1. Introduction
Vector-borne diseases represent a serious health risk to human and animals in many parts of the world. In Europe, pathogens transmitted by
Ixodes ricinus (
I. ricinus) ticks, namely agents of Lyme borreliosis (LB) and tick-borne encephalitis (TBE), are the most widespread vector-borne diseases of human [
1,
2]. To be able to take appropriate measures to limit the level of exposure of human populations to vector-borne pathogens, detailed knowledge of the distribution of the risk of infection in space and time is needed.
Lyme borreliosis is caused by spirochetes of the
Borrelia burgdorferi sensu lato complex (
Spirochaetales,
Spirochaetaceae) and TBE by the tick-borne encephalitis virus (
Flavivirus,
Flaviviridae). Both the diseases are zoonoses maintained in geographically more or less specified areas in nature, called “natural foci”. Humans are accidental, predominantly dead-end hosts, which get infected when entering the natural focus. In these foci, causative agents of vector-borne diseases are maintained by circulation between arthropod vectors and transmission competent hosts. Therefore, the epidemiology of these infections is influenced not only by the intrinsic features of the pathogen, but also by the behavior and ecology of its vectors and hosts. Moreover, all three components of vector-borne pathogen natural cycles are influenced by numerous environmental factors of abiotic (temperature, air humidity, access of sunlight, altitude etc.) [
3,
4,
5,
6] or biotic (natural host availability, transmission competence of the host, vector-host contact rate, host immunity etc.) [
7,
8,
9] nature. Because the environment influences all three components of the natural circulation, environmental variables may be used for the prediction of risk of infection for humans entering certain areas [
10,
11].
The existing models for tick-borne pathogens created for ecological or epidemiological purposes are usually based on the estimation of host-seeking tick density and/or pathogen prevalence in the field, collection of different environmental data, and use of various statistical approaches (multiple linear regression, classification and regression trees, generalized linear models etc.) in order to find a mathematical expression of the relationship between the environmental variables and characteristics of tick and pathogen distribution [
6,
12,
13,
14,
15,
16,
17].
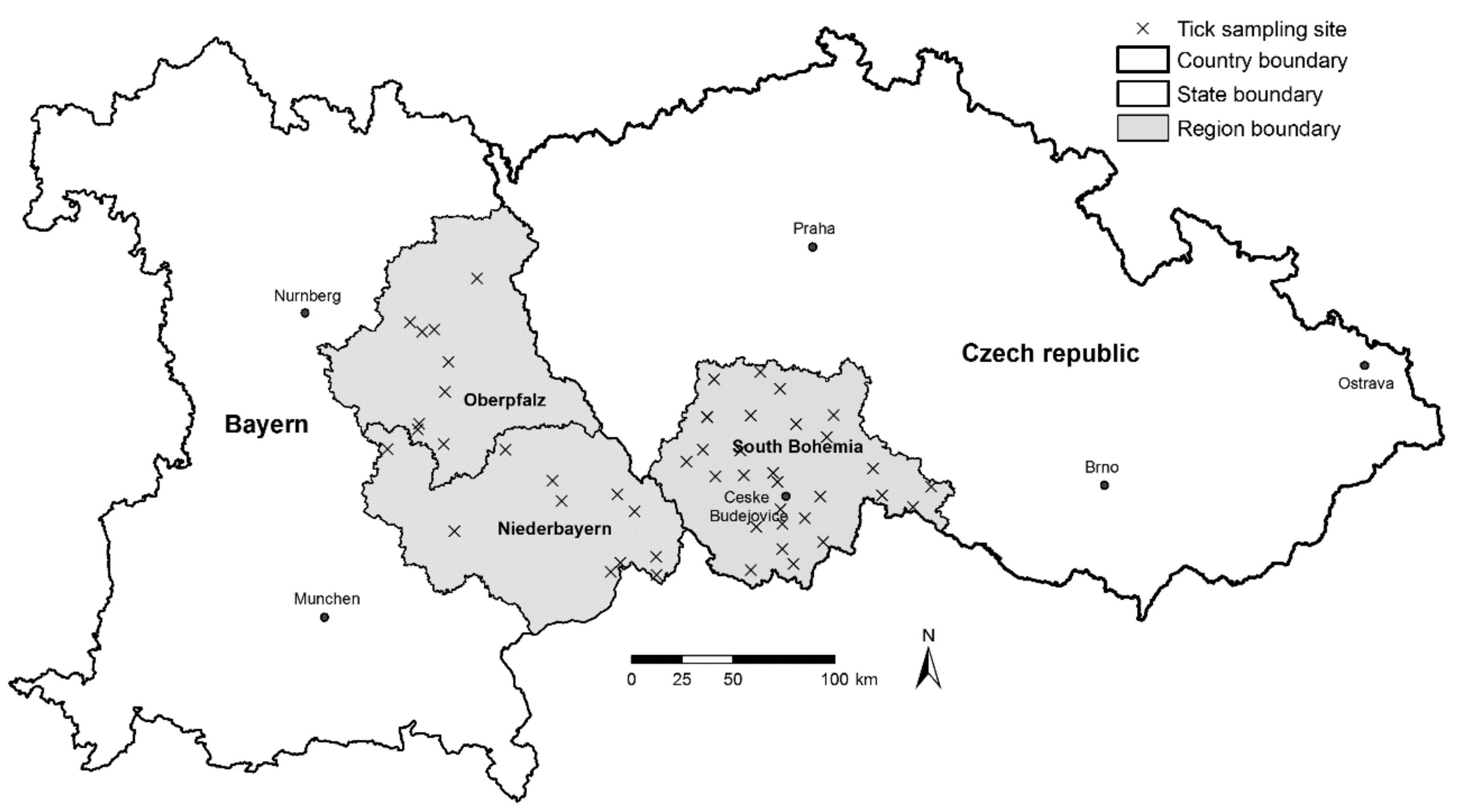
The main aim of this study was to use a set of tick abundance and pathogen prevalence data to prepare summarized, easy to access information on the risk of infection by tick-borne diseases for the general public. Based on the results of an extensive field sampling and laboratory analysis of the tick populations [
