Avian Scavengers as Bioindicators of Antibiotic Resistance Due to Livestock Farming Intensification
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Areas
2.2. Fieldwork
2.3. Microflora Culture and Antibiotic Resistance Identification
2.4. Statistical Analyzes
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Angulo, F.J.; Nargund, V.N.; Chiller, T.C. Evidence of an association between use of anti-microbial agents in food animals and anti-microbial resistance among bacteria isolated from humans and the human health consequences of such resistance. J. Vet. Med. Ser. B 2004, 51, 374–379. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antibiotic resistance, and human health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef]
- Davis, M.F.; Price, L.B.; Liu, C.M.-H. An ecological perspective on U.S. industrial poultry production: The role of anthropogenic ecosystems on the emergence of drug-resistant bacteria from agricultural environments. Curr. Opin. Microbiol. 2011, 14, 244–250. [Google Scholar] [CrossRef]
- Koch, B.J.; Hungate, B.A.; Price, L.B. Food-animal production and the spread of antibiotic resistance: The role of ecology. Front. Ecol. Environ. 2017, 15, 309–318. [Google Scholar] [CrossRef]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Avizanda, A.; Blanco, G.; De Vault, T.L.; Markandya, A.; Virani, M.Z.; Donázar, J.A. Supplementary feeding and endangered avian scavengers: Benefits, caveats and controversies. Front. Ecol. Environ. 2016, 14, 191–199. [Google Scholar] [CrossRef]
- Plaza, P.I.; Blanco, G.; Madariaga, M.J.; Boeri, E.; Teijeiro, M.L.; Bianco, G.; Lambertucci, S.A. Scavenger birds exploiting rubbish dumps: Pathogens at the gates. Transbound. Emerg. Dis. 2019, 66, 873–881. [Google Scholar] [CrossRef]
- Stedt, J.; Bonnedahl, J.; Hernandez, J.; McMahon, B.J.; Hasan, B.; Olsen, B.; Drobni, M.; Waldenström, J. Antibiotic resistance patterns in Escherichia coli from gulls in nine European countries. Infect. Ecol. Epidemiol. 2014, 4, 21565. [Google Scholar] [CrossRef]
