Prevalence of Comorbidities in Individuals Diagnosed and Undiagnosed with Alzheimer’s Disease in León, Spain and a Proposal for Contingency Procedures to Follow in the Case of Emergencies Involving People with Alzheimer’s Disease
Abstract
1. Introduction
- Failures or memory loss, which make everyday activities difficult;
- Difficulty to face and/or solve problems;
- Disorientation;
- Difficulty understanding visual images and spoken language;
- Problems with oral and/or written language;
- Placing objects out of place;
- Diminished and absent capacity of judgment;
- Loss of initiative;
- Personality changes, including apathy and depression;
- Higher anxiety levels, restlessness, and sleep disorders;
- Development of a state of increased dependency.
1.1. Pathophysiology of AD
- Amyloid theory The essential element of extracellular deposits is the protein β-amyloid, which forms fibrils that aggregate and cause the development of diffuse and neuritic plaques. The β-amyloid protein is produced by an abnormal cleavage of the amyloid precursor protein (APP). Normally, the product of secretase α action is a soluble peptide that can be easily removed from the body. In AD, the cleavage is performed by β- and γ-secretases producing insoluble peptides that are removed from neurons. Microglial cells unsuccessfully attempt their removal, and this results in inflammation and nerve damage.
- Tau protein theory The tau protein is the main component of intracellular deposits in neurons. It is a microtubule-associated protein, with microtubules being cytoplasmic structures involved in the assembly and function of the cytoskeletal network of cells including neurons. Tau acts as a microtubule stabilizer. In AD, Tau hyper-phosphorylation prevents its binding to tubulin and results in autoaggregation and formation of neurotoxic intraneuronal precipitates.
- Cholinergic theory A decrease in the levels of the neurotransmitter acetylcholine in patients with AD causes a diminished performance of neural connections.
1.2. Risk and Protective Factors
- Non-modifiable factors include age, gender, genetics-related (karyotype alterations, gene mutations, etc.), parental education, family background;
- Modifiable factors include low educational or socioeconomic level, obesity, type II diabetes mellitus, cardiovascular diseases (hypertension, atherosclerosis, heart disease, atrial fibrillation, and dyslipidemia), smoking, stroke, depression, alcohol abuse, and pneumonia;
- Environmental factors [24] include aluminum, pesticides, pollution.
- Protective factors [21]
- physical, educational, intellectual and social activities, moderate consumption of alcoholic beverages, and a Mediterranean diet.
- To compare the sociodemographic characteristics of the individuals in the two populations under scrutiny;
- To establish and compare the comorbidities associated with the individuals of each of the two populations;
- To know, if any, the protocols of action employed by the Alzheimer Center of León or the León University Hospital in emergency situations concerning people with AD.
2. Materials and Methods
2.1. Population Study
2.2. Literature Search
- Publications from 2014 to 2019;
- Publications in English or Spanish;
- Data concerning the adult population aged ≥65 years diagnosed with AD;
- Both primary (original articles) and secondary (systematic reviews) sources;
- Full texts accessible through the University of León Library;
- Keywords employed (combined with Boolean operators AND and OR), in English included Alzheimer’s disease, comorbidity, elderly, and aged and, in Spanish, “Enfermedad de Alzheimer”, “comorbilidad”, “adulto mayor”, and “anciano”.
2.3. Data Collection
- Alzheimer’s disease diagnosis (this diagnosis has been previously identified by the Psychiatric Service of the León University Hospital);
- Age ≥65 years;
- Residency in the city of León, Spain;
- Not diagnosed with AD;
- Age ≥65 years;
- Residency in the city of León, Spain.