18,
19], we have developed a model for the prediction of the density of host-seeking
I. ricinus ticks, LB spirochete and TBEV prevalence, and density of LB spirochete/TBEV-infected ticks using commonly available environmental data. Using tools of geographic information systems (GIS), we have integrated the results of the research, including the risk models, in map outputs which have been made available to the public through an interactive internet-based map portal.
4. Discussion
Tick-borne diseases are recognized as a well-known health threat in the Northern Hemisphere [
33]. Our study was carried out in a Czech-German cross-border area offering two regions with relatively similar environmental conditions in two neighboring countries. The epidemiological survey has shown a high difference in the occurrence of human TBE disease cases. Far higher numbers of TBE cases were reported from South Bohemia than from regions of Lower Bavaria and Upper Palatinate in Bavaria, both in absolute numbers as well as in relative numbers per 100,000 inhabitants or km
2. The possible reasons for this may lie in a higher abundance of tick populations, higher prevalence of TBEV, or higher activity of infection-susceptible humans within the TBEV foci. Differences in the case recognition and case reporting do not seem very likely, because of the well-established case definition and reporting system based on the localization of tick attack (instead of place of patient´s residence). Moreover, the region of South Bohemia has in the long term, by far the highest incidence of TBE among the regions of the Czech Republic, which share the same reporting system [
20]. Additionally, there is no striking difference in the vaccination rate [
34,
35] between the two areas. One of the previously described specific factors, which in fact increases human activity in South Bohemia, is its high popularity as a place for weekend/vacation leisure activities (mostly associated with spending time outdoors) [
36,
37]. Due to frequent visits of people with residence outside the region, the number of people active in risk areas is much higher than the number of inhabitants used for the relative comparison above. On the other hand, it was shown that in the vast majority of the disease cases, the area of infection coincides with the patient’s residence [
37]. The authors hypothesize that the trend in counter-urbanization (change of residence from urban to rural environments) may have an impact on tick-borne disease incidence in the Czech Republic [
38].
