Detection and Colonization of Multidrug Resistant Organisms in a Regional Teaching Hospital of Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation
2.2. Phenotypic Antibiotic Susceptibility Testing
2.3. Antibiotic Susceptibility and Species Identification of Bacterial Isolates
2.4. Statistical Analysis
3. Results
3.1. Case Analysis of MDRO-Infected Patients
3.2. Clinical and Epidemiological Characteristics of Patients
3.3. MDRO Distribution in ICU, CNU, RCW
3.4. MDRO Distribution in Non-Hospitalized and Hospitalized Patients
4. Discussion
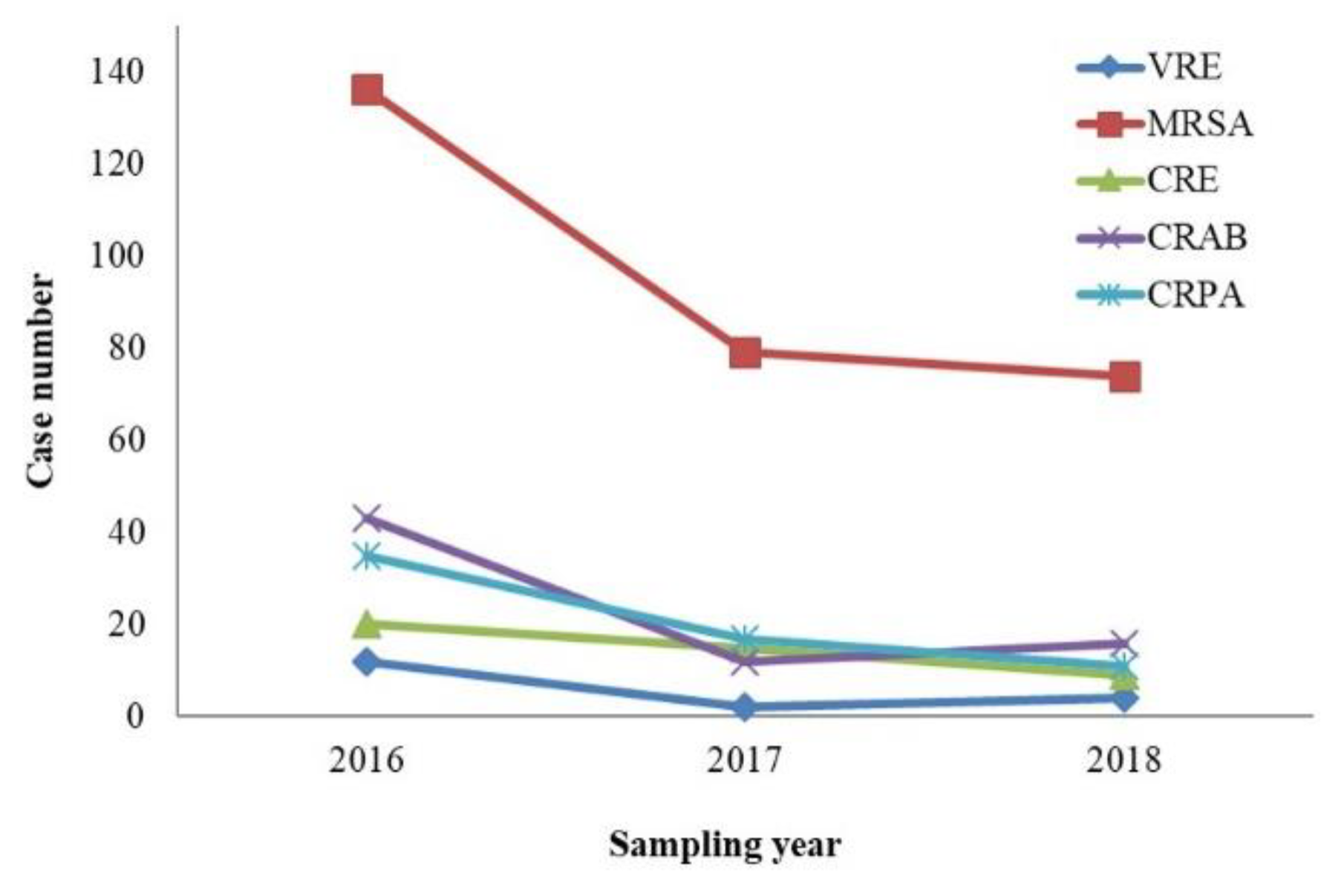
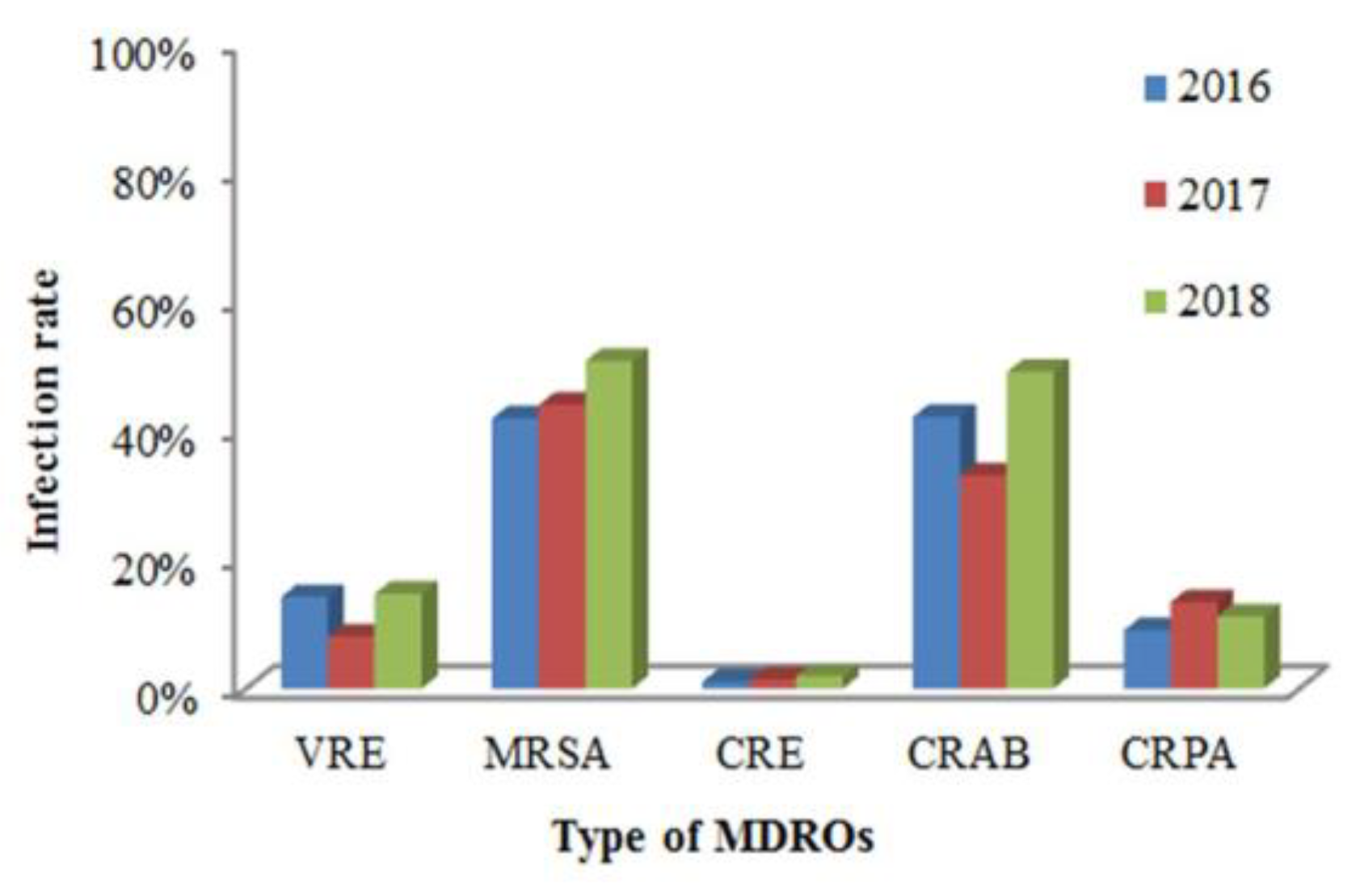
4.1. Resistance of MDROs Isolated from Patients with HAIs Over Time
4.2. Association of Clinical and Epidemiologic Characteristics of Patients with MDRO
4.3. Association of Community-Associated MRSA (CA-MRSA) and Hospital-Acquired MRSA (HA-MRSA)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boldt, A.C.; Schwab, F.; Rohde, A.M.; Kola, A.; Bui, M.T.; Märtin, N.; Kipnis, M.; Schröder, C.; Leistner, R.; WiesePosselt, M.; et al. Admission prevalence of colonization with third-generation cephalosporin-resistant Enterobacteriaceae and subsequent infection rates in a German university hospital. PLoS ONE 2018, 13, e0201548. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chetrit, E.; Wiener-Well, Y.; Lesho, E.; Kopuit, P.; Broyer, C.; Bier, L.; Assous, M.V.; Benenson, S.; Cohen, M.J.; McGann, P.T.; et al. An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: Long-term impact for the ICU and hospital. Crit. Care 2018, 22, 319. [Google Scholar] [CrossRef]
- Cobos-Trigueros, N.; Solé, M.; Castro, P.; Torres, J.L.; Hernández, C.; Rinaudo, M.; Fernández, S.; Soriano, Á.; Nicolás, J.M.; Mensa, J.; et al. Acquisition of Pseudomonas aeruginosa and its resistance phenotypes in critically ill medical patients: Role of colonization pressure and antibiotic exposure. Crit. Care 2015, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Bal, A.M.; Gould, I.M. Antibiotic resistance in Staphylococcus aureus and its relevance in therapy. Expert Opin. Pharm. 2005, 6, 2257–2269. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, L.C.; Chang, Y.J.; Chang, C.Y. Clinical and microbiological characteristics of vancomycin-resistant Enterococcus faecium bloodstream infection in Central Taiwan. Medicine 2017, 96, e9000. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Sader, H.S.; Flamm, R.K.; Castanheira, M.; Mendes, R.E. Oritavancin in vitro activity against gram-positive organisms from European and United States medical centers: Results from the SENTRY Antimicrobial Surveillance Program for 2010–2014. Diagn. Microbiol. Infect. Dis. 2018, 91, 199–204. [Google Scholar] [CrossRef]