2.3.1. Alzheimer’s Disease (AD) Group
2.3.2. Control Group
2.3.3. Data Processing
2.4. Significance Studies
3. Results
3.1. Sociodemographics
3.1.1. Gender Distribution
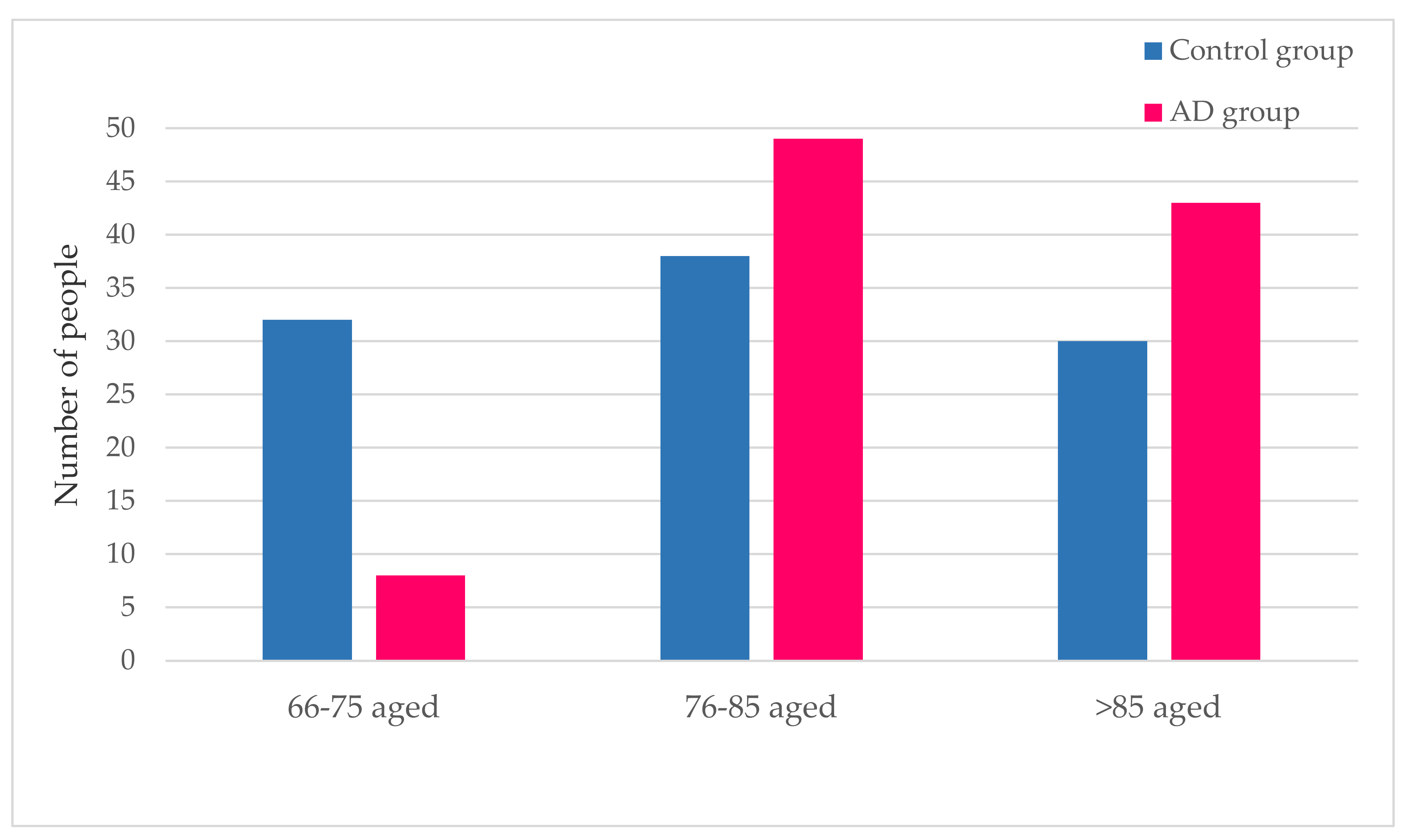
3.1.2. Age Distribution
3.2. Pathologies
3.2.1. Pathologies with a Higher Incidence in the AD Group
3.2.2. Pathologies with a Lower Incidence in the AD Group
3.2.3. Pathologies with a Similar Incidence in both Populations
3.3. Medication
4. Operating Procedures in the Case of Emergencies
Procedures in the Event of a Fall
5. Discussion
6. Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Custodio, N.; Montesinos, R. Enfermedad de Alzheimer Conociendo a la Enfermedad, que Llegó para Quedarse; 2015; p. 274. Available online: https://www.alzheimeruniversal.eu/wp-content/uploads/2015/09/libroenfermedaddealzheimer-150925020156-lva1-app7263.pdf (accessed on 23 April 2020).