- Blanco, G. Supplementary feeding as a source of multiresistant Salmonella in endangered Egyptian vultures. Transbound. Emerg. Dis. 2018, 65, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Dolejska, M.; Literak, I. Wildlife is overlooked in the epidemiology of medically important antimicrobial resistant bacteria. Antimicrob. Agents Chemother. 2019, 63, e01167-19. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.B.; Zeng, Z.L.; Yang, X.W.; Huang, Y.; Liu, J.H. The role of wildlife (wild birds) in the global transmission of antibiotic resistance genes. Zool. Res. 2017, 38, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Ramey, A.M.; Ahlstrom, C.A. Antibiotic resistant bacteria in wildlife: Perspectives on trends, acquisition and dissemination, data gaps, and future directions. J. Wildl. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Junza, A.; Segarra, D.; Barbosa, J.; Barrón, D. Wildlife contamination with fluoroquinolones from livestock: Widespread prevalence of enrofloxacin and marbofloxacin in vultures. Chemosphere 2016, 144, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Junza, A.; Barrón, D. Food safety in scavenger conservation: Diet-associated exposure to livestock pharmaceuticals and opportunist mycoses in threatened cinereous and Egyptian vultures. Ecotoxicol. Environ. Saf. 2017, 135, 292–301. [Google Scholar] [CrossRef]
- Blanco, G.; Junza, A.; Barrón, D. Occurrence of veterinary pharmaceuticals in golden eagle nestlings: Unnoticed scavenging on livestock carcasses and other potential exposure routes. Sci. Total Environ. 2017, 586, 355–361. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Da Costa, P.M.; Loureiro, L.; Matos, A.J.F. Transfer of multidrug-resistant bacteria between intermingled ecological niches: The interface between humans, animals and the environment. Int. J. Environ. Res. Public Health 2013, 10, 278–294. [Google Scholar] [CrossRef]
- Blyton, M.D.J.; Pi, H.; Vangchhia, B.; Abraham, S.; Trott, D.J.; Johnson, J.R.; Gordon, D.M. The genetic structure and antimicrobial resistance of Escherichia coli and cryptic clades in birds with diverse human associations. Appl. Environ. Microbiol. 2015, 81, 5123–5133. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.M.; Ferree, E.; Simon, D.M.; Yeh, P.J. Patterns of bird–bacteria associations. EcoHealth 2018, 15, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, P.; Jiménez-Montalbán, P.J.; Delgado, D.; Martínez-López, E.; María-Mojica, P.; Godino, A.; García-Fernández, A.J. Development of a QuEChERS method for simultaneous analysis of antibiotics in carcasses for supplementary feeding of endangered vultures. Sci. Total Environ. 2018, 626, 319–327. [Google Scholar] [CrossRef]
