The Thermal Effects of Water Immersion on Health Outcomes: An Integrative Review
Abstract
1. Introduction
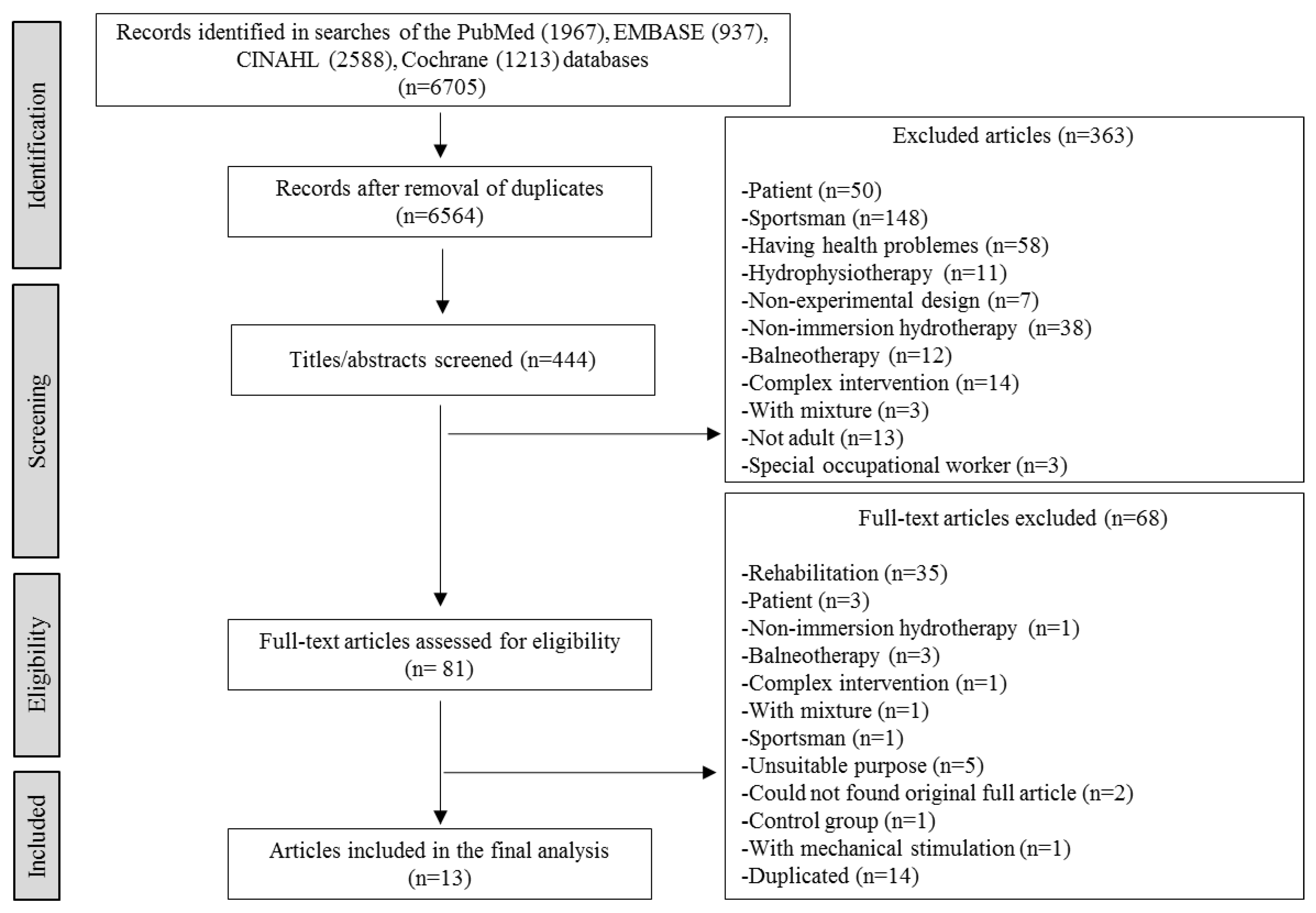
2. Materials and Methods
2.1. Problem Identification Stage
2.1.1. Inclusion Criteria
- Articles published outside Korea between January 2008 and October 2018
- Articles with application of hydrotherapy in the form of water immersion using pure water (liquid state)
- Journal articles published after review by the editorial board
- Published articles in full text form, including the abstract, and written in English
- Research articles excluding systematic or literature reviews
2.1.2. Exclusion Criteria
- Articles with application of hydrotherapy other than water immersion
- Articles with application of mechanical stimulation using water (whirlpool, etc.)
