Chemical and Biological Components of Urban Aerosols in Africa: Current Status and Knowledge Gaps
Abstract
1. Introduction
2. Overview of Ambient Particulate Matter in Africa
3. Chemical and Biological Components of Particulate Matter Worldwide
3.1. Chemical Components of Airborne Particulate Matter
3.1.1. Particulate Matter-Associated Polycyclic Aromatic Hydrocarbons and their Nitro-Derivatives
3.1.2. Toxicity of Polycyclic Aromatic Hydrocarbons and their Nitro-Derivatives
3.2. Biological Components of Airborne Particulate Matter
3.2.1. Particulate Matter-Associated Airborne Fungi
3.2.2. Particulate Matter-Associated Airborne Bacteria
3.2.3. Particulate Matter-Associated Airborne Viruses
4. Chemical Composition of Ambient Particulate Matter in Africa
4.1. Atmospheric Concentrations of Polycyclic Aromatic Hydrocarbons and Their Nitro-Derivatives in Africa
4.2. Source and Risk Assessment of Particulate Matter-Bound Polycyclic Aromatic Hydrocarbons and Their Nitro-Derivatives in Africa
5. Current Understanding of Bioaerosols Associated Particulate Matter in Africa
6. Conclusions
- Exposure of human population to chemical and biological aerosols is of particular concern in Africa.
- Major chemical components of PM include carcinogenic PAHs and NPAHs and major the biological components in PM, including pathogenic fungi and bacteria, although information is scarce in Africa.
- The association of chemical and biological components of PM has been linked to synergistic health effects in other continents. However, the interrelationship of these factors is complex and deserves a comprehensive research in Africa.
- Chemical component of aerosols arises largely from automobiles and wood burning as the major sources of PAHs and NPAHs in Africa.
- Major knowledge gaps persist, particularly for the sub-Saharan region of Africa.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects: The 2017 Revision, Key Findings and Advance and Tables. 2017. Available online: https://www.un.org/development/desa/publications/world-population-prospects-the-2017-revision.html (accessed on 24 February 2019).
- World Health Organization. Household Air Pollution and Health; WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed on 18 February 2019).
- Rajendra, K.C.; Shukla, S.D.; Gautam, S.S.; Hansbro, P.M.; O’Toole, R.F. The role of environmental exposure to non-cigarette smoke in lung disease. Clin. Transl. Med. 2018, 7, 39. [Google Scholar]
- World Health Organization. Burden of Disease of Household Air Pollution for 2016. 2018. Available online: https://www.who.int/airpollution/data/HAP_BoD_results_May2018_final.pdf (accessed on 24 February 2019).
- OECD 2050: The Consequences of Inaction Key Findings on Health and Environment 2012. Available online: https://www.oecd-ilibrary.org/environment/oecd-environmental-outlook-to-2050/health-and-environment_env_outlook-2012-9-en (accessed on 25 February 2019).
- World Health Organization. Health Effects of Particulate Matter. Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2013; Available online: http://www.euro.who.int/__data/assets/pdf_file/0006/189051/Health-effects-of-particulate-matter-final-Eng.pdf?ua=1 (accessed on 24 February 2019).
- IARC-International Agency for Research on Cancer. The Carcinogenicity of Outdoor Air Pollution; IARC-International Agency for Research on Cancer: Lyon, France, 2013. Available online: http://www.scienzainrete.it/files/the_carcinogenity_of_outdoor_air_pollution_0. (accessed on 24 February 2019).
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, composition, and lung diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhou, W.; Pickett, S.T.A.; Li, W.; Qian, Y. Multicontaminant air pollution in Chinese cities. Bull. World Health Organ. 2018, 96, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Sippula, O.; Rintala, H.; Happo, M.; Jalava, P.; Kuuspalo, K.; Virén, A.; Hirvonen, M.R. Characterization of chemical and microbial species from size-segregated indoor and outdoor particulate samples. Aerosol. Air Qual. Res. 2013, 13, 1212–1230. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.I.; Park, J. Approach of molecular methods for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2016, 51, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Morakinyo, O.M.; Mokgobu, M.I.; Mukhola, M.S.; Hunter, R.P. Health Outcomes of Exposure to Biological and Chemical Components of Inhalable and Respirable Particulate Matter. Int. J. Environ. Res. Public Health 2016, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Lu, J.; Li, S.; Tong, Y.; Xie, C.; Zheng, X. Assessment of microbial communities in PM1 and PM10 of Urumqi during winter. Environ. Pollut. 2016, 16, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Hara, K.; Kobayashi, F.; Kurosaki, Y.; Kakikawa, M.; Matsuki, A.; Iwasaka, Y. Vertical distribution of airborne bacterial communities in an Asian-dust downwind area, Noto Peninsula. Atmos. Environ. 2015, 119, 282–293. [Google Scholar] [CrossRef]
- Harrison, R.M.; Yin, J. Particulate matter in the atmosphere: Which particle properties are important for its effects on health. Sci. Total Environ. 2000, 249, 85–101. [Google Scholar] [CrossRef]
- Brodie, E.L.; DeSantis, T.Z.; Parker, J.P.M.; Zubietta, I.X.; Piceno, Y.M.; Andersen, G.L. Urban aerosols harbor diverse and dynamic bacterial populations. Proc. Natl. Acad. Sci. USA 2007, 104, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Bootdee, S.; Chantara, S.; Prapamontol, T. Determination of PM2.5 and polycyclic aromatic hydrocarbons from incense burning emission at shrine for health risk assessment. Atmos. Pollut. Res. 2016, 7, 680–689. [Google Scholar] [CrossRef]