- Blanco, G.; Cortés-Avizanda, A.; Frías, Ó.; Arrondo, E.; Donázar, J.A. Livestock farming practices modulate vulture diet-disease interactions. Glob. Ecol. Conserv. 2019, 17, e00518. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, 1002158. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist. Updates 2012, 15, 162–172. [Google Scholar] [CrossRef]
- Salyers, A.A.; Whitt, D.D. Revenge of the Microbes: How Bacterial Resistance is Undermining the Antibiotic Miracle; American Society for Microbiology Press: Washington, DC, USA, 2005. [Google Scholar]
- Margalida, A.; Sánchez-Zapata, J.A.; Blanco, G.; Hiraldo, F.; Donázar, J.A. Diclofenac approval as a threat to Spanish vultures. Conserv. Biol. 2014, 28, 631. [Google Scholar] [CrossRef]
- Pitarch, A.; Gil, C.; Blanco, G. Oral mycoses in avian scavengers exposed to antibiotics from livestock farming. Sci. Total Environ. 2017, 605, 139–146. [Google Scholar] [CrossRef]
- Pitarch, A.; Gil, C.; Blanco, G. Vultures from different trophic guilds show distinct oral pathogenic yeast signatures and co-occurrence networks. Sci. Total Environ. 2020, 723, 138–166. [Google Scholar] [CrossRef]
- Blanco, G. Can livestock carrion availability influence diet of wintering red kites? Implications of sanitary policies in ecosystem services and conservation. Popul. Ecol. 2014, 56, 593–604. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Gómara, B.; González, M.J.; Baos, R.; Hiraldo, F.; Abad, E.; Rivera, J.; Jiménez, B. Unexpected high PCB and total DDT levels in the breeding population of red kite (Milvus milvus) from Doñana National Park, south-western Spain. Environ. Int. 2008, 34, 73–78. [Google Scholar] [CrossRef]
- Tenan, S.; Adrover, J.; Muñoz-Navarro, A.; Sergio, F.; Tavecchia, G. Demographic consequences of poison-related mortality in a threatened bird of prey. PLoS ONE 2012, 7, e49187. [Google Scholar] [CrossRef]
- Blanco, G.; Cardells, J.; Garijo-Toledo, M.M. Supplementary feeding and endoparasites in threatened avian scavengers: Coprologic evidence from red kites in their wintering stronghold. Environ. Res. 2017, 155, 22–30. [Google Scholar] [CrossRef]