- Articles with application of hydrotherapy combined with aquatic exercise
- Articles with application of hydrotherapy combined with physical therapy
- Articles with application of hydrotherapy for patients with specific diagnoses
- Articles with application of hydrotherapy that included hot springs or mixtures (oil, mud, sulfur, salt, minerals, etc.)
- Articles with hydrotherapy applied as part of a multimodal intervention
- Articles with application of cooling therapy as hydrotherapy after artificially inducing high body temperature
- Articles with application of hydrotherapy for the purpose of recovery after exercise and/or physical activity
- Articles with hydrotherapy applied for sports athletes or specific occupational groups (firefighters, divers, etc.)
- Articles with a research objective that did not match that of the present study or articles with full text that could not be accessed
2.2. Literature Search Stage
2.3. Data Evaluation Stage
2.4. Data Analysis Stage
2.5. Presentation
3. Results
3.1. Study Design/Time for the Measurement/Comparative Group
3.2. Intervention
3.3. Participants
3.4. Time Per Session/Total Session or Period
3.5. Outcome Variables
3.6. Conclusion and Implementation of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geytenbeek, J. Evidence for effective hydrotherapy. Physiotherapy 2002, 88, 514–529. [Google Scholar] [CrossRef]
- Almassmoum, S.M.; Balahmar, E.A.; Almutairi, S.T.; Albuainain, G.S.; Ahmad, R.; Naqvi, A.A. Current clinical status of hydrotherapy; an evidence based retrospective six-years (2012–2017) systematic review. Bali Med. J. 2018, 7, 578–586. [Google Scholar] [CrossRef]
- Mooventhan, A.; Nivethitha, L. Scientific evidence-based effects of hydrotherapy on various systems of the body. N. Am. J. Med. Sci. 2014, 6, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Regnard, J.; Robinet, C.; Mourot, L.; Gomez-Merino, D.; Chennaoui, M.; Jammes, Y.; Dumoulin, G.; Desruelle, A.V.; Melin, B. Whole body immersion and hydromineral homeostasis: Effect of water temperature. Eur. J. Appl. Physiol. 2010, 108, 49–58. [Google Scholar] [CrossRef]
- Wilcock, I.M.; Cronin, J.B.; Hing, W.A. Physiological response to water immersion: A method for sport recovery? Sports Med. 2006, 36, 747–765. [Google Scholar] [CrossRef]
- Kraft, K. Complementary/alternative medicine in the context of prevention of disease and maintenance of health. Prev. Med. 2009, 49, 88–92. [Google Scholar] [CrossRef]
- Verhagen, A.P.; Bierma-Zeinstra, S.M.; Boers, M.; Cardoso, J.R.; Lambeck, J.; De Bie, R.; De Vet, H.C. Balneotherapy (or spa therapy) for rheumatoid arthritis. An abridged version of Cochrane Reviews. Eur. J. Phys. Rehabil. Med. 2015, 51, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Bidonde, J.; Busch, A.J.; Webber, S.C.; Schachter, C.L.; Danyliw, A.; Overend, T.J.; Richards, R.S.; Rader, T. Aquatic exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2014, CD011336. [Google Scholar] [CrossRef]
- Mehrholz, J.; Kugler, J.; Pohl, M. Water-based exercises for improving activities of daily living after stroke. Cochrane Database Syst. Rev. 2011, CD008186. [Google Scholar] [CrossRef]
- Bartels, E.M.; Juhl, C.B.; Christensen, R.; Hagen, K.B.; Danneskiold-Samsøe, B.; Dagfinrud, H.; Lund, H. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst. Rev. 2016, CD005523. [Google Scholar] [CrossRef]
- Han, E.Y.; Kim, B.R.; Im, S.H.; Choi, J.H.; Kim, S.M. Effects of adjuvant hydrotherapy on functional status and mental relaxation in patients with knee osteoarthritis: Preliminary study. J. Korean Geriatr. Soc. 2014, 18, 153–161. [Google Scholar] [CrossRef][Green Version]
- Mizuno, K.; Tanaka, M.; Tajima, K.; Okada, N.; Rokushima, K.; Watanabe, Y. Effects of mild-stream bathing on recovery from mental fatigue. Med. Sci. Monit. 2010, 16, CR8–CR14. [Google Scholar]
- Sa, C.; Palmeira, A. Results of a hydrotherapy program on balance, risk of falls, fear of falling and quality of life in older people. Physiotherapy 2015, 101, eS1307. [Google Scholar] [CrossRef][Green Version]