- Richards, M.J.; Edwards, J.R.; Culver, D.H.; Gaynes, R.P. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infection Control & Hospital Epidemiology. Infect. Control Hosp. Epidemiol. 2000, 21, 510–515. [Google Scholar] [PubMed]
- Tseng, S.H.; Lee, C.M.; Lin, T.Y.; Chang, S.C.; Chuang, Y.C.; Yen, M.Y.; Hwang, K.P.; Leu, H.S.; Yen, C.C.; Chang, F.Y. Combating antimicrobial resistance: Antimicrobial stewardship program in Taiwan. J. Microbiol. Immunol. 2012, 45, 79–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Duan, X.; Wu, H.; Zhou, Q. Surveillance and correlation of antimicrobial usage and resistance of Pseudomonas aeruginosa: A hospital population-based study. PLoS ONE 2013, 8, e78604. [Google Scholar] [CrossRef]
- Dermota, U.; Zdovc, I.; Strumbelj, I.; Grmek-Kosnik, I.; Ribic, H.; Rupnik, M.; Golob, M.; Zajc, U.; Bes, M.; Laurent, F.; et al. Detection of methicillin-resistant Staphylococcus aureus carrying the mecC gene in human samples in Slovenia. Epidemiol. Infect. 2015, 143, 1105–1108. [Google Scholar] [CrossRef]
- Wei, H.M.; Hsu, Y.L.; Lin, H.C.; Hsieh, T.H.; Yen, T.Y.; Lin, H.C.; Su, B.H.; Hwang, K.P. Multidrug-resistant Acinetobacter baumannii infection among neonates in a neonatal intensive care unit at a medical center in central Taiwan. J. Microbiol. Immunol. 2015, 48, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Gomez-Simmonds, A.; Sullivan, S.B.; Giddins, M.J.; Ferguson, S.A.; Korakavi, G.; Leeds, D.; Park, S.; Shim, K.; Sowash, M.G.; et al. Genomic Surveillance Reveals Diversity of Multidrug-Resistant Organism Colonization and Infection: A Prospective Cohort Study in Liver Transplant Recipients. Clin. Infect. Dis. 2018, 67, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.A. Nosocomial infection update. Emerg. Infect. Dis. 1998, 4, 416. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Agreiter, I.; Orlando, L.; Hutt, D. BMT Settings, Infection and Infection Control. In the European Blood and Marrow Transplantation Textbook for Nurses; Springer: Cham, Switzerland, 2018; pp. 97–134. [Google Scholar]