- Valenti, R.; Pantoni, L.; Markus, H.S. Treatment of vascular risk factors in patients with a diagnosis of Alzheimer’s disease: A systematic review. BMC Med. 2014, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Mattsson-Carlgren, N.; Weiner, M.W.; Initiative, A.D.N. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimer’s Dement. 2014, 10, S122–S145. [Google Scholar] [CrossRef]
- Alzheimer’s Association; Chételat, G.; Villemagne, V.; Villain, N.; Jones, G.; Ellis, K.; Ames, D.; Martins, R.; Head, R.; Masters, C.; et al. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2011, 7, 208–244. [Google Scholar] [CrossRef]
- Miranda, A.; Gómez-Gaete, C.; Mennickent, S. Dieta mediterránea y sus efectos benéficos en la prevención de la enfermedad de Alzheimer. Revista Médica Chile 2017, 145, 501–507. [Google Scholar] [CrossRef]
- Cabrera-Pivaral, C.E.; Báez-Báez, M.G.L.; Rosa, A.D.J.C.-D.L. Mortalidad por enfermedad de Alzheimer en México de 1980 a 2014. Gac. Med. Mex 2018, 154, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.M.; Tarasov, V.V.; Makhmutovа, A.; Chubarev, V.N.; Avila-Rodriguez, M.; Bachurin, S.O.; Aliev, G. The Possibility of an Infectious Etiology of Alzheimer Disease. Mol. Neurobiol. 2018, 56, 4479–4491. [Google Scholar] [CrossRef]
- Mancino, R.; Martucci, A.; Cesareo, M.; Giannini, C.; Corasaniti, M.T.; Bagetta, G.; Nucci, C. Glaucoma and Alzheimer Disease: One Age-Related Neurodegenerative Disease of the Brain. Curr. Neuropharmacol. 2018, 16, 971–977. [Google Scholar] [CrossRef]
- Alzheimer’s Association 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [CrossRef]
- Garcez, M.L.; Falchetti, A.C.B.; Mina, F.; Budni, J. Alzheimer´s Disease associated with Psychiatric Comorbidities. Anais Academia Brasileira Ciências 2015, 87, 1461–1473. [Google Scholar] [CrossRef]
- Haaksma, M.L.; Vilela, L.R.; Marengoni, A.; Calderón-Larrañaga, A.; Leoutsakos, J.-M.S.; Rikkert, M.G.M.O.; Melis, R.J.F. Comorbidity and progression of late onset Alzheimer’s disease: A systematic review. PLoS ONE 2017, 12, e0177044. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Roy, A.; Shalat, S.L.; Von Stein, R.T.; Hossain, M.M.; Buckley, B.; Gearing, M.; Levey, A.I.; German, D.C. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 2014, 71, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, C.M.; Mimenza, A.J.; Navarro, S.A.; Ávila, P.A.; Gutiérrez, L.G.; Arellano, S.J.; Avila-Funes, J.A. Factores asociados a la demencia mixta en comparación con demencia tipo Alzheimer en adultos mayores mexicanos. Neurología 2017, 32, 309–315. [Google Scholar] [CrossRef]
- Monzani, F.; Pasqualetti, G.; Tognini, S.; Calsolaro, V.; Polini, A. Potential drug–drug interactions in Alzheimer patients with behavioral symptoms. Clin. Interv. Aging 2015, 10, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, D.; Pekel, N.B.; Kiliç, A.K.; Tolgay, E.N.; Tufan, F. Malnutrition is associated with dementia severity and geriatric syndromes in patients with Alzheimer disease. Turk. J. Med. Sci. 2015, 45, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialog Clin. Neurosci. 2016, 18, 437–446. [Google Scholar]
- Borrell, F. Enfermedad de Alzheimer y Factores de Riesgo Ambientales|Armenteros Borrell|Revista Cubana de Enfermería. Available online: http://www.revenfermeria.sld.cu/index.php/enf/article/view/1024/239 (accessed on 21 April 2020).
