4) | 1026 (90) | 66 (6) | 57 (5) | 1015 (89) | 69 (6) | 46 (4) | 1019 (89) | 76 (7) | 57 (5) | 1023 (90) | 61 (5) |
| Gender, % | Males | 34.7 | 42.9 | 39.4 | 38.6 | 42.9 | 37.7 | 41.3 | 42.7 | 38.2 | 49.1 | 42.0 | 41.0 |
| Females | 65.3 | 57.1 | 60.6 | 61.4 | 57.1 | 62.3 | 58.7 | 57.3 | 61.8 | 50.9 | 58.0 | 59.0 | |
| Age class, % | 35–44 | 22.4 | 22.4 | 21.2 | 17.5 | 22.3 | 27.5 | 17.4 | 22.3 | 26.3 | 21.1 | 22.7 | 18.0 |
| 45–54 | 36.7 | 30.3 | 27.3 | 33.3 | 30.0 | 33.3 | 21.7 | 30.9 | 28.9 | 29.8 | 29.9 | 39.3 | |
| 55–64 | 30.6 | 29.4 | 36.4 | 33.3 | 30.0 | 26.1 | 43.5 | 29.1 | 31.6 | 38.6 | 29.7 | 24.6 | |
| >= 65 | 10.2 | 17.8 | 15.2 | 15.8 | 17.7 | 13.0 | 17.4 | 17.7 | 13.2 | 10.5 | 17.7 | 18.0 | |
| Educational levels, % | None/elementary | 8.2 | 9.3 | 4.5 | 14.0 | 8.6 | 10.1 | 15.2 | 8.5 | 10.5 | 7.0 | 9.3 | 4.9 |
| Lower secondary school | 34.7 | 30.4 | 24.2 | 35.1 | 30.0 | 30.4 | 34.8 | 30.2 | 27.6 | 33.3 | 29.8 | 34.4 | |
| Upper secondary school | 40.8 | 51.1 | 60.6 | 43.9 | 51.3 | 55.1 | 41.3 | 51.6 | 51.3 | 52.6 | 51.2 | 49.2 | |
| Graduate/higher qualification | 16.3 | 9.3 | 10.6 | 7.0 | 10.1 | 4.3 | 8.7 | 9.6 | 10.5 | 7.0 | 9.7 | 11.5 | |
| Occupational status, % | Employed | 55.1 | 53.5 | 48.5 | 52.6 | 53.3 | 53.6 | 45.7 | 54.0 | 48.7 | 63.2 | 53.0 | 49.2 |
| Unemployed | 4.1 | 5.4 | 1.5 | 1.8 | 5.3 | 4.3 | 4.3 | 5.0 | 6.6 | 3.5 | 5.0 | 8.2 | |
| Housewife | 32.7 | 19.9 | 27.3 | 28.1 | 20.4 | 21.7 | 30.4 | 20.2 | 23.7 | 17.5 | 20.7 | 26.2 | |
| Retired/invalid | 8.2 | 21.2 | 22.7 | 17.5 | 21.0 | 20.3 | 19.6 | 20.8 | 21.1 | 15.8 | 21.3 | 16.4 | |
| Smoking status, % | Current smoker | 30.6 | 24.0 | 25.8 | 17.5 | 25.1 | 18.8 | 17.4 | 24.4 | 27.6 | 14.0 | 25.4 | 16.4 |
| Never-smoker | 46.9 | 39.2 | 39.4 | 56.1 | 38.3 | 43.5 | 41.3 | 39.7 | 35.5 | 40.4 | 39.6 | 37.7 | |
| Ex-smoker | 22.4 | 36.8 | 34.8 | 26.3 | 36.6 | 37.7 | 41.3 | 35.8 | 36.8 | 45.6 | 35.0 | 45.9 | |
| Body Mass Index, % | Underweight (<18.49) | 0.0 | 1.0 | 0.0 | 1.8 | 0.8 | 1.4 | 0.0 | 0.9 | 1.3 | 0.0 | 1.0 | 0.0 |
| Normal weight (18.5–24.99) | 26.5 | 32.8 | 28.8 | 31.6 | 32.2 | 34.8 | 21.7 | 32.7 | 34.2 | 21.1 | 32.7 | 36.1 | |
| Overweight (25–29.99) | 57.1 | 41.9 | 36.4 | 38.6 | 42.2 | 46.4 | 52.2 | 41.5 | 46.1 | 42.1 | 42.2 | 42.6 | |