- Sergio, F.; Tanferna, A.; Chicano, J.; Blas, J.; Tavecchia, G.; Hiraldo, F. Protected areas under pressure: Decline, redistribution, local eradication and projected extinction of a threatened predator, the red kite, in Doñana National Park, Spain. Endanger. Species Res. 2019, 38, 189–204. [Google Scholar] [CrossRef]
- Diputación de Segovia. Propuestas del Plan Provincial de Gestión de Residuos Ganaderos de Segovia; Diputación Provincial de Segovia: Segovia, Spain, 2006. [Google Scholar]
- Blanco, G. Influence of diet on the gastrointestinal flora of wintering Red Kites. Eur. J. Wildl. Res. 2014, 60, 695–698. [Google Scholar] [CrossRef][Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: 18th Informational Supplement; CLSI Document M100–S18; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows (Version 25); IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- SAS Institute Inc. JMP (Version 12); SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2017; EMA/294674/2019; EMA: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sandegren, L.; Stedt, J.; Lustig, U.; Bonnedahl, J.; Andersson, D.I.; Järhult, J.D. Long-term carriage and rapid transmission of extended spectrum beta-lactamase-producing E. coli within a flock of Mallards in the absence of antibiotic selection. Environ. Microbiol. Rep. 2018, 10, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jia, A.; Wan, Y.; Liu, H.; Wang, K.; Peng, H.; Dong, Z.; Hu, J. Occurrences of three classes of antibiotics in a natural river basin: Association with antibiotic-resistant Escherichia coli. Environ. Sci. Technol. 2014, 48, 14317–14325. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, Y.; Zarazaga, M.; Briñas, L.; Lantero, M.; Ruiz-Larrea, F.; Torres, C. Antibiotic resistance in Escherichia coli isolates obtained from animals, foods and humans in Spain. Int. J. Antimicrob. Agents 2001, 18, 353–358. [Google Scholar] [CrossRef]
- Mesa, R.J.; Blanc, V.; Blanch, A.R.; Cortés, P.; González, J.J.; Lavilla, S.; Miró, E.; Muniesa, M.; Saco, M.; Tórtola, M.T.; et al. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 2006, 58, 211–215. [Google Scholar] [CrossRef]