- Goto, Y.; Hayasaka, H.; Shigeo, K.; Nakamura, Y. Physical and mental effects of bathing: A randomized intervention study. J. Evid. Based Complement. Altern. Med. 2018, 2018, 9521086. [Google Scholar] [CrossRef]
- Malanga, G.; Yan, N.; Stark, J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad. Med. 2014, 127, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Garra, G.; Singer, A.J.; Leno, R.; Taira, B.R.; Gupta, N.; Mathaikuty, B.; Thode, H.J. Heat or cold packs for neck and back stain: A randomized controlled trial of efficacy. Acad. Emerg. Med. 2010, 17, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef]
- Brunt, V.E.; Howard, M.J.; Francisco, M.A.; Ely, B.R.; Minson, C.T. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J. Physiol. 2016, 594, 5329–5342. [Google Scholar] [CrossRef] [PubMed]
- Shimodozono, M.; Matsumoto, S.; Ninomiya, K.; Miyata, R.; Ogata, A.; Etoh, S.; Watanabe, S.; Kawahira, K. Acute effects of a single warm-water bath on serum adiponectin and leptin levels in healthy men: A pilot study. Int. J. Biometeorol. 2012, 56, 933–939. [Google Scholar] [CrossRef]
- Brunt, V.E.; Jeckell, A.T.; Ely, B.R.; Howard, M.J.; Thijssen, D.H.; Minson, C.T. Acute hot water immersion is protective against impaired vascular function following forearm ischemia-reperfusion in young healthy humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1060–R1067. [Google Scholar] [CrossRef]
- Herrera, E.; Sandoval, M.C.; Camargo, D.M.; Salvini, T.F. Motor and sensory nerve conduction are affected differently by ice pack, ice massage, and cold water immersion. Phys. Ther. 2010, 90, 581–591. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Wijayanto, T.; Kuroki, H.; Lee, J.Y.; Tochihara, Y. The effect of repeated mild cold water immersions on the adaptation of the vasomotor responses. Int. J. Biometeorol. 2012, 56, 631–637. [Google Scholar] [CrossRef]
- Bailey, T.G.; Cable, N.T.; Miller, G.D.; Sprung, V.S.; Low, D.A.; Jones, H. Repeated warm water immersion induces similar cerebrovascular adaptations to 8 weeks of moderate-intensity exercise training in females. Int. J. Sports Med. 2016, 37, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhu, W.; Zhu, Y.; Zheng, L.; Hughson, R.L. Acute effects of warm footbath on arterial stiffness in healthy young and older women. Eur. J. Appl. Physiol. 2012, 112, 1261–1268. [Google Scholar] [CrossRef]
- Kojima, D.; Nakamura, T.; Banno, M.; Umemoto, Y.; Kinoshita, T.; Ishida, Y.; Tajima, F. Head-out immersion in hot water increases serum BDNF in healthy males. Int. J. Hyperth. 2018, 34, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, L.; Seyyedrasooli, A.; Zamanazadeh, V.; Nasiri, K. Comparing the effects of reflexology and footbath on sleep quality in the elderly: A controlled clinical trial. Iran. Red Crescent Med. J. 2015, 17, e20111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, Y.; Sohng, K.Y. The effects of footbath on sleep among the older adults in nursing home: A quasi-experimental study. Complement. Ther. Med. 2016, 26, 40–46. [Google Scholar] [CrossRef]
- Ayme, K.; Gavarry, O.; Rossi, P.; Desruelle, A.; Regnard, J.; Boussuges, A. Effect of head-out water immersion on vascular function in healthy subjects. Appl. Physiol. Nutr. Metab. 2014, 39, 425–431. [Google Scholar] [CrossRef]
- Tei, C.; Horikiri, Y.; Park, J.C.; Jeong, J.W.; Chang, K.S.; Toyama, Y.; Tanaka, N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 1995, 91, 2582–2590. [Google Scholar] [CrossRef]
- Crinnion, W.J. Sauna as a valuable clinical tool for cardiovascular, autoimmune, toxicant-induced and other chronic health problems. Altern. Med. Rev. 2011, 16, 215–225. [Google Scholar]