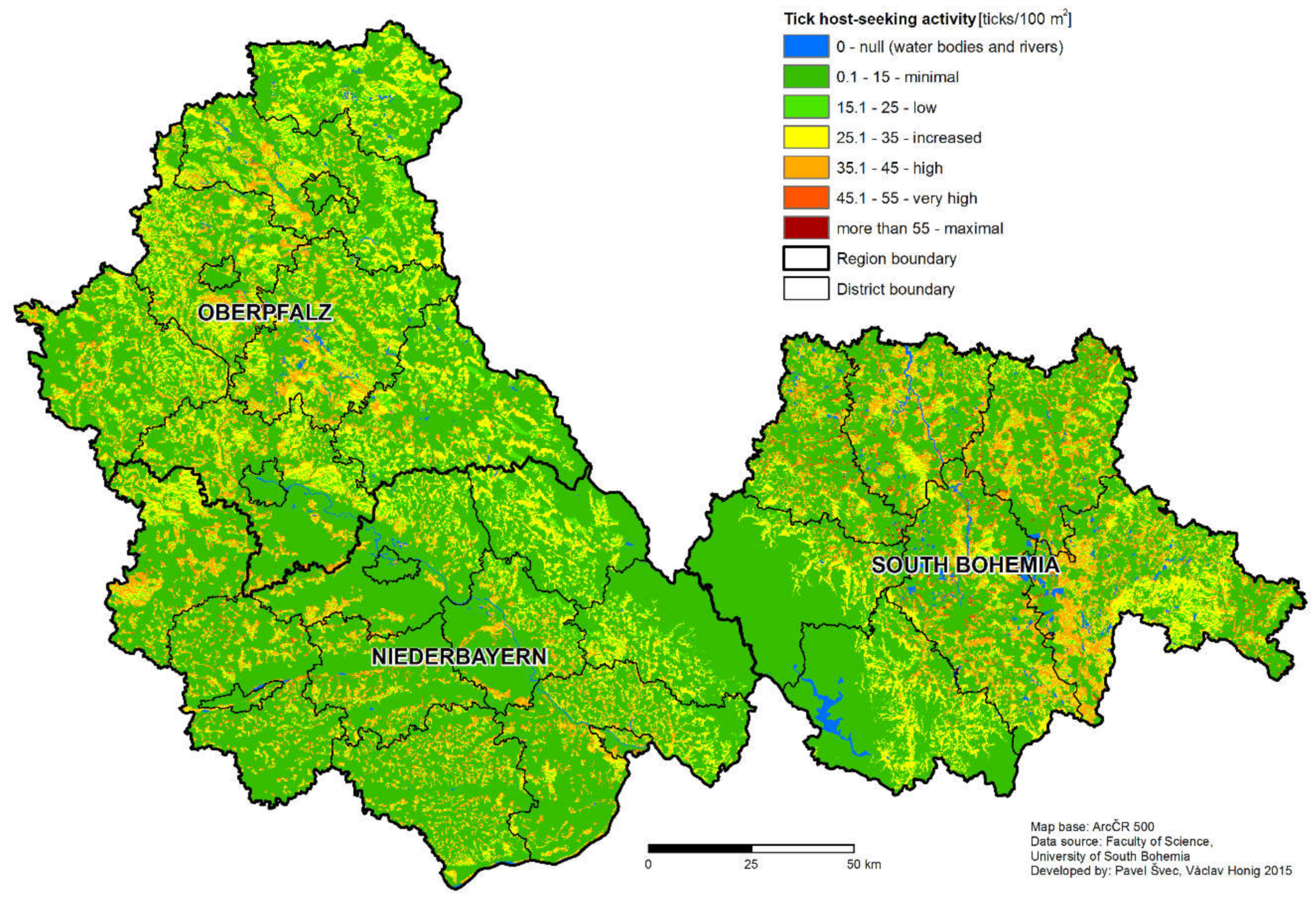
The non-human associated factors that may influence the difference in the rate of TBE disease case incidence between the two regions will be discussed further. Despite the fact that the number of sampling sites differs in Germany and the Czech Republic, the density of sampling sites still allows the comparison of each area and is comparable to similar studies. The average density of host-seeking ticks was statistically significantly higher in the South Bohemian than in Bavarian study sites. This finding was further supported by the result of the tick density model, predicting higher portions of areas classified as high, very high, and maximum risk for South Bohemia than Bavaria (
Table 5). As the risk of vector-borne human disease case occurrence corresponds with the rate of contact between a susceptible person and infected vector [
11,
39], increased activity of the vector itself increases the risk. This is especially valid in the case of Lyme borreliosis, where the pathogen seems to be present in most of the
I. ricinus populations. Presence of
B. burgdorferi s.l. was confirmed for all the sampling plots in our study [
18,
19]. It was previously noticed that the transmission cycles of TBEV are more fragile and tend to be scattered in smaller geographically-restricted areas [
40]. On the other hand, in the case of TBEV, a high tick density may be a crucial factor in natural focus development and stability [
41,
42]. Tick-borne encephalitis virus positive sites show a higher host-seeking tick density than TBEV negative ones [
18,
43]. Apart from the higher prevalence of
Borrelia in adult ticks from Bavaria, no other differences in the prevalence rate of pathogens in ticks were found in the comparison of the two regions. In general, the variability in the density of host-seeking ticks was much higher than the variability in pathogen prevalence. Therefore, in this situation, the risk of infection is rather influenced by the tick abundance compared to the pathogen prevalence. The difference in the incidence of TBE in South Bohemia and Bavaria may then be assigned not only to differences in human behavior, but also to different levels of (infected) tick density, and thus general suitability of the area for