- Blanco, G. Multiresistant Salmonella serovar Typhimurium monophasic in wintering red kites Milvus milvus in Segovia, central Spain. J. Raptor Res. 2015, 49, 339–341. [Google Scholar] [CrossRef]
- Blanco, G.; Díaz de Tuesta, J.A. Culture-and molecular-based detection of swine-adapted Salmonella shed by avian scavengers. Sci. Total Environ. 2018, 634, 1513–1518. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.; Dantas, G. The shared antibiotic resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef]
- Arnold, K.E.; Williams, N.J.; Bennett, M. ‘Disperse abroad in the land’: The role of wildlife in the dissemination of antimicrobial resistance. Biol. Lett. 2016, 12, 20160137. [Google Scholar] [CrossRef]
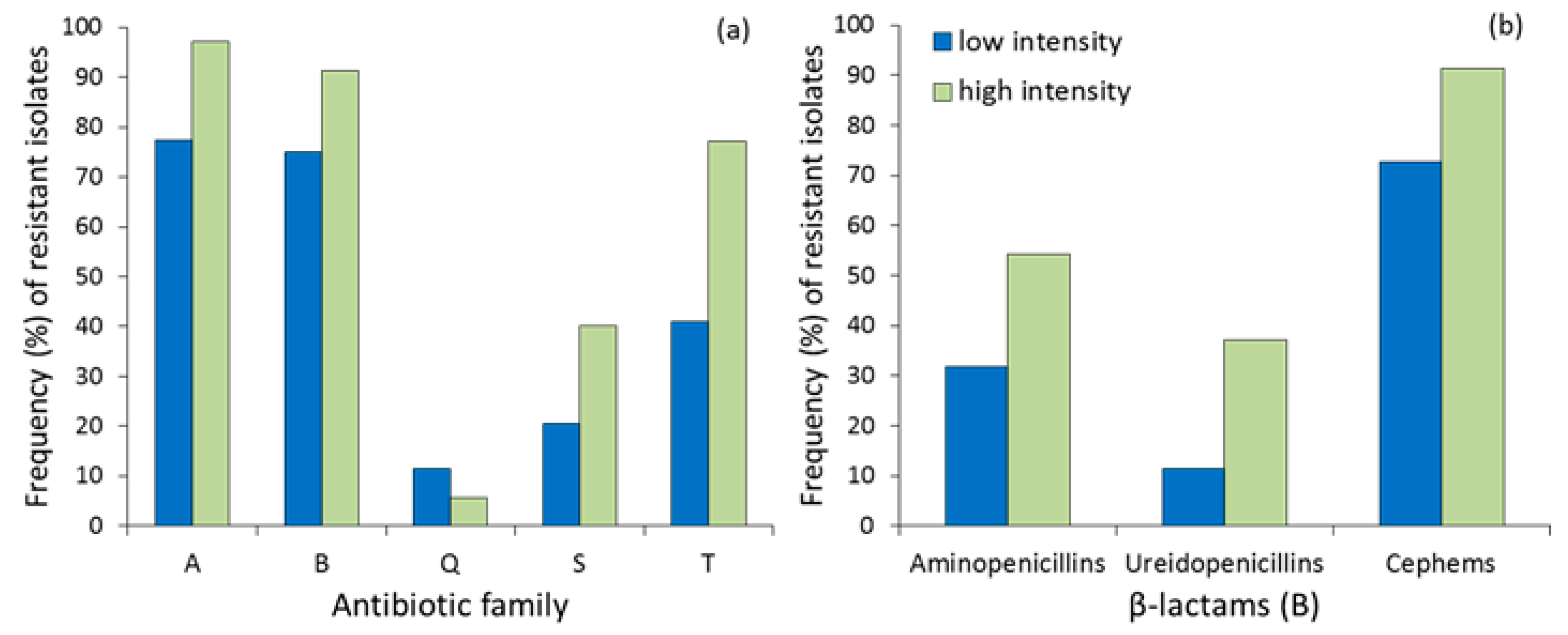
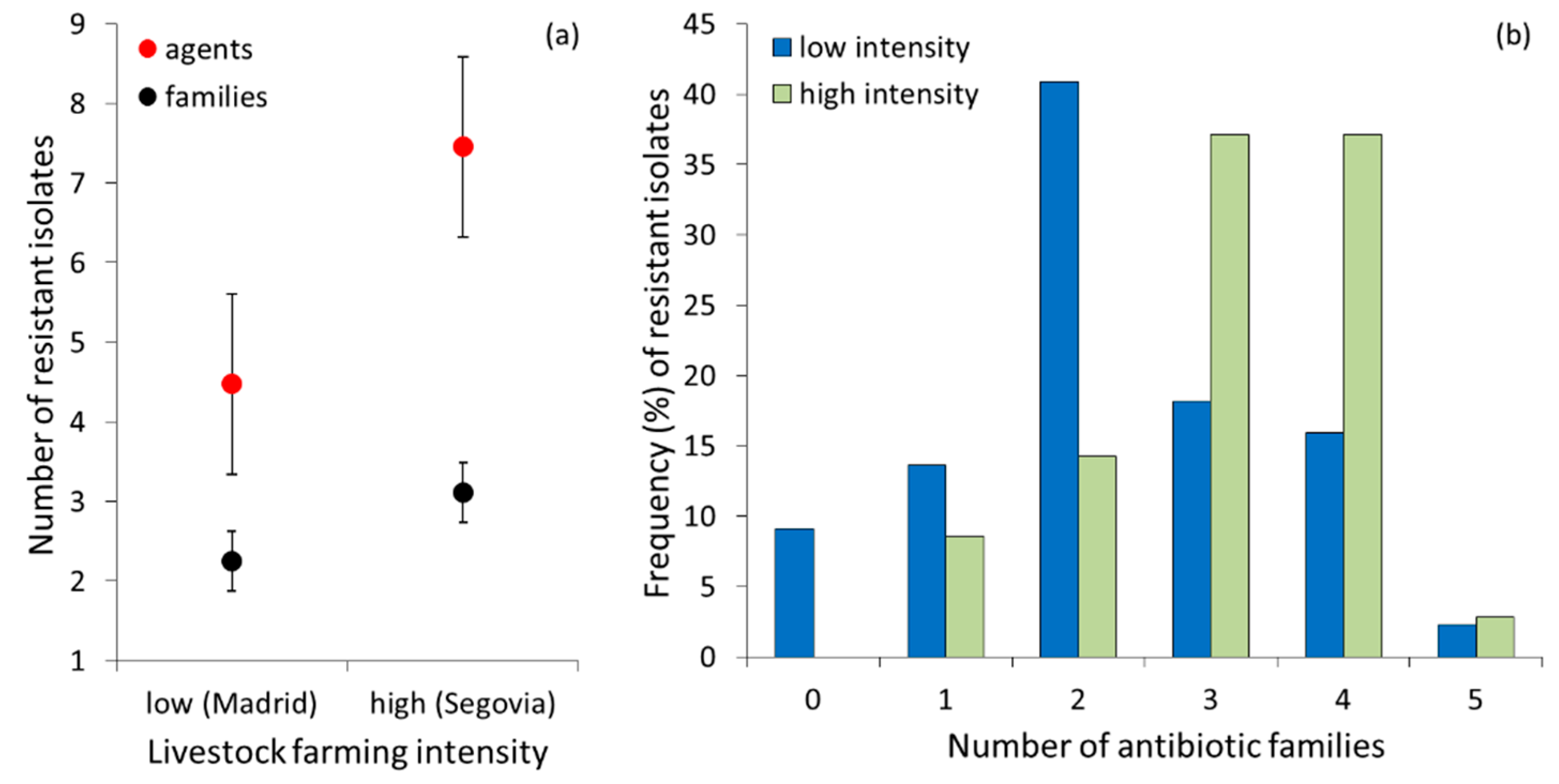
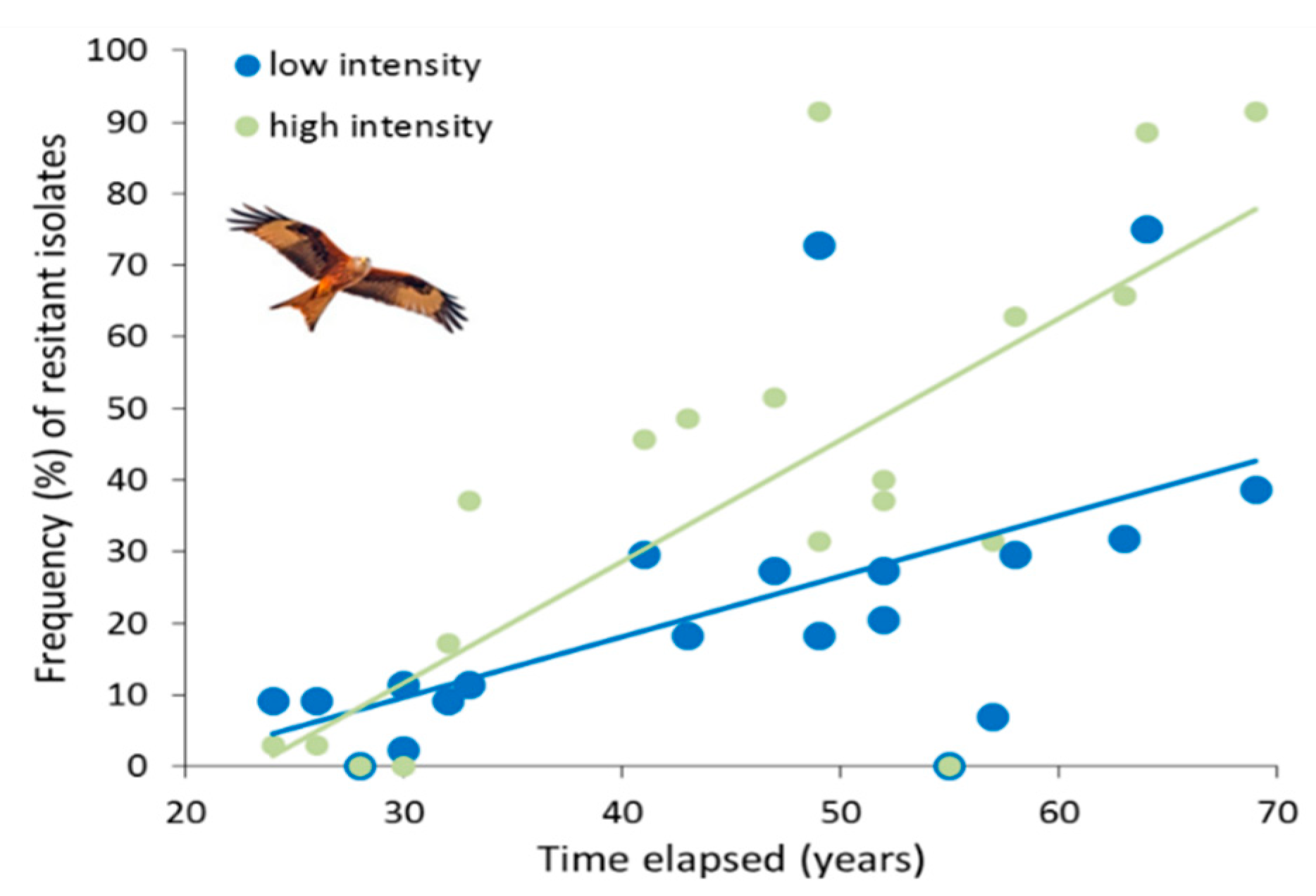
| Antibiotics | Livestock Intensification | ||||
|---|---|---|---|---|---|
| Family (Acronym) Subfamily | Agent | Low Madrid (n = 44) | High Segovia (n = 35) | Fisher Exact Test | |
| No. resistant (%) | No. resistant (%) | p | RR (95% CI) | ||
| Aminoglycosides (A) | Gentamycin | 8 (18.18) | 11 (31.43) | 0.194 | 2.063 (0.724−5.876) |
| Kanamycin | 3 (6.82) | 11 (31.43) | 0.007 | 6.264 (1.588−24.710) | |
| Streptomycin | 17 (38.64) | 32 (91.43) | <0.0001 | 16.941 (4.481−64.052) | |
| Neomycin | 33 (75.00) | 31 (88.57) | 0.156 | 2.583 (0.744−8.971) | |
| β-lactams (B) | |||||
| Aminopenicillins | Amoxicillin | 13 (29.55) | 16 (45.71) | 0.163 | 2.008 (0.794−5.081) |
| Amoxicillin/clavulanic | 4 (9.09) | 6 (17.14) | 0.325 | 2.069 (0.535−8.000) | |
| Ampicillin | 12 (27.27) | 13 (37.14) | 0.466 | 1.576 (0.607−4.091) | |
| Ureidopenicillins | Piperacillin | 5 (11.36) | 13 (37.14) | 0.014 | 4.609 (1.450−14.648) |
| Cephalosporins | Cephalothin | 32 (72.73) | 32 (91.43) | 0.045 | 4.000 (1.030−15.534) |
| Cephalexin | 8 (18.18) | 17 (48.57) | 0.007 | 4.250 (1.543−11.704) | |
| Ceftazidime | 1 (2.27) | 0 (0.00) | 1.000 | 0.551 (0.451−0.673) | |
| Carbapenems | Imipenem | 0 (0.00) | 0 (0.00) | – | – |
| Polypeptides (P) | Colistin | 0 (0.00) | 0 (0.00) | – | – |
| Quinolones (Q) | Norfloxacin | 5 (11.36) | 0 (0.00) | 0.063 | 0.527 (0.425−0.654) |
| Ciprofloxacin | 4 (9.09) | 1 (2.86) | 0.376 | 0.294 (0.031−2.759) | |
| Enrofloxacin | 4 (9.09) | 1 (2.86) | 0.376 | 0.294 (0.031−2.759) | |