- Costello, J.T.; Donnelly, A.E. Effects of cold water immersion on knee joint position sense in healthy volunteers. J. Sports Sci. 2011, 29, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.T.; Donnelly, A. Cryotherapy and joint position sense in healthy participants: A systematic review. J. Athl. Train. 2010, 45, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, R.; Someh, M.; Ghafarinejad, F. The effect of cryotherapy on the normal ankle joint position sense. Asian J. Sports Med. 2011, 2, 91–98. [Google Scholar] [CrossRef]
- Wassinger, C.A.; Myers, J.B.; Gatti, J.M.; Conley, K.M.; Lephart, S.M. Proprioception and throwing accuracy in the dominant shoulder after cryotherapy. J. Athl. Train. 2007, 42, 84–89. [Google Scholar]
- Algafly, A.A.; George, K.P. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br. J. Sports Med. 2007, 41, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.G.; MaCneil, S.A.; Holt, L.E.; MaCkinnon, N.C.; Rasmussen, R.L. The effect of hot or cold water immersion on isometric strength training. J. Strength Cond. Res. 2000, 14, 21–25. [Google Scholar] [CrossRef]
- Streff, A.; Kuehl, L.K.; Michaux, G.; Anton, F. Differential physiological effects during tonic painful hand immersion tests using hot and ice water. Eur. J. Pain 2010, 14, 266–272. [Google Scholar] [CrossRef]
- Wijayanto, T.; Toramoto, S.; Tochihara, Y. Passive heat exposure induced by hot water leg immersion increased oxyhemoglobin in pre-frontal cortex to preserve oxygenation and did not contribute to impaired cognitive functioning. Int. J. Biometeorol. 2013, 57, 557–567. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 2018, 5, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Sramek, P.; Simeckova, M.; Jansky, L.; Savlikova, J.; Vybiral, S. Human phsycological responses to immersion into water of different temperature. Eur. J. Appl. Physiol. 2000, 81, 436–452. [Google Scholar] [CrossRef]
- Tarlochan, F.; Ramesh, S. Heat transfer model for predicting survival time in cold water immersion. Biomed. Eng. Appl. Basis Commun. 2005, 17, 159–166. [Google Scholar] [CrossRef]
- Runitz, K.; Jensen, T.H. Heat stroke and burns resulting from use of sauna. Ugeskr Laeger 2009, 26, 305–306. [Google Scholar]
- Laukkanen, J.A.; Laukkanen, T.; Kunutsor, S.K. Cardiovascular and other health benefits of sauna bathing: A review of the evidence. Mayo Clin. Proc. 2018, 93, 111–1121. [Google Scholar] [CrossRef]
- Becker, B.E.; Hildenbrand, K.; Whitcomb, R.K.; Sanders, J.P. Biophysiologic effects of warm water immersion. Int. J. Aquatic Res. Educ. 2009, 3, 24–37. [Google Scholar] [CrossRef]
- Charkoudian, N.; Stachenfeld, N.S. Reproductive hormone influences on thermoregulation in women. Compr. Physiol. 2014, 4, 793–804. [Google Scholar] [CrossRef]
- Krauchi, K. How is the circadian rhythm of core body temperature regulated? Clin. Auton. Res. 2002, 12, 147–149. [Google Scholar] [CrossRef]
| Author | Exclusion Criteria | Safety Considerations | |
|---|---|---|---|
| Pre-Intervention Preparation | Post-Intervention Management | ||
| Brunt et al. [18] | • Health problems; cardiovascular disease, diabetes mellitus, hypertension, hyperlipidemia, recent surgery, dermatological conditions, and history of heat-related illness • All medication for 24 h, alcohol and caffeine intake for 12 h, and heavy exercise for 24 h prior to experiment • Pregnant woman | • If the first morning urine specific gravity was >1.024, subjects drank 5 mL/kg water • Rectal thermistors were used as a safety precaution | • Subjects were transferred to a recovery chair for at least 10 min, or until rectal temperature had fallen below 38.5 °C |
| Shimodozono et al. [19] | • Health problems and cardiovascular disease • All medication and supplements • Women; to avoid hormonal effect of the menstrual cycle | • Water immersion was performed after overnight fast • Subjects drank only 300 mL of water at 7 A.M. | • Subjects were kept warm and were wrapped in a blanket for 30 min |
| Bailey et al. [20] | • Health problems, cardiovascular and metabolic diseases • All medication (including hormonal contraceptives) and smoking • Women with irregular menstrual cycles (~28 days) | • Checking alcohol intake, exercise for 24 h and caffeine intake for 12 h prior to experiment | • Laboratory temperature was controlled (21 °C and 45% relative humidity) |