I. ricinus occurrence.
The main aim of our study was to offer the general public easy access to information on the risk of infection by tick-borne diseases. Infection risk estimation based on disease incidence has several advantages—provides information about epidemiological significance, takes into account insusceptible or immune persons, application of preventive measures, etc. [
10,
44,
45]. On the other hand, such systems are, to a large extent, affected by the rate of human activity, which may result in undervaluation of the real acarological risk in less commonly visited natural areas [
44]. Similarly, in regions with an extremely high vaccination rate (like Austria in the case of TBEV), the incidence-based systems are not applicable for disease risk estimation at all [
46]. Furthermore, there are other sources of distortion associated with low accuracy in the geographic localization of the infected vector attack, differences in case definitions, differences in the awareness of the medical doctors, and loss of resolution due to legal or other reasons [
10,
44,
45]. Recently, as an alternative approach, surveillance systems based on the estimation of TBE seroprevalence in sentinel animals were proposed [
47]. The authors also mention that the detection of TBEV in ticks is not a suitable indicator for TBE infection risk estimation. Our study shows that at least in areas with high TBEV occurrence, this approach brings valuable data for disease risk estimation.
The close relationship between the abundance of vector species and various environmental variables, together with the increased availability of high-resolution sources of geo-referenced environmental data and growing capabilities of GIS tools, have made it possible to construct (infected) vector-based models [
44,
48]. Such systems allow the prediction of infection risk that is encountered by a susceptible person entering a certain geographical area. In the case of our model, this means prediction of the density of infected ticks. We have proposed a model predicting the density of host-seeking ticks and probability of tick infection by either LB spirochetes or TBEV.