- Mohammed, M.O.A.; Song, W.; Ma, Y.; Liu, L.; Ma, W.; Li, W.L.; Khan, A.U. Distribution patterns, infiltration and health risk assessment of PM2.5-bound PAHs in indoor and outdoor air in cold zone. Chemosphere 2016, 155, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.; Maharaj, K.; Lakhani, A. Chemical characteristics and mutagenic activity of PM2.5 at a site in the Indo-Gangetic plain, India. Ecotoxicol. Environ. Saf. 2015, 114, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Masala, S.; Lim, H.; Bergvall, C.; Johansson, C.; Westerholm, R. Determination of semi-volatile and particle-associated polycyclic aromatic hydrocarbons in Stockholm air with emphasis on the highly carcinogenic dibenzopyrene isomers. Atmos. Environ. 2016, 140, 370–380. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Fadda, E.; Marzia, V. Polycyclic aromatic hydrocarbons and cancer in man. Environ. Health Perspect. 1996, 104, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, R. Abundance of cellular material and proteins in the atmosphere. Science 2005, 308, 73. [Google Scholar] [CrossRef] [PubMed]
- Womiloju, T.O.; Miller, J.D.; Mayer, P.M.; Brook, J.R. Methods to determine the biological composition of particulate matter collected from outdoor air. Atmos. Environ. 2003, 37, 4335–4344. [Google Scholar] [CrossRef]
- Matti Maricq, M. Chemical characterization of particulate emissions from diesel engines: A review. J. Aerosol Sci. 2007, 38, 1079–1118. [Google Scholar] [CrossRef]
- Kellogg, C.A.; Griffin, D.W. Aerobiology and the global transport of desert dust. Trends. Ecol. Evol. 2006, 21, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Després, V.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.; Buryak, G.; Jaenicke, R. Primary biological aerosol particles in the atmosphere: A review. Tellus B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Morawska, L.; Thomas, S.; Jamriska, M.; Johnson, G. The modality of particle size distributions of environmental aerosols. Atmos. Environ. 1999, 33, 4401–4411. [Google Scholar] [CrossRef]
- Adhikari, A.; Sen, M.M.; Gupta-Bhattacharya, S.; Chanda, S. Airborne viable, non-viable, and allergenic fungi in a rural agricultural area of India: A 2-year study at five outdoor sampling stations. Sci. Total Environ. 2004, 326, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Boreson, J.; Dillner, A.M.; Peccia, J. Correlating bioaerosol load with PM2.5 and PM10cf concentrations: A comparison between natural desert and urban-fringe aerosols. Atmos. Environ. 2004, 38, 6029–6041. [Google Scholar] [CrossRef]
- Skóra, J.; Matusiak, K.; Wojewódzki, P.; Nowak, A.; Sulyok, M.; Ligocka, A.; Gutarowska, B. Evaluation of microbiological and chemical contaminants in poultry farms. Int. J. Environ. Res. Public Health 2016, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Petkova, E.P.; Jack, D.W.; Volavka-Close, N.H.; Kinney, P.L. Particulate matter pollution in African cities. Air Qual. Atmos. Health 2013, 6, 603–614. [Google Scholar] [CrossRef]
- Naidja, L.; Ali-Khodja, H.; Khardi, S. Particulate matter from road traffic in Africa. J. Earth Sci. Geotech. Eng. 2017, 7, 289–304. [Google Scholar]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Kalisa, E.; Nagato, E.G.; Bizuru, E.; Lee, K.C.; Tang, N.; Pointing, S.B.; Hayakawa, K.; Archer, S.D.J.; Lacap-bugler, D.C. Characterization and Risk Assessment of Atmospheric PM2.5 and PM10 Particulate-Bound PAHs and NPAHs in Rwanda, Central-East Africa. Environ. Sci. Technol. 2018, 52, 12179–12187. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Noma, H.; Kurai, J.; Hantan, D.; Burioka, N.; Nakamoto, S.; Shimizu, E. Association between Outdoor Fungal Concentrations during Winter and Pulmonary Function in Children with and without Asthma. Int. J. Environ. Res. Public Health 2016, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhang, T.; Su, J.; Zhao, L.L.; Wang, H.; Fang, X.M.; Yu, L.Y. Diversity and composition of airborne fungal community associated with particulate matters in Beijing during haze and non-haze days. Front. Microbiol. 2016, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.C.; Brar, M.S.; Chan, Y.; Lau, M.C.Y.Y.; Leung, F.C.C.C.; Scott, J.A.; Pointing, S. Temporal variation in airborne microbial populations and microbially-derived allergens in a tropical urban landscape. Atmos. Environ. 2013, 74, 291–300. [Google Scholar] [CrossRef]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal Variability in Bacterial and Fungal Diversity of the Near- Surface Atmosphere. Env. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef] [PubMed]
- Deguillaume, L.; Leriche, M.; Amato, P.; Ariya, P.A.; Delort, A.M.; Pöschl, U.; Morris, C.E. Microbiology and atmospheric processes: Chemical interactions of Primary Biological Aerosols. Biogeosci. Discuss. 2008, 5, 841–870. [Google Scholar] [CrossRef]
- Briggs, D. Environmental pollution and the global burden of disease. Br. Med. Bull. 2003, 68, 1–24. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Polycyclic Aromatic Hydrocarbons (PAHs)—EPA Fact Sheet; National Center for Environmental Assessment: Washington, DC, USA, 2008. Available online: https://ec.europa.eu/jrc/sites/jrcsh/files/Factsheet%20PAH_0.pdf (accessed on 24 February 2019).
- Coker, E.; Kizito, S. A narrative review on the human health effects of ambient air pollution in sub-saharan africa: An urgent need for health effects studies. Int. J. Environ. Res. Public Health 2018, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. The Global Burden of Disease: Generating Evidence, Guiding Policy Sub-Saharan Africa Region Edition. The World BANK, Institute for Health METRICS and Evaluation. 2013. Available online: http://www.healthdata.org/policy-report/global-burden-disease-generating-evidence-guiding-policy-%E2%80%93-sub-saharan-africa-regional (accessed on 24 February 2019).
- World Health Organization. Ambient (Outdoor) Air Quality and Health; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 24 February 2019).