- Oosterveld, S.M.; Kessels, R.P.; Hamel, R.; Ramakers, I.H.G.B.; Aalten, P.; Verhey, F.R.J.; Sistermans, N.; Smits, L.L.; Pijnenburg, Y.A.; Van Der Flier, W.M.; et al. The Influence of Co-Morbidity and Frailty on the Clinical Manifestation of Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 42, 501–509. [Google Scholar] [CrossRef]
- Marfany, A.; Sierra, C.; Camafort, M.; Domenech, M.; Coca, A. High blood pressure, Alzheimer disease and antihypertensive treatment. Panminerva Med. 2018, 60, 8–16. [Google Scholar]
- Muñoz, C.G.; Navarro, N.G. Manejo de pacientes con enfermedad de Alzheimer: ¿cambio en el paradigma actual? Revista Científica Sociedad Española Enfermería Neurológica 2017, 45, 30–31. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29763097/ (accessed on 21 April 2020).
- Attems, J.; Jellinger, K.A. The overlap between vascular disease and Alzheimer’s disease–lessons from pathology. BMC Med. 2014, 12, 206. [Google Scholar] [CrossRef]
- Culqui, D.R.; Linares, C.; Ortiz, C.; Carmona, R.; Diaz, J. Association between environmental factors and emergency hospital admissions due to Alzheimer’s disease in Madrid. Sci. Total. Environ. 2017, 592, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Malek-Ahmadi, M.H.; Monsell, S.E.; Kukull, W.A.; Woodruff, B.K.; Reiman, E.M.; Beach, T.G.; Wilson, J. A Cross-Sectional Analysis of Late-Life Cardiovascular Factors and Their Relation to Clinically Defined Neurodegenerative Diseases. Alzheimer Dis. Assoc. Disord. 2016, 30, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.K.; Tierney, M.C. The puzzle of sex, gender and Alzheimer’s disease: Why are women more often affected than men? Women’s Health 2018, 14, 174550651881799. [Google Scholar] [CrossRef]
- Nucera, A.; Hachinski, V. Cerebrovascular and Alzheimer disease: Fellow travelers or partners in crime? J. Neurochem. 2018, 144, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Sachs, M.C.; Zhou, Y.; Monsell, S.E.; Saver, J.L.; Vinters, H.V. Clinical predictors of severe cerebral amyloid angiopathy and influence of APOE genotype in persons with pathologically verified Alzheimer disease. JAMA Neurol. 2014, 71, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Campdelacreu, J. Enfermedad de Parkinson y enfermedad de Alzheimer: Factores de riesgo ambientales. Neurología 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Domínguez, R.; Pagano, M.; Marschoff, E.; González, S.; Repetto, M.; Serra, J. Enfermedad de Alzheimer y deterioro cognitivo asociado a la diabetes mellitus de tipo 2: Relaciones e hipótesis. Neurología 2014, 29, 567–572. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Beiser, A.; Himali, J.J.; Seshadri, S.; O’Donnell, C.J.; Manning, W.J.; Wolf, P.A.; Au, R.; Benjamin, E.J. Low cardiac index is associated with incident dementia and Alzheimer disease: The Framingham Heart Study. Circulation 2015, 131, 1333–1339. [Google Scholar] [CrossRef]
- Cunningham, J.B.; McCrum-Gardner, E. Power, effect and sample size using GPower: Practical issues for researchers and members of research ethics committees Joseph. Evid. Based Midwifery 2007, 5, 132. [Google Scholar]
- Alzheimer León Alza su Voz el 21 de Septiembre|Leonoticias. Available online: https://www.leonoticias.com/leon/alzheimer-leon-alza-20180915185052-nt.html (accessed on 21 April 2020).
- Escala de Downton—Enfermería Creativa. Available online: https://enfermeriacreativa.com/2019/07/08/escala-de-downton/ (accessed on 22 April 2020).