| Sulfonamides (S) | Sulfamethoxazole–trimethoprim | 9 (20.45) | 14 (40.00) | 0.081 | 2.593 (0.957−7.026) |
| Tetracyclines (T) | Tetracycline | 13 (29.55) | 22 (62.86) | 0.006 | 4.036 (1.571−10.363) |
| Oxytetracycline | 14 (31.82) | 23 (65.71) | 0.003 | 4.107 (1.599−10.548) | |
| Doxycycline | 12 (27.27) | 18 (51.43) | 0.037 | 2.824 (1.105−7.213) | |
| Livestock Intensification | |||
|---|---|---|---|
| Resistance Pattern by Antibiotic Family a | Low (Madrid) No. (%) | High (Segovia) No. (%) | Total No. (%) |
| Susceptible | 4 (9.09) | – | 4 (5.06) |
| A | 1 (2.27) | 3 (8.57) | 4 (5.06) |
| B | 4 (9.09) | – | 4 (5.06) |
| T | 1 (2.27) | – | 1 (1.27) |
| A-B | 13 (29.55) | 5 (14.29) | 18 (22.78) |
| A-Q | 1 (2.27) | – | 1 (1.27) |
| A-S | 1 (2.27) | – | 1 (1.27) |
| A-T | 2 (4.55) | – | 2 (2.53) |
| B-T | 1 (2.27) | – | 1 (1.27) |
| A-B-Q | 1 (2.27) | – | 1 (1.27) |
| A-B-S | 1 (2.27) | – | 1 (1.27) |
| A-B-T | 6 (13.64) | 12 (34.29) | 18 (22.78) |
| B-S-T | – | 1 (2.86) | 1 (1.27) |
| A-B-Q-T | 1 (2.27) | 1 (2.86) | 2 (2.53) |
| A-B-S-T | 5 (11.36) | 12 (34.29) | 17 (21.52) |
| A-Q-S-T | 1 (2.27) | – | 1 (1.27) |
| A-B-Q-S-T | 1 (2.27) | 1 (2.86) | 2 (2.53) |
| Total | 44 (100) | 35 (100) | 79 (100) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, G.; Bautista, L.M. Avian Scavengers as Bioindicators of Antibiotic Resistance Due to Livestock Farming Intensification. Int. J. Environ. Res. Public Health 2020, 17, 3620. https://doi.org/10.3390/ijerph17103620
Blanco G, Bautista LM. Avian Scavengers as Bioindicators of Antibiotic Resistance Due to Livestock Farming Intensification. International Journal of Environmental Research and Public Health. 2020; 17(10):3620. https://doi.org/10.3390/ijerph17103620
Chicago/Turabian StyleBlanco, Guillermo, and Luis M. Bautista. 2020. "Avian Scavengers as Bioindicators of Antibiotic Resistance Due to Livestock Farming Intensification" International Journal of Environmental Research and Public Health 17, no. 10: 3620. https://doi.org/10.3390/ijerph17103620
APA StyleBlanco, G., & Bautista, L. M. (2020). Avian Scavengers as Bioindicators of Antibiotic Resistance Due to Livestock Farming Intensification. International Journal of Environmental Research and Public Health, 17(10), 3620. https://doi.org/10.3390/ijerph17103620