- Terrouche, A.; Ali-Khodja, H.; Kemmouche, A.; Bouziane, M.; Derradji, A.; Charron, A. Identification of sources of atmospheric particulate matter and trace metals in Constantine, Algeria. Air Qual. Atmos. Health 2016, 9, 69–82. [Google Scholar] [CrossRef]
- Xu, H.; Léon, J.F.; Liousse, C.; Guinot, B.; Yoboué, V.; Akpo, A.B.; Adon, J.; Ho, K.F.; Ho, S.S.H.; Li, L.; et al. Personal exposure to PM2.5 emitted from typical anthropogenic sources in Southern West Africa (SWA): Chemical characteristics and associated health risks. Atmos. Chem. Phys. Discuss. 2018. [Google Scholar] [CrossRef]
- Boman, J.; Lindén, J.; Thorsson, S.; Holmer, B.; Eliasson, I. A tentative study of urban and suburban fine particles (PM2.5) collected in Ouagadougou, Burkina Faso. X-ray Spectrom. 2009, 38, 354–362. [Google Scholar] [CrossRef]
- Li, C.S. Sampling performance of impactors for bacterial bioaerosols. Aerosol Sci. Technol. 1999, 30, 280–287. [Google Scholar] [CrossRef]
- Arku, R.E.; Vallarino, J.; Dionisio, K.L.; Willis, R.; Choi, H.; Wilson, J.G.; Ezzati, M. Characterizing air pollution in two low-income neighborhoods in Accra, Ghana. Sci. Total Environ. 2008, 402, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, K.L.; Rooney, M.S.; Arku, R.E.; Friedman, A.B.; Hughes, A.F.; Vallarino, J.; Ezzati, M. Within-neighborhood patterns and sources of particle pollution: Mobile monitoring and geographic information system analysis in four communities in Accra, Ghana. Environ. Health Perspect. 2010, 118, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Pope, F.D.; Gatari, M.; Ng’ang’, A.D.; Poynter, A.; Blake, R. Airborne particulate matter monitoring in Kenya using calibrated low-cost sensors. Atmos. Chem. Phys. 2018, 18, 15403–15418. [Google Scholar] [CrossRef]
- Garrison, V.H.; Majewski, M.S.; Konde, L.; Wolf, R.E.; Otto, R.D.; Tsuneoka, Y. Inhalable desert dust, urban emissions, and potentially biotoxic metals in urban Saharan-Sahelian air. Sci. Total Environ. 2014, 500–501, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Zghaid, M.; Noack, Y.; Bounakla, M.; Benyaich, F. Pollution atmosphérique particulaire dans la ville de Kenitra (Maroc) [Atmospheric particulate pollution in Kenitra (Morocco)]. Poll. Atmos. 2009, 51, 313–324. [Google Scholar]
- De Longueville, F.; Hountondji, Y.C.; Ozer, P.; Marticorena, B.; Chatenet, B.; Henry, S. Saharan Dust Impacts on Air Quality: What Are the Potential Health Risks in West Africa? Hum. Ecol. Risk Assess. Int. J. 2013, 19, 1595–1617. [Google Scholar] [CrossRef]
- Dieme, D.; Cabral-Ndior, M.; Garçon, G.; Verdin, A.; Billet, S.; Cazier, F.; Shirali, P. Relationship between physicochemical characterization and toxicity of fine particulate matter (PM2.5) collected in Dakar city (Senegal). Environ. Res. 2012, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, J.P.; Swanepoel, L.; Chow, J.C.; Watson, J.G.; Egami, R.T. PM2.5 and PM10 concentrations from the Qalabotjha low-smoke fuels macro-scale experiment in South Africa. Environ. Monit. Assess. 2001, 69, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Worobiec, A.; Potgieter-Vermaak, S.S.; Berghmans, P.; Winkler, H.; Burger, R.; Grieken, R.V. Air particulate emissions in developing countries: A case study in South Africa. Anal. Lett. 2011, 44, 1907–1924. [Google Scholar] [CrossRef]
- Mkoma, S.L.; Chi, X.; Maenhaut, W. Characteristics of carbonaceous aerosols in ambient PM10 and PM2.5 particles in Dar es Salaam, Tanzania. Sci. Total Environ. 2010, 408, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Kirenga, B.; Meng, Q.; van Gemert, F.; Aanyu-Tukamuhebwa, H.; Chavannes, N.; Katamba, A.; Mohsenin, V. The State of Ambient Air Quality in Two Ugandan Cities: A Pilot Cross-Sectional Spatial Assessment. Int. J. Environ. Res. Public Health 2015, 12, 8075–8091. [Google Scholar] [CrossRef] [PubMed]
- Mentz, G.; Robins, T.G.; Batterman, S.; Naidoo, R.N. Acute respiratory symptoms associated with short term fluctuations in ambient pollutants among schoolchildren in Durban, South Africa. Environ. Pollut. 2018, 233, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Guo, Y.; Zheng, Y.; Zhao, X.; Cao, Z.; Rigdon, S.E.; Wu, F. Exposure to ambient PM2.5 associated with overall and domain-specific disability among adults in six low- and middle-income countries. Environ. Int. 2017, 104, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Makamure, M.T.; Reddy, P.; Chuturgoon, A.; Naidoo, R.N.; Mentz, G.; Batterman, S.; Robins, T.G. Interaction between ambient pollutant exposure, CD14 (-159) polymorphism and respiratory outcomes among children in Kwazulu-Natal, Durban. Hum. Exp. Toxicol. 2017, 36, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ana, G.; Odeshi, T.A.; Sridhar, M.K.C.; Ige, M.O. Outdoor respirable particulate matter and the lung function status of residents of selected communities in Ibadan, Nigeria. Perspect. Public Health 2014, 134, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Voyi, K. Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001–2006. Int. J. Environ. Res. Public Health 2012, 9, 3978–4016. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, B.A.; Blangiardo, M.; Briggs, D.J.; Hansell, A.L. Traffic air pollution and other risk factors for respiratory illness in schoolchildren in the niger-delta region of Nigeria. Environ. Health Perspect. 2011, 119, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Kaphingst, K.A.; Persky, S.; Lachance, C. Ambient pollution and respiratory outcomes among schoolchildren in Durban, South Africa. S. Afr. J. Child Health 2010, 14, 384–399. [Google Scholar]