- Xu, X.; Zou, J.; Geng, W.; Wang, A. Association between glaucoma and the risk of Alzheimer’s disease: A systematic review of observational studies. Acta Ophthalmol. 2019, 97, 665–671. [Google Scholar] [CrossRef]
- Ehrenberg, A.J.; Suemoto, C.K.; Resende, E.D.P.F.; Petersen, C.; Leite, R.E.P.; Rodriguez, R.D.; Ferretti-Rebustini, R.E.D.L.; You, M.; Oh, J.; Nitrini, R.; et al. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 66, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.; Kang, J.-H.; Lin, H.-C.; Huang, C.-C.; Lee, H.-C.; Chung, S.-D. Rheumatoid Arthritis Was Negatively Associated with Alzheimer’s Disease: A Population-Based Case-Control Study. PLoS ONE 2016, 11, e0168106. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V. The convergence of stroke and dementia TT—A convergência do acidente vascular cerebral e da demência. Arq. Neuropsiquiatr. 2018, 76, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Dubreuil, M.; Zhang, Y.; Neogi, T.; Rai, S.K.; Ascherio, A.; Hernan, M.; Choi, H.K. Gout and the risk of Alzheimer’s disease: A population-based, BMI-matched cohort study. Ann. Rheum. Dis. 2015, 75, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Washida, K. Linking Atrial Fibrillation with Alzheimer’s Disease: Epidemiological, Pathological, and Mechanistic Evidence. J. Alzheimer’s Dis. 2018, 62, 61–72. [Google Scholar] [CrossRef]
- Annweiler, C.; Dursun, E.; Féron, F.; Gezen-Ak, D.; Kalueff, A.V.; Littlejohns, T.; Llewellyn, D.J.; Millet, P.; Scott, T.; Tucker, K.L.; et al. ‘Vitamin D and cognition in older adults’: Updated international recommendations. J. Intern. Med. 2014, 277, 45–57. [Google Scholar] [CrossRef]
- Lo, R.Y.; Chen, Y.-H. Alzheimer’s disease and osteoporosis. Tzu Chi Med. J. 2017, 29, 138–142. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Li, C.-C.; Juan, Y.-S.; Chang, Y.-H.; Yeh, H.-C.; Tsai, C.-C.; Chueh, K.-S.; Wu, W.-J.; Yang, Y.-H. Urinary Incontinence in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dementiasr 2016, 32, 51–55. [Google Scholar] [CrossRef]
- Swords, G.; Nguyen, L.; Mudar, R.A.; Llano, D.A. Auditory system dysfunction in Alzheimer disease and its prodromal states: A review. Ageing Res. Rev. 2018, 44, 49–59. [Google Scholar] [CrossRef]