- Hara, G.L.; Kanj, S.S.; Pagani, L.; Abbo, L.; Endimiani, A.; Wertheim, H.F.L.; Amábile-Cuevas, C.; Tattevin, P.; Mehtar, S.; Cardoso, F.L.; et al. Ten key points for the appropriate use of antibiotics in hospitalised patients: A consensus from the Antimicrobial Stewardship and Resistance Working Groups of the International Society of Chemotherapy. Int. J. Antimicrob. Agents 2016, 48, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.L.; Apisarnthanarak, A.; Madriaga, G. The burden of healthcare-associated infections in Southeast Asia: A systematic literature review and meta-analysis. Clin. Infect. Dis. 2015, 60, 1690–1699. [Google Scholar] [CrossRef]
- Marchaim, D.; Chopra, T.; Bogan, C.; Bheemreddy, S.; Sengstock, D.; Jagarlamudi, R.; Malani, A.; Lemanek, L.; Moshos, J.; Lephart, P.R.; et al. The burden of multidrug-resistant organisms on tertiary hospitals posed by patients with recent stays in long-term acute care facilities. Am. J. Infect. Control 2012, 40, 760–765. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Lessa, F.C.; Belflower, R.; Mu, Y.; Wise, M.; Nadle, J.; Bamberg, W.M.; Petit, S.; Ray, S.M.; Harrison, L.H.; et al. Invasive methicillin-resistant Staphylococcus aureus infections among patients on chronic dialysis in the United States, 2005–2011. Clin. Infect. Dis. 2013, 57, 1393–1400. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Walters, M.S.; Weiner, L.M.; Edwards, J.; Brown, A.C.; Huang, J.Y.; Malik, S.; Slayton, R.B.; Paul, P.; Capers, C.; et al. Containment of Novel Multidrug-Resistant Organisms and Resistance Mechanisms-United States, 2006–2017. MMWR-Morbid. Mortal. W. 2018, 67, 396. [Google Scholar] [CrossRef]
- Chang, A.; Schyve, P.M.; Croteau, R.J.; O’leary, D.S.; Loeb, J.M. The JCAHO patient safety event taxonomy: A standardized terminology and classification schema for near misses and adverse events. Int. J. Qual. Health Care 2005, 17, 95–105. [Google Scholar] [CrossRef]
- Rello, J.; Lode, H.; Cornaglia, G.; Masterton, R. A European care bundle for prevention of ventilator-associated pneumonia. Intensive Care Med. 2010, 36, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Torres, A.; Rinaudo, M.; Terraneo, S.; de Rosa, F.; Ramirez, P.; Diaz, E.; Fernández-Barat, L.; Li bassi, G.L.; Ferrer, M. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J. Infect. 2015, 70, 213–222. [Google Scholar]