- Amegah, A.K.; Agyei-Mensah, S. Urban air pollution in Sub-Saharan Africa: Time for action. Environ. Pollut. 2017, 220, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Jing, X.; Chang, W.J.; Chen, Z.-X.; Zeng, H. Cumulative health risk assessment of halogenated and parent polycyclic aromatic hydrocarbons associated with particulate matters in urban air. Ecotoxicol. Environ. 2015, 113, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Diemer, J.; Dietrich, S.; Cyrys, J.; Heinrich, J.; Lang, W.; Twardella, D. Chemical and morphological properties of particulate matter (PM10, PM2.5) in school classrooms and outdoor air. Atmos. Environ. 2008, 42, 6597–6605. [Google Scholar] [CrossRef]
- Raes, F.; Dingenen, R.; Van Vignati, E.; Wilson, J.; Putaud, J.P.; Seinfeld, J.H.; Adams, P. Formation and cycling of aerosols in the global troposphere. Atmos. Environ. 2000, 34, 4215–4240. [Google Scholar] [CrossRef]
- Polymenakou, P.N. Atmosphere: A source of pathogenic or beneficial microbes? Atmosphere 2012, 3, 87–102. [Google Scholar] [CrossRef]
- Marley, N.A.; Gaffney, J.S. Introduction to urban aerosols and their impacts. In ACS Symposium Series; Oxford University Press: Oxford, UK, 2005; Volume 919, pp. 2–22. [Google Scholar]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2015, 25, 107–123. [Google Scholar] [CrossRef]
- Hayakawa, K. Environmental Behaviors and Toxicities of Polycyclic Aromatic Hydrocarbons and Nitropolycyclic Aromatic Hydrocarbons. Chem. Pharm. Bull. 2016, 64, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, S.; Sato, T.; Ota, Y.; Wang, X.; Tang, N.; Hayakawa, K. Long-range transport of polycyclic aromatic hydrocarbons (PAHs) from the eastern Asian continent to Kanazawa, Japan with Asian dust. Atmos. Environ. 2007, 41, 2580–2593. [Google Scholar] [CrossRef]
- Wu, S.; Yang, B.; Wang, X.; Hong, H.; Yuan, C. Diurnal variation of nitrated polycyclic aromatic hydrocarbons in PM10 at a roadside site in Xiamen, China. J Environ Sci (China) [Internet]. The Research Centre for Eco-Environmental Sciences, Chinese Academy of Sciences. J. Environ. Sci. 2012, 24, 1767–1776. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2018. Available online: https://monographs.iarc.fr/ (accessed on 24 February 2019).
- Albinet, A.; Leoz-garziandia, E.; Budzinski, H.; Viilenave, E. Polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs and oxygenated PAHs in ambient air of the Marseilles area (South of France): Concentrations and sources. Sci. Total Environ. 2007, 384, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Chen, X.N. Antineutrophil cytoplasmic antibodies-associated glomerulonephritis: From bench to bedside. Chronic Dis. Transl. Med. 2018, 4, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, R.; Chen, Z.; Li, Q.; Huang, S.; Zhu, Z.; Zhou, L.F. Fine particulate matter (PM2.5): The culprit for chronic lung diseases in China. Chronic Dis. Transl. Med. 2018, 4, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K. Polycyclic Aromatic Hydrocarbons: Environmental Behavior and Toxicity in East Asia; Springer: Singapore, 2018. [Google Scholar]
- Keith, L.; Telliard, W. Priority pollutants: I-a perspective view. Environ. Sci. Technol. 1979, 13, 416–423. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tang, N.; Nagato, E.G.; Toriba, A.; Sakai, S.; Kano, F.; Goto, S.; Endo, O.; Arashidani, K.I.; Kakimoto, H. Long term trends in atmospheric concentrations of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons: A study of Japanese cities from 1997 to 2014. Environ. Pollut. 2018, 233, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.T.; Kameda, T.; Toriba, A.; Hayakawa, K. Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in particulates emitted by motorcycles. Environ. Pollut. 2013, 183, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Hattori, T.; Taga, R.; Igarashi, K.; Yang, X.; Tamura, K.; Kakimoto, H.; Mishukov, V.F.; Toriba, A.; Kizu, R.; et al. Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in urban air particulates and their relationship to emission sources in the Pan–Japan Sea countries. Atmos. Environ. 2005, 39, 5817–5826. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, J.; Yang, Y.; Li, B.; Shen, G.; Su, Y.; Tao, S. Occurrence and exposure to polycyclic aromatic hydrocarbons and their derivatives in a rural Chinese home through biomass fuelled cooking. Environ. Pollut. 2012, 169, 160–166. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Air Quality Guidelines for Europe. 2000. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/74732/E71922.pdf (accessed on 24 February 2019).