- Rouch, I.; Dorey, J.-M.; Boublay, N.; Henaff, M.-A.; Dibie-Racoupeau, F.; Makaroff, Z.; Harston, S.; Benoit, M.; Barrellon, M.-O.; Fédérico, D.; et al. Personality, Alzheimer’s disease and behavioural and cognitive symptoms of dementia: The PACO prospective cohort study protocol. BMC Geriatr. 2014, 14, 110. [Google Scholar] [CrossRef]
- Verbrugge, L.M. Women, men, and osteoarthritis. Arthritis Rheum. 1995, 8, 212–220. [Google Scholar] [CrossRef] [PubMed]
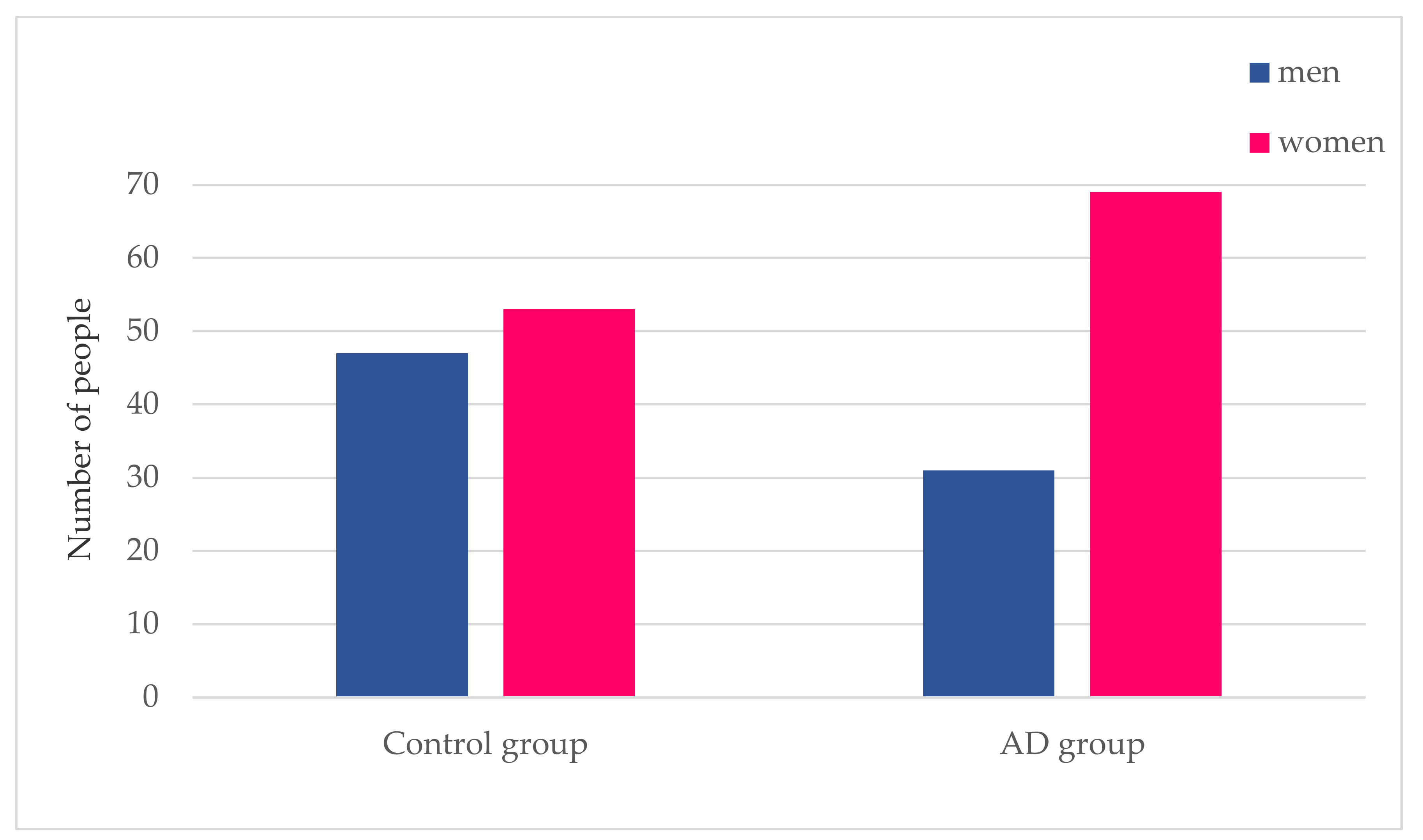

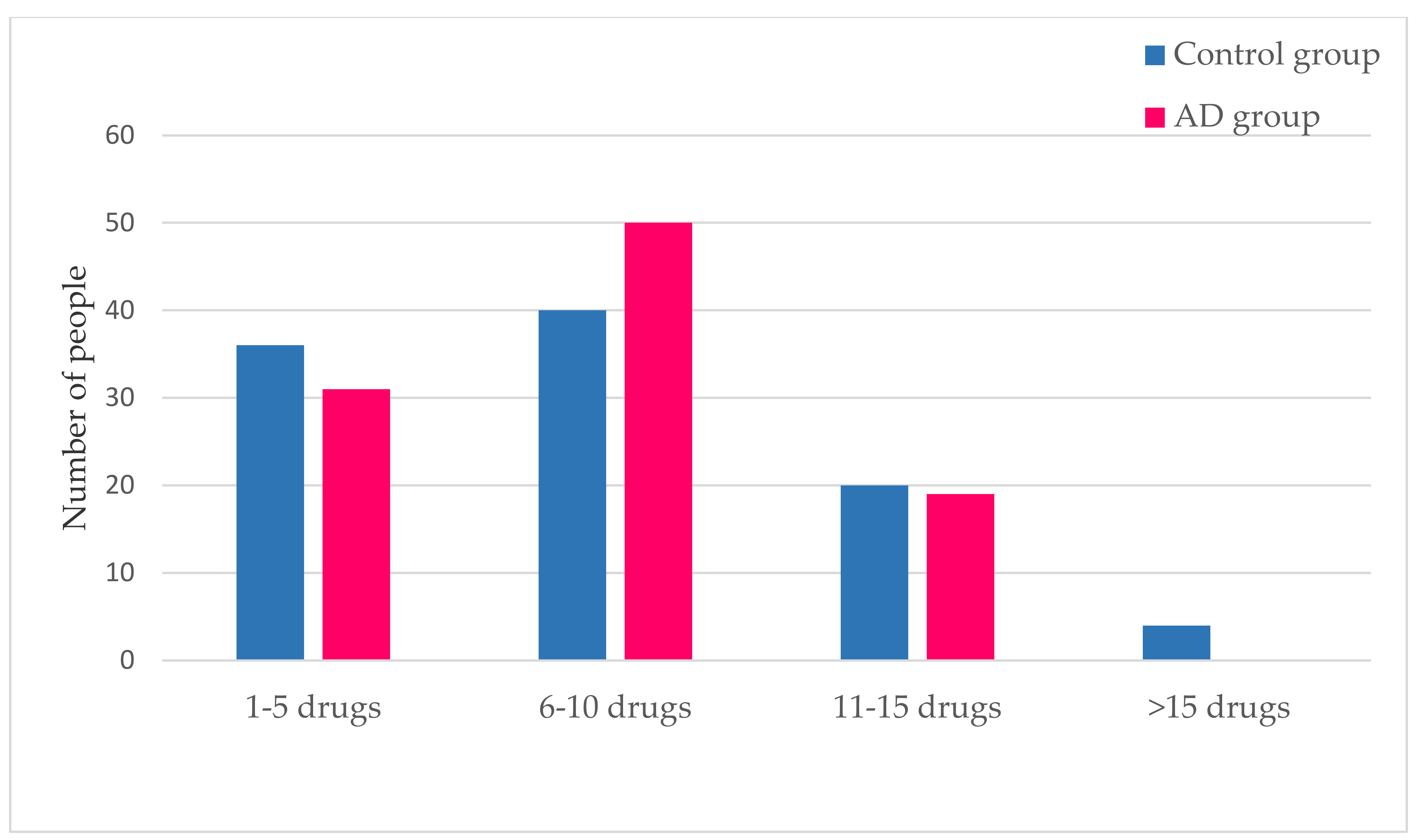
| Control Group | AD Group | |
|---|---|---|
| Medical condition | % | % |
| Cataract | 12.0% | 21.0% |
| Urinary incontinence | 16.0% | 38.0% |
| Vitamin D deficiency | 5.0% | 11.0% |
| Osteoarthritis | 9.0% | 26.0% |
| Hypoacusis | 1.0% | 13.0% |
| Osteoporosis | 1.0% | 20.0% |
| Personality disorders | 1.0% | 12.0% |
| Medical Condition | Chi-Square | df | Sig. |
|---|---|---|---|
| Cataracts | 2.940 | 1 | 0.086 * |
| Urinary Incontinence | 12.278 | 1 | 0.000 * |
| Vitamin D deficiency | 2.446 | 1 | 0.118 |
| Degenerative joint disease | 10.009 | 1 | 0.002 * |
| Hearing loss | 11.060 | 1 | 0.001 * |
| Osteoporosis | 19.207 | 1 | 0.000 * |
| Personality disorders | 9.955 | 1 | 0.002 * |
| Control Group | AD Group | |
|---|---|---|
| Medical condition | % | % |
| Eye strain | 9.0% | 2.0% |
| Ictus | 5.0% | 1.0% |
| Vertigo | 11.0% | 2.0% |
| Hyperuricemia | 14.0% | 9.0% |
| Circulatory insufficiency | 34.0% | 21.0% |
| Atrial fibrillation | 11.0% | 7.0% |
| Respiratory failure | 25.0% | 4.0% |
| Medical Condition | Chi-Square | df | Sig. |
|---|---|---|---|
| Eye strain | 4.714 | 1 | 0.030 * |
| Ictus | 2.749 | 1 | 0.097 *b |
| Vertigo | 6.664 | 1 | 0.010 * |
| Hyperuricemia | 1.228 | 1 | 0.268 |
| Circulatory insufficiency | 4.238 | 1 | 0.040 * |
| Atrial fibrillation | 0.977 | 1 | 0.323 |
| Respiratory failure | 17.786 | 1 | 0.000 * |
| Control Group | AD Group | |
|---|---|---|
| Medical condition | % | % |
| Type 2 diabetes mellitus | 20.0% | 19.0% |
| Glaucoma | 6.0% | 6.0% |
| Depression | 26.0% | 27.0% |
| Obesity | 7.0% | 7.0% |