| Obese (30–34.99) | 12.2 | 17.3 | 25.8 | 22.8 | 17.6 | 11.6 | 19.6 | 17.8 | 13.2 | 29.8 | 17.1 | 13.1 | |
| Severely obese (>34) | 4.1 | 7.0 | 9.1 | 5.3 | 7.2 | 5.8 | 6.5 | 7.2 | 5.3 | 7.0 | 6.9 | 8.2 | |
| Exposure to VGDF *at work, % | 12.2 | 24.5 | 19.7 | 28.1 | 23.3 | 26.1 | 23.9 | 23.7 | 22.4 | 36.8 | 23.5 | 14.8 | |
| Asthma, % | 4.1 | 8.6 | 12.1 | 5.3 | 8.7 | 10.1 | 6.5 | 8.3 | 13.2 | 10.5 | 8.0 | 16.4 | |
| Emphysema, % | 0.0 | 2.4 | 0.0 | 1.8 | 2.2 | 2.9 | 0.0 | 2.5 | 0.0 | 0.0 | 2.2 | 3.3 | |
| Chronic Bronchitis, % | 2.0 | 4.9 | 1.5 | 0.0 | 0.0 | 0.0 | 6.5 | 4.7 | 1.3 | 3.5 | 4.4 | 8.2 | |
| COPD #, % | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.2 | 0.0 | |
| PM10 from Thermoelectric Power Plant | NOx from Traffic | PM10 from Harbour | PM10 from Biomass combustion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5th (<0.02) | 5°–95° (0.02–0.04) | >95th (>0.04) | <5th (1.89) | 5°–95° (1.89–16.58) | >95th (>16.58) | <5th (<0.00) | 5°–95° (0.00–0.04) | >95th (>0.04) | <5th (<1.65) | 5°–95° (1.65–33.98) | >95th (>33.98) | ||
| Total Cohort, n | 1141 | 49 | 1026 | 66 | 57 | 1015 | 69 | 46 | 1019 | 76 | 57 | 1023 | 61 |
| Wheezing, % | 4.1 | 10.4 | 13.6 | 14.0 | 9.9 | 14.5 | 8.7 | 10.5 | 9.2 | 8.8 | 10.5 | 9.8 | |
| Dyspnea, % | 10.2 | 10.6 | 7.6 | 3.5 | 10.6 | 13.0 | 15.2 | 10.6 | 5.3 | 12.3 | 10.3 | 11.5 | |
| Cough with phlegm, % | 46.9 | 43.3 | 36.4 | 38.6 | 43.1 | 46.4 | 47.8 | 42.4 | 48.7 | 47.4 | 42.6 | 45.9 | |
| FVC, L (SD) | 3.81 (1.08) | 3.85 (0.99) | 3.83 (0.93) | 3.81 (0.89) | 3.85 (0.89) | 3.77 (1.02) | 3.86 (0.95) | 3.84 (1.00) | 3.86 (0.88) | 4.06 (1.07) | 3.83 (0.99) | 3.91 (0.97) | |
| FEV1, L, (SD) | 3.05 (0.84) | 3.05 (0.80) | 3.03 (0.72) | 3.05 (0.72) | 3.06 (0.80) | 2.96 (0.91) | 3.02 (0.70) | 3.05 (0.81) | 3.06 (0.75) | 3.19 (0.78) | 3.04 (0.80) | 3.10 (0.80) | |
| FEF25–75, L (SD) | 2.97 (0.96) | 2.97 (1.03) | 2.85 (0.88) | 2.98 (0.80) | 2.96 (1.02) | 2.93 (1.14) | 2.74 (0.78) | 2.97 (1.03) | 2.93 (1.01) | 3.00 (0.83) | 2.97 (1.03) | 2.82 (0.91) | |
| FEV1/FVC * (SD) | 80 (4.98) | 79.51 (7.79) | 79.37 (5.89) | 80.00 (7.40) | 79.56 (7.21) | 78.63 (12.13) | 78.65 (5.17) | 79.59 (7.70) | 79.36 (7.40) | 79.21 (6.06) | 79.57 (7.72) | 79.30 (6.62) | |