- Sakoulas, G.; Eliopoulos, G.M.; Fowler, V.G.; Moellering, R.C.; Novick, R.P.; Lucindo, N.; Yeaman, M.R.; Bayer, A.S. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob. Agents Chemother. 2005, 49, 2687–2692. [Google Scholar] [CrossRef]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Nesme, X.; Etienne, J.; Vandenesch, F. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Zhang, H.; Li, X.; Huang, W.; Fu, Q.; Li, M. Molecular Characteristics of Community-Associated Staphylococcus aureus Isolates from Pediatric Patients with Bloodstream Infections Between 2012 and 2017 in Shanghai, China. Front. Microbiol. 2018, 9, 1211. [Google Scholar] [CrossRef]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233. [Google Scholar]
- Tewolde, R.; Dallman, T.; Schaefer, U.; Sheppard, C.L.; Ashton, P.; Pichon, B.; Ellington, M.; Swift, C.; Green, J.; Underwood, A. MOST: A modified MLST typing tool based on short read sequencing. PeerJ 2016, 4, e2308. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Dammhayn, C.; Hackel, M.; Seifert, H. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2009, 65, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Maree, C.L.; Daum, R.S.; Boyle-Vavra, S.; Matayoshi, K.; Miller, L.G. Community-associated methicillin-resistant Staphylococcus aureus isolates and healthcare-associated infections. Emerg. Infect. Dis. 2007, 13, 236. [Google Scholar] [CrossRef] [PubMed]
| Epidemiologic Variable | Total MDRO | VRE | MRSA | CRE | CRAB | CRPA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | Adjusted OR | 95% CI | Adjusted OR | 95% CI | Adjusted OR | 95% CI | Adjusted OR | 95% CI | Adjusted OR | 95% CI | ||
| Gender | female | ref. | ref. | ref. | ref. | ref. | ref. | ||||||
| male | 1.23 | 1.01–1.5 * | 1.31 | 0.48–3.55 | 1.10 | 0.85–1.42 | 1.84 | 0.98–3.47 | 1.62 | 0.95–2.75 | 1.00 | 0.58–1.70 | |
| Age (years) | <40 | ref. | ref. | ref. | ref. | ref. | ref. | ||||||
| 40–59 | 0.85 | 0.63–1.16 | NA | 0.60 | 0.42–0.84 * | 1.88 | 0.61–5.75 | 3.13 | 0.59–16.4 | 1.49 | 0.39–5.69 | ||
| ≥60 | 1.75 | 1.37–2.23 * | NA | 0.85 | 0.64–1.14 | 4.08 | 1.67–9.96 * | 15.8 | 3.78–66.00 * | 7.67 | 2.98–19.70 * | ||
| Sample | Urine | ref. | ref. | ref. | ref. | ref. | ref. | ||||||
| Pus | 9.83 | 7.52–12.8 * | 0.34 | 0.07–1.57 | 51.90 | 31.3–86.00 * | 1.29 | 0.57–2.88 | 2.39 | 1.01–5.64* | 0.70 | 0.26–1.88 | |
| Sputum | 4.33 | 3.08–6.10 * | NA | 8.51 | 4.29–16.80 * | 0.65 | 0.23–1.83 | 6.77 | 3.33–13.70* | 2.29 | 1.17–4.46 * | ||