| Costello et al. [21] | • Health problems; Raynaud’s disease, ankle or knee injuries for 12 months prior to experiment, and history of ear or vestibular conditions | - | • Subjects wore clothes and were transferred to the laboratory |
| Herrera et al. [22] | • Health problems; peripheral vascular disease, cardiovascular disease, diabetes, neurological disorders, skeletal muscle disorders, recent trauma or injury to leg, local hot or cold insensitivity, cold adverse reactions, Raynaud’s phenomenon, and pregnancy | • Subjects attended intervention at the same time (e.g., 2–6 P.M.) • Checking alcohol, caffeine, or chocolate intake for 2 h, and exercise for 4 h prior to experiment | • Subjects were transferred to a warm room at 24 °C • Leg was quickly dried without friction |
| Hu et al. [23] | • Health problems and any disease known to affect the cardiovascular system • Smoking and regular physical activity • Medication for disease treatment; diabetes, metabolic disease, and cardiovascular disease • Estrogen-replacement therapy • Regular thermal therapy; sauna, bathing, and footbath • Young women not were scheduled in the follicular phase of their menstrual cycle • Older women before menopause | • Checking alcohol or caffeine intake, vigorous exercise, and bathing for 24 h prior to experiment | - |
| Wakabayashi et al. [24] | • Health problems and history of repeated cold exposure or cold-induced illness | • All measurements were conducted at the same time of the day to reduce circadian effects | - |
| Brunt et al. [25] | • Health problems and history of cardiovascular-related diseases • All medication (including hormonal contraceptives) • Pregnancy and using urine human chorionic gonadotropin (only women) | • Subjects were confirmed to be in a state of euhydration via urine-specific gravity (<1.02) • If the specific gravity was >1.02, subjects drank 5 mL/kg water | • Subjects were monitored for 10 min, or until rectal temperature had fallen below 38.5 °C • Dry nude body weight of subjects was measured to calculate the mean whole-body sweat rate, after correcting for water intake during immersion |
| Streff et al. [26] | • Health problems; any medical, neurological, psychiatric, or psychological disorders, and substance abuse (e.g., nicotine) • All medication (except oral contraceptives) • Alcohol intake for 24 h prior to experiment | - | - |
| Wijayanto et al. [27] | - | • Checking eating, intake of alcohol or caffeinated beverages, smoking, and exercise for at least 2 h prior to experiment | - |
| Kojima et al. [28] | • Medication for disease treatment and medical or psychological condition • Smoking and regular exercise | • Checking eating and drinking of any fluid except tap water | - |
| Valizadeh et al. [29] | • Health problems; enuresis, use the other complementary treatment except hypnotic drugs, and diabetes for more than 10 years | - | - |
| Kim et al. [30] | • Health problems; foot injuries, sensory disorder, acute disease, peripheral vascular disease, schizophrenia, pain or infection, and difficulties in communicating | - | • Subjects were examined for any redness or pain while completely drying feet • Subjects wore socks to keep their feet warm |
| Authors | Design | Experimental Group | Comparative Group | Outcome Variables | Conclusion and Implementation of Evidence | Effect of Water | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Time (T) for Measurement | Participants | Time Per Session | Total Session and Period | ||||||