| Arthritis | 9.0% | 8.0% |
| High blood pressure | 64.0% | 51.0% |
| Dyslipidemia | 39.0% | 45.0% |
| Anxiety | 12.0% | 14.0% |
| Heart disease | 26.0% | 31.0% |
| Medical Condition | Chi-Square | df | Sig. |
|---|---|---|---|
| Type 2 diabetes mellitus | 0.032 | 1 | 0.858 |
| Glaucoma | 0.000 | 1 | 1.000 |
| Depression | 0.026 | 1 | 0.873 |
| Obesity | 0.000 | 1 | 1.000 |
| Arthritis | 0.064 | 1 | 0.800 |
| High blood pressure | 3.458 | 1 | 0.063* |
| Dyslipidemia | 0.739 | 1 | 0.390 |
| Anxiety | 0.177 | 1 | 0.674 |
| Heart disease | 0.613 | 1 | 0.434 |
| Comprehensive Care Center of León | León University Hospital | |
|---|---|---|
| Target | Prevention and response in case of falls in patients with Alzheimer’s disease | Activities to be performed by the nursing staff according to the different nursing diagnosis lists of the NANDA that the individual with Alzheimer’s disease has |
| Coincidences | -Use of specific support measures for wandering, i.e., walking sticks or support rails -Specific protocol for physical restraint, if necessary, under medical order (with information to relatives) -Specific nursing care monitoring skin integrity or reassessment of the need for restraints -Increase environmental safety by avoiding slippery floors and architectural barriers, placing objects more easily accessible, positioning beds at a lower height and with handrails to prevent falls | |
| The differences | -Directed to all employees | -Directed only to the nursing staff |
| Key points | -The use of the J.H. Downton scale -It assesses the presence of certain intrinsic factors (age, drugs, or associated comorbidities) and extrinsic factors (of an environmental nature as inappropriate soil or equipment in each case) -There is an action protocol in case of falls -1st aid + 2nd reassures and secures the rest -Evaluate general condition and emergency equipment needs, report on the fall (questionnaire) | -Individualized protocol for the nursing staff |