- El-Bayoumy, K.; Hecht, S.S. Mutagenicity of K-region derivatives of 1-nitropyrene; remarkable activity of 1- and 3-nitro-5H-phenanthro[4,5-bcd]pyran-5-one. Mutat. Res. Toxicol. 1986, 170, 31–40. [Google Scholar] [CrossRef]
- Jones, A.M.; Harrison, R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total. Environ. 2004, 326, 151–180. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G. Environmental urban factors (air pollution and allergens) and the rising trends in allergic respiratory diseases. Allergy 2002, 57, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Peccia, J.; Hospodsky, D.; Bibby, K. New directions: A revolution in DNA sequencing now allows for the meaningful integration of biology with aerosol science. Atmos. Environ. 2011, 45, 1896–1897. [Google Scholar] [CrossRef]
- Donnison, A.; Ross, C.; Noonan, M.; Fisher, G.; Waller, J. Bacterial survival and dispersal in spray irrigation aerosols. N. Z. J. Agric. Res. 2004, 47, 575–585. [Google Scholar] [CrossRef]
- Kalisa, E.; Fadlallah, S.; Amani, M.; Nahayo, L.; Habiyaremye, G. Temperature and air pollution relationship during heatwaves in Birmingham, UK. Sustain. Cities Soc. 2018, 43, 111–1120. [Google Scholar] [CrossRef]
- Davis, J. Modeling the Long-Range Transport of Plant Pathogens in the Atmosphere. Annu. Rev. Phytopathol. 1987, 25, 169–188. [Google Scholar] [CrossRef]
- Driver, C.R.; Valway, S.E.; Morgan, W.M.; Onorato, I.M.; Castro, K.G. Transmission of Mycobacterium tuberculosis associated with air travel. Annu. Rev. Phytopathol. 1994, 272, 1031–1035. [Google Scholar]
- Zimmermann, R. Aerosols and health: A challenge for chemical and biological analysis. Anal. Bioanal. Chem. 2015, 407, 5863–5867. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.Z. Air Pollution and Global Warming: History, Science, and Solutions, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012; p. 398. [Google Scholar]
- Newson, R.; Strachan, D.; Corden, J.; Millington, W. Fungal and other spore counts as predictors of admissions for asthma in the Trent region. Occup. Environ. Med. 2000, 57, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qi, J.; Zhang, H.; Huang, S.; Li, L.; Gao, D. Concentration and size distribution of bioaerosols in an outdoor environment in the Qingdao coastal region. Sci. Total Environ. 2011, 409, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Engling, G.; Chan, C.Y.; Zhang, Y.N.; Zhang, Z.S.; Lin, M.; Li, Y.S. Contribution of fungal spores to particulate matter in a tropical rainforest. Environ. Res. Lett. 2010, 5, 024010. [Google Scholar] [CrossRef]
- Glikson, M.; Rutherford, S.; Simpson, R.W.; Mitchell, C.A.; Yago, A. Microscopic and submicron components of atmospheric particulate matter during high asthma periods in Brisbane, Queensland, Australia. Atmos. Environ. 1995, 29, 549–562. [Google Scholar] [CrossRef]