The potential explanatory variables were similar to those in other tick-borne disease risk models [
6,
13,
17]. All the three models share some of the significant predictors: land surface temperature and NDVI (two of them altitude, orientation of the slope), although the specific forms of the variable expression differ (mean, minimum, month of NDVI reading). NDVI and other remote sensing data-derived indices describing the vegetation cover are frequently used predictors in tick (tick-borne disease) ecological and epidemiological studies [
12,
13,
15,
49]. Information on vegetation cover contains not only information on climatic and to some extent even microclimatic conditions, but also information on probable tick host species’ richness and abundance. Nevertheless, characteristics of vegetation cover are usually combined with climatic factors to increase the model accuracy [
13,
50], as it was shown that variation in climatic conditions may influence the relationship between tick abundance and NVDI [
50,
51]. Furthermore, data on (adult tick) host availability (typically deer abundance) are frequently powerful predictors of tick density [
16,
52]. Such data, with a sufficient resolution and comparable quality for the whole area, were not available at the time of the study.
The purpose of this study was to model the tick-borne disease risk and hence the environmental variables were used as technical correlates. Thus, causality of the relationships is not necessarily direct. A positive correlation of density of host-seeking ticks with land surface temperatures and negative correlation with altitude were recorded, as expected [
4,
5]. In the case of the prediction of probability of infection, we would assume an indirect interaction mediated by host species composition and ratio between the abundance of transmission competent and incompetent hosts influencing the tick abundance and prevalence of the pathogens [