| Brunt et al. [18] | One group pre- and post-test | • Hot warm water immersion of the shoulder (40.5 °C) for 25–30 min • Sitting up in waist-level water in a tub for 60 min | • Pre-treatment (T1) • Post-treatment (T2) • Post-I/R (T3) | • Young, healthy men (n = 5) and women (n = 5) • Aged 23 ± 6 years (mean) • Sample size was calculated using SigmaPlot 11.0 | 60 min | Single | - | • Brachial artery flow-mediated dilation as endothelial function • Rectal temperature • Heart rate | • There was a significant interaction effect of intervention × time point on FMD% • Rectal temperature increased to a peak of 38.9 ± 0.2 °C • Heart rate increased from 81 ± 18 beats/min at T1 to 127 ± 18 beats/min during hot warm immersion • Hot water immersion results in potential protective effects against I/R-induced vascular dysfunction | Thermal effect |
| Shimodozono et al. [19] | One group pre- and post-test | • Warm immersion to subclavicular level (41 °C) • Subjects were reclined on a stretcher at an angle of 36° in a bathtub | • Pre-treatment (T1) • Immediately post-treatment (T2) • 30 min after treatment (T3) | • Healthy men (n = 7) • Aged 39.7 ± 6 years (mean) • No mention of calculation of sample size | 10 min | Single | - | • Adiponectin and leptin, as adipocyte-derived hormones • Glucose • Insulin • Lipids (T-chol, LDL-C, HDL-C, TG, and FFA) • CBC (RBC, Hb, Ht, WBC, and Plt) | • Leptin levels significantly increased at T2 and T3 after warm immersion • Some parameters (insulin, T-Chol, RBC, Hb, Ht, and WBC) significantly increased immediately (T2) after warm immersion • A single warm immersion for 10 min may modulate leptin and adiponectin profiles in healthy men | Thermal effect |
| Bailey et al. [20] | Randomized controlled trial | • Warm water immersion (42 °C) • Subjects were seated in a tank with water up to top-sternal level | • Pre-treatment (T1) • Post-treatment (T2) | • Healthy women (n = 9) • Aged 25 ± 5 years (mean) • No mention of calculation of sample size | 30 min | 24 times for 8 weeks | • Control (n = 9): cycling (70% HRmax) | • Brachial artery flow-mediated dilation • Cardiorespiratory fitness | • Two outcome variables improved after both warm water intervention and cycling • Passive heat training through warm water intervention can be a useful alternative to exercise training. | Thermal effect |
| Costello et al. [21] | Randomized crossover trial | • Cold water immersion (14 ± 1 °C) • Subjects were seated in tank with water up to umbilicus level | • Healthy men (n = 8) and women (n = 6) • Aged 21.9–25.1 years (mean) • No mention of calculation of sample size | 30 min | Single | • Self-control (crossover) (n = 14): tepid water immersion (28 ± 1 °C) | • Knee joint position sense | • No significant difference between pre- and post-test for both cold and tepid water • Cold water immersion cannot reduce knee joint position sense | Thermal effect | |
| Herrera et al. [22] | Quasi-experimental | • Cold water immersion (10 °C) • Subjects were seated in an acrylic container with water below the level of the head of the fibula | • Pre-treatment (T1) • Post-treatment (T2) | • Healthy men (n = 18) and women (n = 18) • Aged 20.5 ± 1.9 years (mean) • Sample size was calculated using Stata | 15 min | Single | • Comparative 1 (n = 12): ice massage • Comparative 2 (n = 12): ice pack | • Skin temperature • Nerve conduction parameters | • Cold water immersion is the most effective modality for changing the nerve conduction parameter | Thermal effect |
| Hu et al. [23] | Randomized crossover trial | • Warm water immersion (41–43 °C) • Subjects were seated with legs and feet in a plastic bucket with the water level below the knees | • Pre-treatment (T1) • Post-treatment (T2) | • Healthy young (n = 16) and older women (n = 16) • Young women aged 25.4 ± 0.4 years (mean) and older women aged 59.8 ± 1.7 years (mean) • No mention of calculation of sample size | 30 min | Single | • Self-control (crossover): sedentary seating in chairs | • Cardio-ankle vascular index indicated arterial stiffness • Tympanic temperature | • Main time effect (+) in both women • HR (↑), diastolic BP (↓) in young women • Tympanic temperature (↑) in both groups of women • Warm water immersion results in transient improvement of systemic arterial stiffness, mediated by elevation of core temperature • Repeated thermal therapy can promote cardiovascular health | Thermal effect |