- Deacon, L.J.; Pankhurst, L.J.; Drew, G.H.; Hayes, E.T.; Jackson, S.; Longhurst, P.J.; Tyrrel, S.F. Particle size distribution of airborne Aspergillus fumigatus spores emitted from compost using membrane filtration. Atmon. Environ. 2009, 43, 5698–5701. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Zhu, T.F. Inhalable Microorganisms in Beijing’s PM2.5 and PM10 Pollutants during a Severe Smog Event. Environ. Sci. Technol. 2014, 48, 1499–14507. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Nowoisky, J.; Pickersgill, D.A.; Després, V.R.; Pöschl, U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA 2009, 106, 12814–12819. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Shamy, M.; Redal, M.A.; Khoder, M.; Awad, A.H.; Elserougy, S. Microorganisms associated particulate matter: A preliminary study. Sci. Total Environ. 2014, 479–480, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J.; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Galler, H.; Luxner, J.; Zarfel, G.; Buzina, W.; Friedl, H.; Reinthaler, F.F. The concentrations of culturable microorganisms in relation to particulate matter in urban air. Atmos. Environ. 2013, 65, 215–222. [Google Scholar] [CrossRef]
- Bowers, R.M.; Sullivan, A.P.; Costello, E.K.; Collett, J.L.; Knight, R.; Fierer, N. Sources of bacteria in outdoor air across cities in the midwestern United States. Int. Arch. Allergy Immunol. 2011, 77, 6350–6356. [Google Scholar] [CrossRef] [PubMed]
- Gavett, S.H.; Koren, H.S. The Role of Particulate Matter in Exacerbation of Atopic Asthma. Int. Arch. Allergy Immunol. 2001, 27711, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Nasir, Z.A.; Colbeck, I. Assessment of bacterial and fungal aerosol in different residential settings. Water Air Soil Pollut. 2010, 211, 367–377. [Google Scholar] [CrossRef]
- Liang, Y.; Fang, L.; Pan, H.; Zhang, K.; Kan, H.; Brook, J.R.; Sun, Q. PM2.5 in Beijing—Temporal pattern and its association with influenza. Environ. Health 2014, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Furuse, Y.; Tamaki, R.; Okamoto, M.; Saito-Obata, M.; Suzuki, A.; Saito, M.; Oshitani, H. Association Between Preceding Viral Respiratory Infection and Subsequent Respiratory Illnesses Among Children: A Prospective Cohort Study in the Philippines. J. Infect. Dis. 2018, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- World Bank. World Development Report 2011: World Development Indicators, Fossil Fuel Energy Consumption; The World Bank: Washington, DC, USA, 2011. [Google Scholar]
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Nassar, H.F.; Tang, N.; Kameda, T.; Toriba, A.; Khoder, M.I.; Hayakawa, K. Atmospheric concentrations of polycyclic aromatic hydrocarbons and selected nitrated derivatives in Greater Cairo, Egypt. Atmos. Environ. 2011, 45, 7352–7359. [Google Scholar] [CrossRef]
- Ladji, R.; Yassaa, N.; Balducci, C.; Cecinato, A.; Meklati, B.Y. Annual variation of particulate organic compounds in PM10 in the urban atmosphere of Algiers. Atmos. Res. 2009, 92, 258–269. [Google Scholar] [CrossRef]
- Muendo, M.; Hanai, Y.; Kameda, Y.; Masunaga, S. Polycyclic aromatic hydrocarbons in urban air: Concentration levels, patterns, and source analysis in Nairobi, Kenya. Environ. Forensics 2006, 7, 147–157. [Google Scholar] [CrossRef]