7,
9].
Different genospecies of the
B. burgdorferi s.l. complex display different potential to cause Lyme disease in human [
53]. In our study, we have used data on the occurrence of
B. burgdorferi s.l., regardless of the particular genospecies. Nevertheless, it was previously reported for a part of the same set of tick samples, that the majority of LB spirochete positive ticks were infected by unequivocally pathogenic species (
B. burgdorferi s. s.,
B. afzelii,
B. garinii,
B. bavariensis,
B. spielmanii) [
53], namely more than 96% in South Bohemia [
18] and 81% in Lower Bavaria and Upper Palatinate [
19]. Furthermore, other genospecies of the
B. burgdorferi complex (
B. bissetti,
B. valaisiana,
B. lusitaniae) may be at least conditionally pathogenic [
53,
54]. Therefore, we have used the data on
B. burgdorferi sensu lato presence in ticks for the model construction.
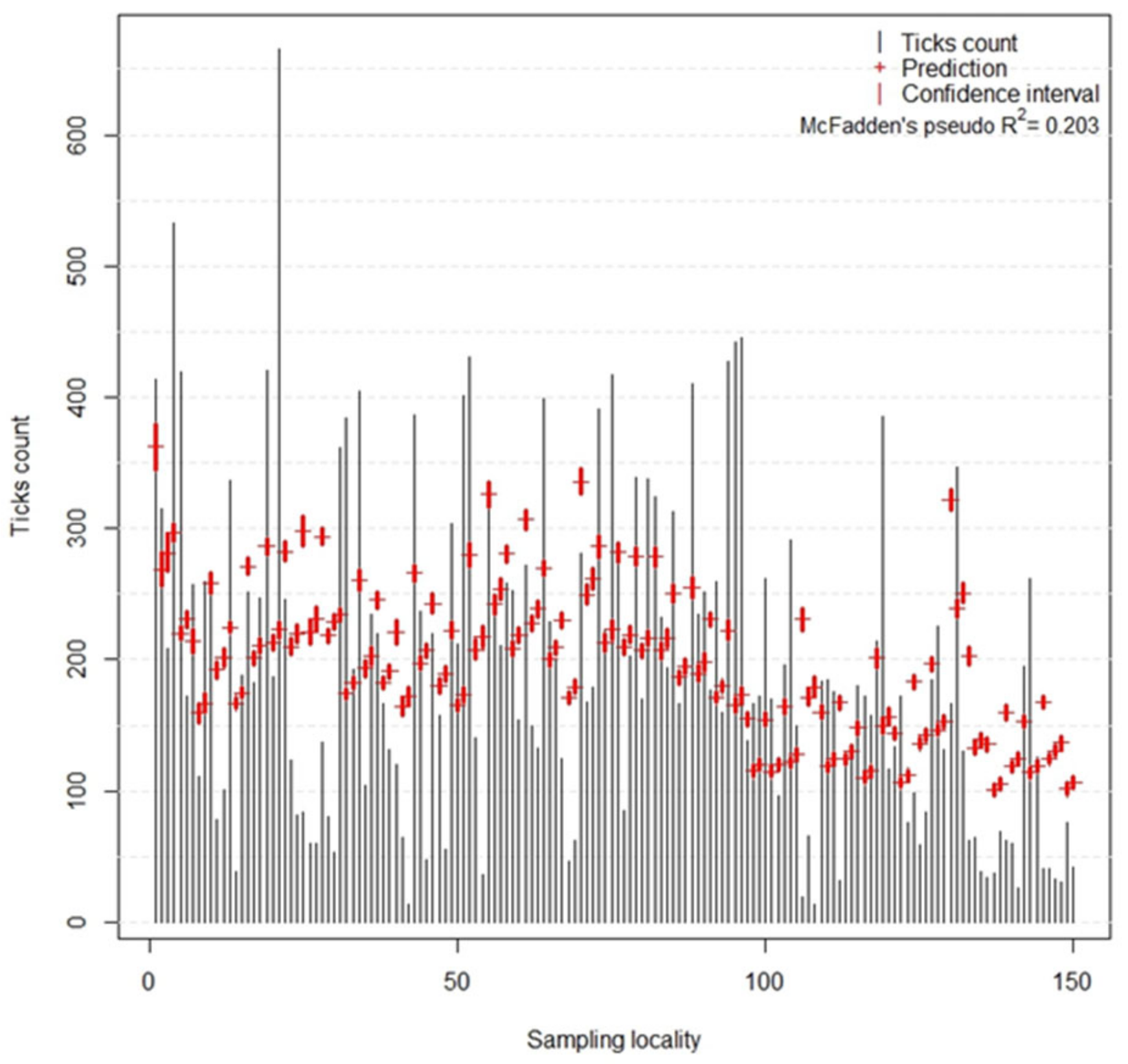
The quality of the prediction of host-seeking tick density by the model was evaluated using McFadden’s pseudo R
2. This goodness-of-fit index can be understood as an alternative to OLS coefficient of determination (R
2); however, as stated by [
55], its values tend to be considerably lower than R
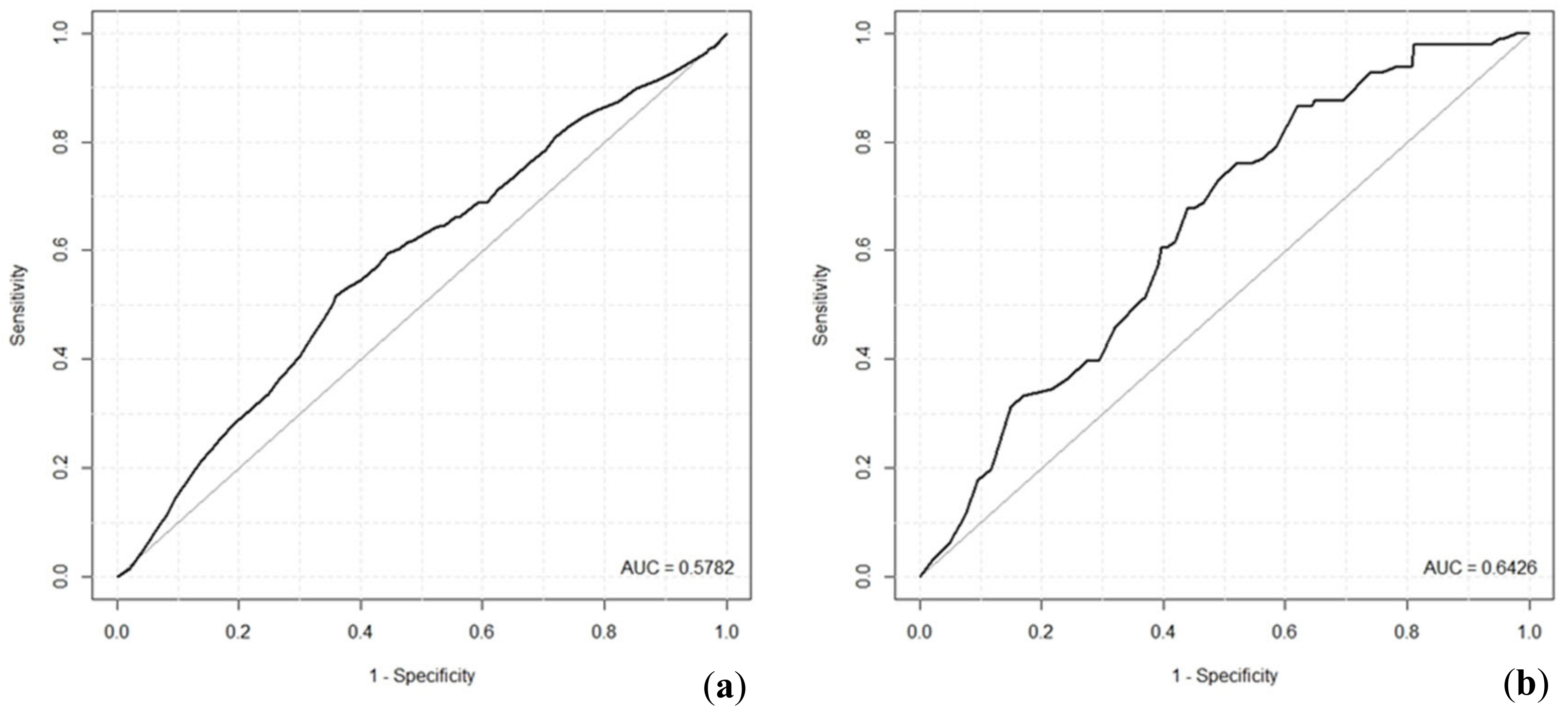
2, for example, values of 0.2 to 0.4 represent an excellent fit of the model. Hence, in our case, the value of 0.203 indicates a model with very good fit. Relatively low ROC values reached by the tick infection probability models indicate that both models are suitable for predictions; however, their predicting ability is limited.
The resolution of the model is crucial in its ability to distinguish small areas of high risk in large low risk areas [
10], which is a typical pattern in diseases with natural focality, like TBE. The spatial structure of TBE focus consists of a microfocus, relatively small core area, where the virus is present continuously and a wider surrounding area, where the virus is spread by vertebrate hosts, predominantly rodents [