| Lacks | -Lack of personalized care by the employees because there is a single protocol for all | -The J.H. Downton scale is not used -It does not take into account both intrinsic and extrinsic factors -There is no protocol for intervention in the event of a fall -Lack of protocols for the rest of the professional staff members working in the hospital |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortajada-Soler, M.; Sánchez-Valdeón, L.; Blanco-Nistal, M.; Benítez-Andrades, J.A.; Liébana-Presa, C.; Bayón-Darkistade, E. Prevalence of Comorbidities in Individuals Diagnosed and Undiagnosed with Alzheimer’s Disease in León, Spain and a Proposal for Contingency Procedures to Follow in the Case of Emergencies Involving People with Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2020, 17, 3398. https://doi.org/10.3390/ijerph17103398
Tortajada-Soler M, Sánchez-Valdeón L, Blanco-Nistal M, Benítez-Andrades JA, Liébana-Presa C, Bayón-Darkistade E. Prevalence of Comorbidities in Individuals Diagnosed and Undiagnosed with Alzheimer’s Disease in León, Spain and a Proposal for Contingency Procedures to Follow in the Case of Emergencies Involving People with Alzheimer’s Disease. International Journal of Environmental Research and Public Health. 2020; 17(10):3398. https://doi.org/10.3390/ijerph17103398
Chicago/Turabian StyleTortajada-Soler, Macrina, Leticia Sánchez-Valdeón, Marta Blanco-Nistal, José Alberto Benítez-Andrades, Cristina Liébana-Presa, and Enrique Bayón-Darkistade. 2020. "Prevalence of Comorbidities in Individuals Diagnosed and Undiagnosed with Alzheimer’s Disease in León, Spain and a Proposal for Contingency Procedures to Follow in the Case of Emergencies Involving People with Alzheimer’s Disease" International Journal of Environmental Research and Public Health 17, no. 10: 3398. https://doi.org/10.3390/ijerph17103398
APA StyleTortajada-Soler, M., Sánchez-Valdeón, L., Blanco-Nistal, M., Benítez-Andrades, J. A., Liébana-Presa, C., & Bayón-Darkistade, E. (2020). Prevalence of Comorbidities in Individuals Diagnosed and Undiagnosed with Alzheimer’s Disease in León, Spain and a Proposal for Contingency Procedures to Follow in the Case of Emergencies Involving People with Alzheimer’s Disease. International Journal of Environmental Research and Public Health, 17(10), 3398. https://doi.org/10.3390/ijerph17103398