- Geldenhuys, G.; Rohwer, E.R.; Naudé, Y.; Forbes, P.B.C. Monitoring of atmospheric gaseous and particulate polycyclic aromatic hydrocarbons in South African platinum mines utilising portable denuder sampling with analysis by thermal desorption-comprehensive gas chromatography-mass spectrometry. J. Chromatogr. 2015, 1380, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Val, S.; Liousse, C.; Galy-Lacaux, C.; Cachier, H.; Marchand, N.; Badel, A.; Baeza-Squiban, A. Physico-chemical characterization of African urban aerosols (Bamako in Mali and Dakar in Senegal) and their toxic effects in human bronchial epithelial cells: Description of a worrying situation. Part. Fibre Toxicol. 2013, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.T.; Wirmvem, M.J.; Sawyerr, V.H.; Nakai, S. Characterization and determination of PM2.5 bound polycyclic aromatic hydrocarbons (PAHS) in indoor and outdoor air in western Sierra Leone. J. Environ. Anal. Toxicol. 2015, 5, 2161-0525. [Google Scholar]
- Arinaitwe, K.; Kiremire, B.T.; Muir, D.C.; Fellin, P.; Li, H.; Teixeira, C.; Mubiru, D.N. Atmospheric concentrations of polycyclic aromatic hydrocarbons in the watershed of Lake Victoria, East Africa. Environ. Sci. Technol. 2012, 46, 11524–11531. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, S.C.; Ho, K.F.; Wang, X.M.; Zou, S.C. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos. Environ. 2003, 37, 5307–5317. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Sexton, K.; Linder, S.H.; Marko, D.; Bethel, H.; Lupo, P.J. Comparative assessment of air pollution-related health risks in Houston. Environ. Health. Perspect. 2007, 115, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, N.; Cuadras, A.; Rovira, E.; Marcé, R.M.; Borrull, F. Risk assessment related to atmospheric polycyclic aromatic hydrocarbons in gas and particle phases near industrial sites. Environ. Health Perspect. 2011, 119, 1110. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.K.; Khoder, M.I. Gas-particle concentration, distribution, and health risk assessment of polycyclic aromatic hydrocarbons at a traffic area of Giza. Egypt. Environ. Monit. Assess. 2012, 184, 3593–3612. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.Y. Numerical Study of the Seasonal Variation of Elevated Dust Aerosols from the Taklimakan Desert. SOLA 2012, 8, 98–102. [Google Scholar] [CrossRef]
- Griffin, D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Diehl, T.; Ginoux, P.; Malm, W. Intercontinental transport of pollution and dust aerosols: Implications for regional air quality. Atmos. Chem. Phys. Discuss. 2007, 7, 5501–5517. [Google Scholar] [CrossRef]
- Sanchez de la Campa, A.; García-Salamanca, A.; Solano, J.; de la Rosa, J.; Ramos, J.L. Chemical and microbiological characterization of atmospheric particulate matter during an intense African dust event in Southern Spain. Environ. Sci. Technol. 2013, 47, 3630–3638. [Google Scholar] [CrossRef] [PubMed]
- Prospero, J.M.; Blades, E.; Naidu, R.; Mathison, G.; Thani, H.; Lavoie, M.C. Relationship between African dust carried in the Atlantic trade winds and surges in pediatric asthma attendances in the Caribbean. Int. J. Biometeorol. 2008, 52, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Matuka, O.; Singh, T.S.; Bryce, E.; Yassi, A.; Kgasha, O.; Zungu, M.; O’Hara, L. Pilot study to detect airborne Mycobacterium tuberculosis exposure in a South African public healthcare facility outpatient clinic. J. Hosp. Infect. 2015, 89, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.M.; McLetchie, S.; Knight, R.; Fierer, N. Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. ISME J. 2011, 5, 601. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.E.; Ibrahim, H.Y.; Yousef, F.A.; Elnasr, A.A.; Saeed, Y.; Hameed, A.A. A study on microbiological contamination on air quality in hospitals in Egypt. Indoor Built Environ. 2018, 27, 963–968. [Google Scholar] [CrossRef]