56]. Based on the longitudinal monitoring of two foci identified based on an analysis of human TBE disease cases, Dobler et al. [
56] estimated the size of a TBE focus to be about 2500 m
2. The final resolution of the model (250 × 250 m pixel size) is, in principal, capable of the distinction of potential tick-borne disease foci of a small size. Compared to similar studies [
3,
6,
13], we have attempted to consider a rather large area in order to provide meaningful information to the public. Despite the relatively lower number of sampling sites, we have included the majority of relevant
I. ricinus habitat types to obtain a reliable risk prediction.
An obvious limitation of the model is that the data were collected in a single season. Annual variations in total tick abundance, as well in the seasonal patterns, were reported previously. Nevertheless, the ratios among different study sites seem to be more stable [
39,
57,
58,
59,
60,
61]. Hence, we consider the proposed model of tick abundance without the influence of season as generally valid in the sense of relative risk prediction, despite the annual variations. Accordingly, the graphical representation of the model shows classes of minimum to maximum risk, with tick density being only an indicative value. Some annual variations were also observed in the prevalence of TBEV and LB spirochetes [
58,
62,
63]. Nevertheless, concerning the overall low prevalence of TBEV, the risk is more related to presence/absence of the pathogen, which is geographically stable, at least for shorter periods of time (several years). Concerning the predicted probability of infection by
B. burgdorferi s.l., it is an image of a single-year situation rather than a prediction valid for several consecutive years.