| Wakabayashi et al. [24] | One group pre- and post-test | • Repeated mild cold water immersion (26 °C) • Subjects were seated in tank with water up to xiphoid level | • Pre-treatment (T1) • Post-treatment (T2) | • Healthy men (n = 7) • Aged 21.3 ± 0.8 years (mean) • No mention of calculation of sample size | 60 min | 12 times over 4 weeks | - | • Body temperature (11 regional skin temperatures) • Skin blood flow on the forearm • Metabolic heat production • Cold-induced vasodilation | • Main effect of pre- and post-test acclimation is observed in mean skin temperature • Skin blood flow was significantly lower post-test than pre-test • Index of cold-induced vasodilation was significantly lower in post-test acclimation than in pre-test acclimation • The repeated cold immersion in 26 °C water was sufficient to induce the insulative-type of cold adaptation. | Thermal effect |
| Brunt et al. [25] | Non-randomized trials | • Warm immersion up to the shoulder (40.5 °C) for 25–30 min and up to the waist for 60 min • Subjects stayed in hot tub until the rectal temperature reached 38.5 °C | • Pre-treatment (T1) • 1 week after treatment (T2) • 2 weeks after treatment (T3) • 4 weeks after treatment (T4) • 6 weeks after treatment (T5) • 8 weeks after treatment (T6) | • Healthy sedentary males (n = 4) and females (n = 6) • Aged 22.0 ± 1.0 years (mean) • No mention of calculation of sample size | 90 min | 36 times over 8 weeks | • Control (n = 10): thermo-neutral water immersion (36 °C) | • Carotid artery wall thickness and stiffness • Pulse wave velocity • Flow-mediated dilatation and post-occlusive reactive hyperemia • Endothelium-dependent dilatation | • Flow-mediated dilatation with passive heat therapy (warm immersion) was significantly elevated at 2, 6, and 8 weeks • Passive heat therapy significantly increased post-occlusive reactive hyperemia by 6 weeks • Passive heat therapy significantly reduced carotid artery wall thickness by 8 weeks • There was a significant main effect of time on the systolic blood pressure • Passive heat therapy using warm immersion results in increased endothelium-dependent dilatation and reduced arterial stiffness, wall thickness, and blood pressure. | Thermal effect |
| Streff et al. [26] | Randomized crossover trial | • Hot water immersion (47–48 °C) • Subjects were immersed in water up to the wrist in a 12-L tank | • Pre-treatment (T1) • Post-treatment with hot or cold water (T2) • Post-alternating treatment with hot and cold water (T3) | • Healthy men (n = 17) and women (n = 18) • Aged 24 years (median) • Sample size was calculated using G*power | 75 min | Single | • Self-control (crossover): cold water immersion (3–4 °C) | • Subjective pain intensity, pain threshold, pain intolerance level using the VAS • Unpleasantness and affectivity • Physiological parameters (BP, HR, RR) | • Both pain thresholds and pain tolerance levels were significantly higher for cold water immersion than for hot water immersion • Hot water immersion produced a slightly higher subjective pain experience and was tolerated for a shorter period of time • BP was significantly higher in the cold water immersion trial • In cold water immersion, the HR parameters varied as the sympathetic activity was higher than that in hot water immersion | Thermal effect |
| Wijayanto et al. [27] | One group pre- and post-test | • Hot water immersion (38 °C, 40 °C, and 42 °C) • Subjects were immersed in water up to the knee level in a chamber | • Pre-treatment (T1) • 15 min after treatment (T2) • 30 min after treatment (T3) • 45 min after treatment (T4) | • Healthy men (n = 11) • Aged 22.1 ± 1.1 years (mean) • No mention of calculation of sample size | 45 min | Single | - | • Short-term memory span • Rectal temperature • BP • Subjective thermal comfort and thermal sensation • Tissue oxygenation index in the pre-frontal cortex • Change in oxy-Hb level • Change in deoxy-Hb level | • Significant main effect of water temperature on change in the rectal temperature and HR after 45 min • Change in oxy-Hb increased in all three different conditions of water temperature (time effect): significantly higher in the 42 °C condition than in the 38 °C condition • Change in deoxy-Hb did not differ among the three conditions • Different temperature conditions induced little effect on cognitive functioning | Thermal effect |