| PM10 from Thermoelectric Power Plant | NOx from Traffic | PM10 from Harbour | PM10 from Biomasscombustion | |
|---|---|---|---|---|
| OR, (95% CI) | OR, (95% CI) | OR, (95% CI) | OR, (95% CI) | |
| Wheezing a | 1.65, (0.66–4.10) | 0.86, (0.37–1.96) | 0.59, (0.26–1.29) | 0.53, (0.24–1.17) |
| Dyspnea a | 0.79, (0.32–1.94) | 1.25, (0.59–2.67) | 0.82, (0.37–1.81) | 0.80, (0.39–1.62) |
| Cough with phlegm a | 0.88, (0.66–1.17) | 1.08, (0.34–1.38) | 1.09, (0.85–1.41) | 1.13, (0.92–1.39) |
| PM10 from Thermoelectric Power Plant | NOx from Traffic | PM10 from Harbour | PM10 from Biomasscombustion | |
|---|---|---|---|---|
| β @, (95% CI) | β @, (95% CI) | β @, (95% CI) | β @, (95% CI) | |
| FEV1 a | 0.00, (−0.08. 0.09) | −0.06, (−0.16. 0.03) | −0.12 *, (−0.21. −0.02) | −0.02, (−0.08. 0.03) |
| FEV1/FVC a | −0.25, (−1.55. 0.04) | −0.07, (−1.54. 1.38) | −1.67 *, (−3.10. −0.23) | −0.59, (−1.49. 0.29) |
| FEF25–75 a | 0.00, (−0.08. 0.09) | −0.06, (−0.13. 0.03) | −0.12 *, (−0.21. −0.02) | −0.02, (−0.08. 0.03) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolocci, G.; Bauleo, L.; Folletti, I.; Murgia, N.; Muzi, G.; Ancona, C. Industrial Air Pollution and Respiratory Health Status among Residents in an Industrial Area in Central Italy. Int. J. Environ. Res. Public Health 2020, 17, 3795. https://doi.org/10.3390/ijerph17113795
Paolocci G, Bauleo L, Folletti I, Murgia N, Muzi G, Ancona C. Industrial Air Pollution and Respiratory Health Status among Residents in an Industrial Area in Central Italy. International Journal of Environmental Research and Public Health. 2020; 17(11):3795. https://doi.org/10.3390/ijerph17113795
Chicago/Turabian StylePaolocci, Giulia, Lisa Bauleo, Ilenia Folletti, Nicola Murgia, Giacomo Muzi, and Carla Ancona. 2020. "Industrial Air Pollution and Respiratory Health Status among Residents in an Industrial Area in Central Italy" International Journal of Environmental Research and Public Health 17, no. 11: 3795. https://doi.org/10.3390/ijerph17113795
APA StylePaolocci, G., Bauleo, L., Folletti, I., Murgia, N., Muzi, G., & Ancona, C. (2020). Industrial Air Pollution and Respiratory Health Status among Residents in an Industrial Area in Central Italy. International Journal of Environmental Research and Public Health, 17(11), 3795. https://doi.org/10.3390/ijerph17113795