| Others | 2.73 | 2.04–3.65 * | 0.11 | 0.01–0.97 * | 10.10 | 5.95–17.10 * | 1.14 | 0.51–2.51 | 2.02 | 0.91–4.44 | 1.13 | 0.56–2.27 | |
| Station | OPD, ER | ref. | ref. | ref. | ref. | ref. | ref. | ||||||
| ICU, RCW, CNU | 2.3 | 1.65–3.20 * | 6.66 | 1.49–29.70 * | 1.62 | 1.01–2.59 * | 3.80 | 1.08–13.30 * | 5.41 | 2.36–12.40 * | 6.28 | 2.74–14.30 * | |
| General Ward | 1.05 | 0.84–1.31 | 1.1 | 0.85–1.42 | 0.64 | 0.49–0.83 * | 4.35 | 1.89–10.00 * | 2.01 | 0.98–4.13 | 2.40 | 1.21–4.77 * | |
| Isolates | Non-Hospitalization | Hospitalization | ||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| GP | (N = 382) | (N = 617) | ||
| Entero. spp. | 93 | (24.3) | 242 | (39.2) |
| VRE | 2 | (0.5) | 16 | (2.6) |
| SA | 161 | (42.1) | 196 | (31.8) |
| MRSA | 126 | (33.0) | 163 | (26.4) |
| GNB | (N = 2135) | (N = 1845) | ||
| E. coli | 1801 | (84.4) | 1333 | (72.2) |
| KP | 282 | (13.2) | 364 | (19.7) |
| E. cloace | 45 | (2.1) | 110 | (6.0) |
| CRE | 7 | (0.3) | 38 | (2.1) |
| GNF | (N = 123) | (N = 395) | ||
| AB | 15 | (12.2) | 51 | (12.9) |
| CRAB | 11 | (8.9) | 60 | (15.2) |
| PA | 85 | (69.1) | 233 | (59.0) |
| CRPA | 12 | (9.8) | 51 | (12.9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-P.; Liang, C.-C.; Chang, R.; Kuo, C.-M.; Hung, C.-H.; Liao, T.-N.; Liao, C.-S. Detection and Colonization of Multidrug Resistant Organisms in a Regional Teaching Hospital of Taiwan. Int. J. Environ. Res. Public Health 2019, 16, 1104. https://doi.org/10.3390/ijerph16071104
Chen Y-P, Liang C-C, Chang R, Kuo C-M, Hung C-H, Liao T-N, Liao C-S. Detection and Colonization of Multidrug Resistant Organisms in a Regional Teaching Hospital of Taiwan. International Journal of Environmental Research and Public Health. 2019; 16(7):1104. https://doi.org/10.3390/ijerph16071104
Chicago/Turabian StyleChen, Yi-Ping, Ching-Chao Liang, Renin Chang, Chen-Min Kuo, Chih-Hsin Hung, Tung-Nan Liao, and Chien-Sen Liao. 2019. "Detection and Colonization of Multidrug Resistant Organisms in a Regional Teaching Hospital of Taiwan" International Journal of Environmental Research and Public Health 16, no. 7: 1104. https://doi.org/10.3390/ijerph16071104
APA StyleChen, Y.-P., Liang, C.-C., Chang, R., Kuo, C.-M., Hung, C.-H., Liao, T.-N., & Liao, C.-S. (2019). Detection and Colonization of Multidrug Resistant Organisms in a Regional Teaching Hospital of Taiwan. International Journal of Environmental Research and Public Health, 16(7), 1104. https://doi.org/10.3390/ijerph16071104