| Kojima et al. [28] | Randomized crossover trial | • Hot water immersion (42 °C) • Subjects sat in a tank with water up to the neck | • Pre-treatment (T1) • Immediately after treatment (T2) • 15 min after treatment (T3) • 30 min after treatment (T4) | • Healthy men (n = 8) • Aged 25.4 ± 3.3 years (mean) • No mention of calculation of sample size | 20 min | - | • Self-control (crossover): thermo-neutral water immersion (35 °C) | • Core temperature • Mean arterial pressure • Heart rate • Serum BDNF level • Serum S100β level • Plasma cortisol level • Plt count • Monocyte count | • Core temperature was significantly higher at T2 and T3 • BDNF level was higher at T2 and T3, and returned to the baseline at T4. • Cortisol level was lower at T2 and returned to pre-test level during the recovery period | Thermal effect |
| Valizadeh et al. [29] | Controlled single-blinded parallel trial | • Hot water immersion (41–42°C) • To place foot in plastic container at a height of 10 cm | • Pre-treat (T1) • Post-treat (T2) | • Healthy elderly subjects (n = 23) • Aged 67.69 ± 4.28 years (mean) • Sample size was calculated | 20 min | 42 times over 6 weeks | • Comparative 1 (n = 23): massage with olive oil • Control (n = 23): no treatment | • Quality of sleep and sleep patterns using PSQI instrument | • Foot bath was effective in all components except sleep efficiency and use of sleep medication • Foot bath caused 22% reduction in the prevalence of sleep disorders compared to 18% reduction with the comparative intervention | Thermal effect |
| Kim et al. [30] | Quasi-experimental | • Hot water immersion (40 °C) • Subjects placed their foot in a footbath machine to a height of 20 cm above the ankle | • Pre-treatment (T1) • 1 week after treatment (T2) • 2 weeks after treatment (T3) • 3 weeks after treatment (T4) • 4 week after treatment (T5) | • Healthy elderly subjects (n = 10) • Aged 81.6 ± 4.5 years (mean) • Sample size was calculated using G power • All groups (experimental, comparative, and control) were divided into the subgroups good-sleep and poor-sleep | 30 min | 28 times over 4 weeks | • Comparative 1 (n = 10): water immersion (36.5 °C) • Control (n = 10): no treatment | • Sleep patterns assessed using ATG machine (Mini-Mitter Co., Inc., Bend, OR, USA) • Sleep-disturbed behaviors were assessed using SDI instrument | • There were no significant differences in total sleep between groups and between measurement times • In the sleep effectiveness of the experimental group, there was a significant interaction between group and time | Thermal effect |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Lee, I.; Yi, Y. The Thermal Effects of Water Immersion on Health Outcomes: An Integrative Review. Int. J. Environ. Res. Public Health 2019, 16, 1280. https://doi.org/10.3390/ijerph16071280
An J, Lee I, Yi Y. The Thermal Effects of Water Immersion on Health Outcomes: An Integrative Review. International Journal of Environmental Research and Public Health. 2019; 16(7):1280. https://doi.org/10.3390/ijerph16071280
Chicago/Turabian StyleAn, Jiyeon, Insook Lee, and Yunjeong Yi. 2019. "The Thermal Effects of Water Immersion on Health Outcomes: An Integrative Review" International Journal of Environmental Research and Public Health 16, no. 7: 1280. https://doi.org/10.3390/ijerph16071280
APA StyleAn, J., Lee, I., & Yi, Y. (2019). The Thermal Effects of Water Immersion on Health Outcomes: An Integrative Review. International Journal of Environmental Research and Public Health, 16(7), 1280. https://doi.org/10.3390/ijerph16071280