- Rahoma, U.A. Estimation of Pathogenic Microorganisms during Atmospheric Tempestat North Africa. Am. Med. J. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Abdel-Rahim, I.R.; Nafady, N.A.; Bagy, M.M.K.; Abd-Alla, M.H.; Abd-Alkader, A.M. Fungi-induced paint deterioration and air contamination in the Assiut University hospital, Egypt. Indoor. Built. Environ. 2018. [Google Scholar] [CrossRef]
- Setlhare, G.; Malebo, N.; Shale, K.; Lues, R. Identification of airborne microbiota in selected areas in a health-care setting in South Africa. BMC Microbiol. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, C.A.; Griffin, D.W.; Garrison, V.H.; Peak, K.K.; Royall, N.; Smith, R.R.; Shinn, E.A. Characterization of aerosolized bacteria and fungi from desert dust events in Mali, West Africa. Aerobiologia 2004, 20, 99–110. [Google Scholar] [CrossRef]
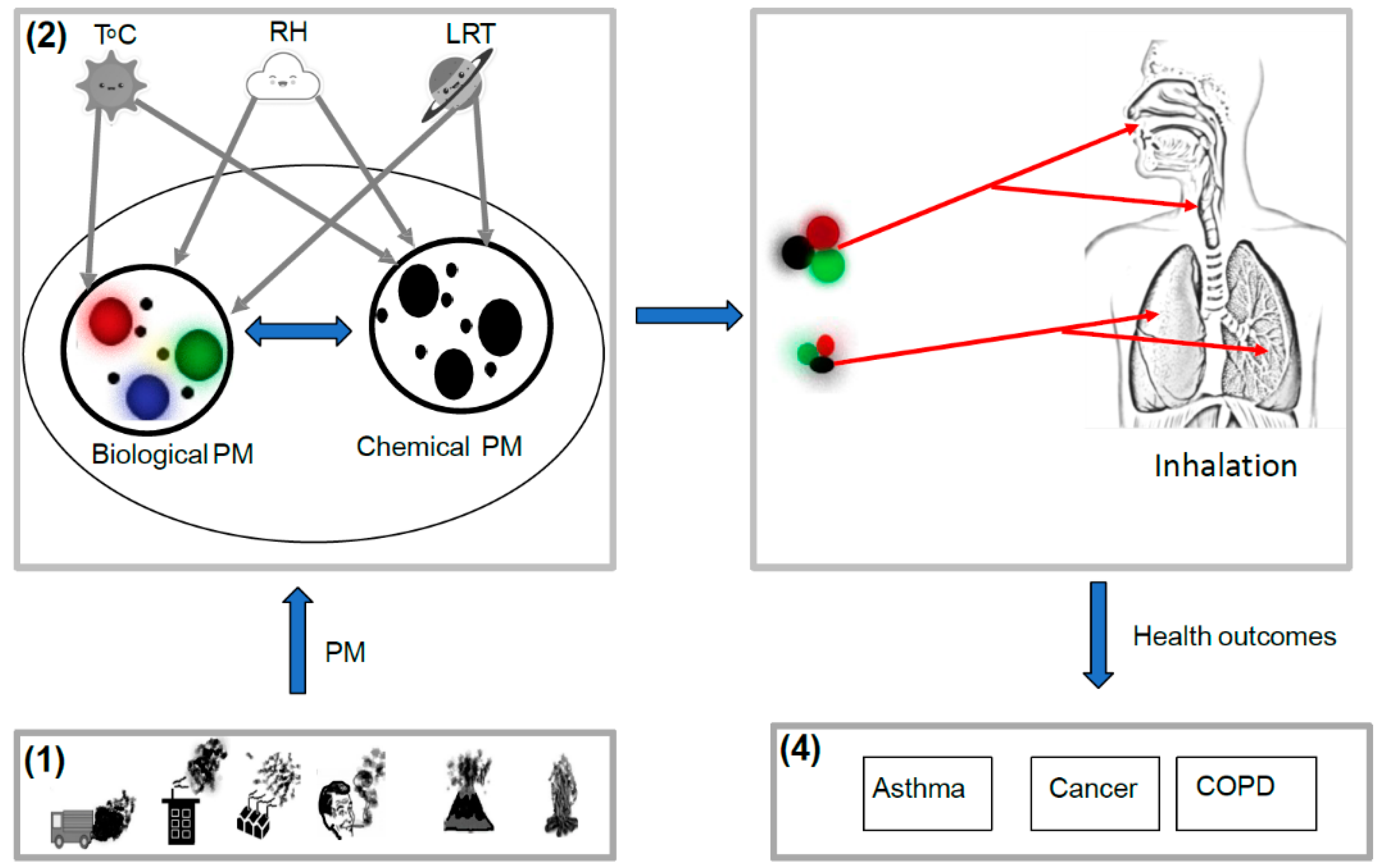
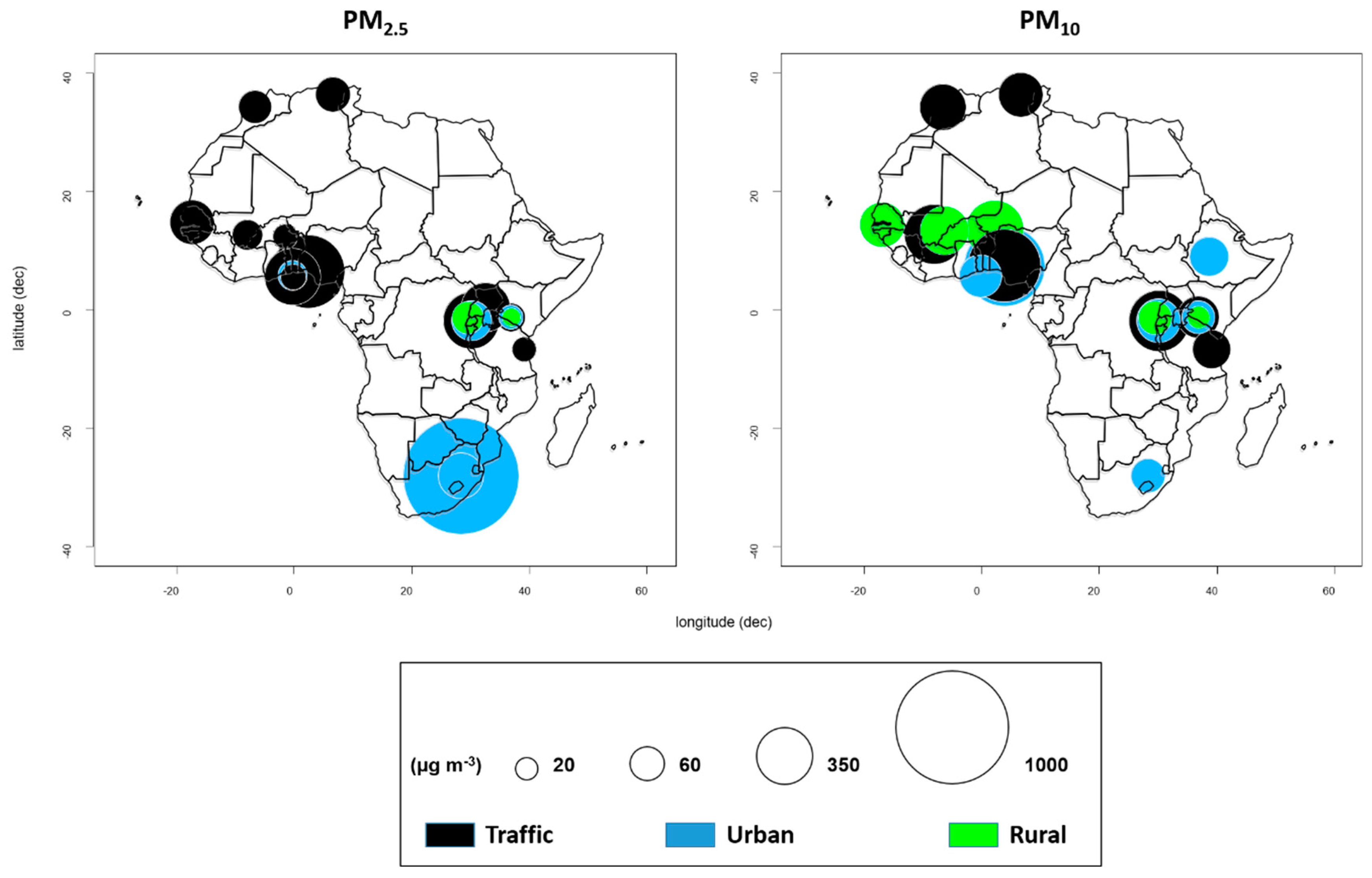
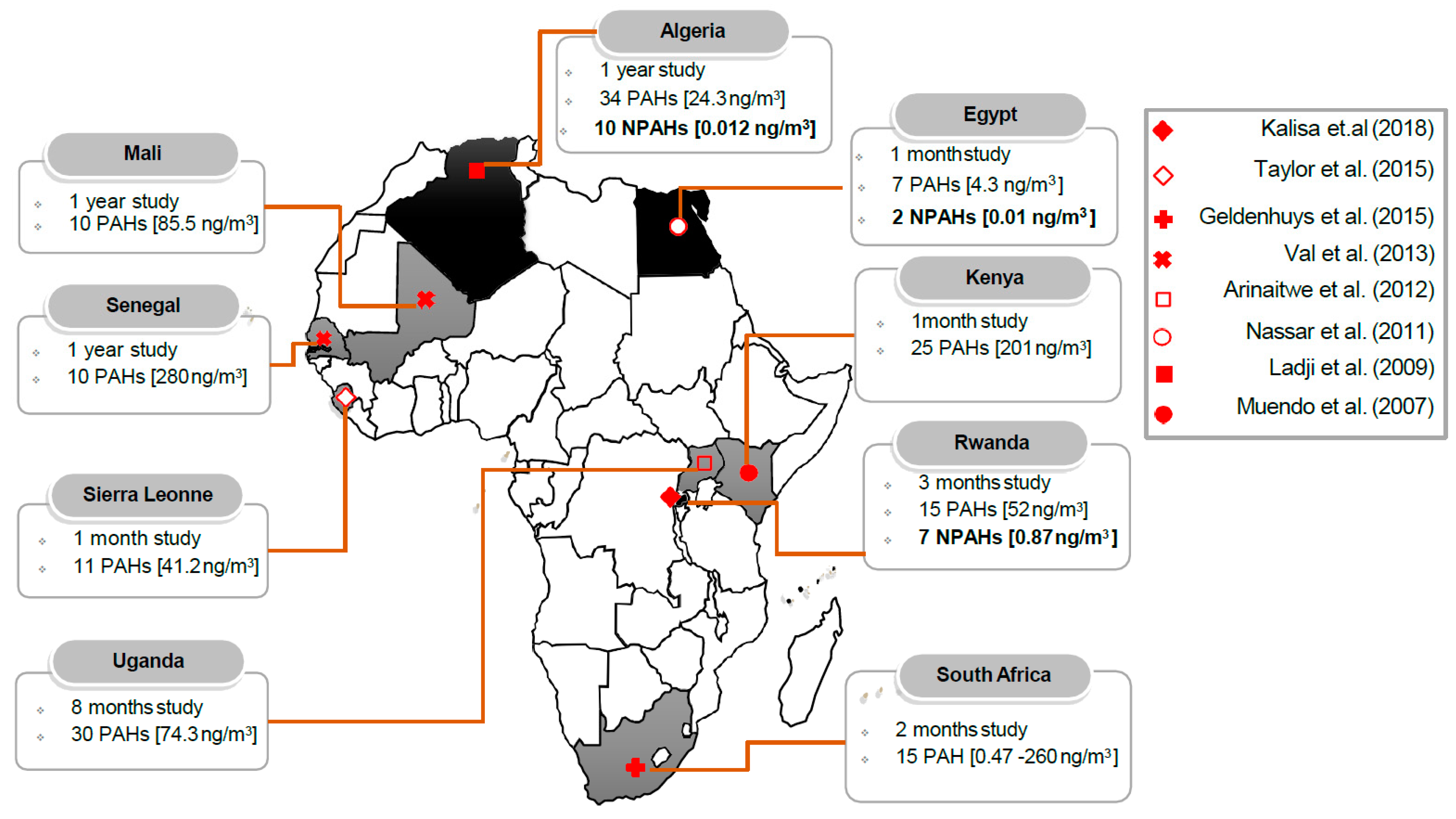
| Study | Study Location | Type of Study | Study Population | Statistical Analysis | PM Size Fraction | Association and Health Outcome |
|---|---|---|---|---|---|---|
| Mentz et al. [60] | Durban, South Africa | Longitudinal | N = 423 school children | Generalized estimating equation (GEE); 0–5 day lags; single lags and distributed lags | PM10 and PM2.5 | Exposure to PM10 was associated with significantly increased occurrence of respiratory symptoms among children (cough, shortness of breath, and chest tightness). |
| Lin et al. [61] | South Africa, Ghana | Cross-sectional | N = 45,625, global aging and adult health | Logistic regression—3-level multilevel model | PM2.5 | PM2.5 was found to be associated with overall disability and with cognition and mobility. |
| Makamure et al. [62] | Kwazulu-Natal, South Africa | Longitudinal/questionnaire | N = 71, Children ages 7–9 | Linear multivariate | PM10 and PM2.5 | Air pollution exposure results in increased expression of cluster of differentiation (CD14) in airway macrophages. |
| Ana et al. [63] | Ibadan, Nigeria | Cross-sectional | N = 140 ages 15–65 years | ANOVA and Spearman-rank correlation | PM10 | Higher PM10 burden was observed to cause declining lung function. |
| Wichmann & Voyi [64] | Cape Town, South Africa | Case-crossover | N = 149,667 (RD = 13,439; CVD = 21,569; CVD = 7594) | Logistic regression | PM10 | PM10 was associated with cardiovascular disease, respiratory disease, cerebrovascular disease, and mortality. |
| Mustapha et al. [65] | Ibadan, Nigeria | Cross-sectional | N = 1397 Schoolchildren (7–14 years) | Logistic regression | TSP, PM2.5 and PM10 | Traffic pollution was associated with respiratory symptoms (wheeze, night cough, phlegm, rhinitis, and asthma in school children). |
| Kaphingst et al. [66] | Durban, South Africa | Longitudinal | N = 873 schoolchildren | Regression models | PM10 and PM2.5 | Schoolchildren living near industries were more likely to develop asthma and airway hyperreactivity rather than those living far away from industries. |
| Reference | City, Country | Type of Site | PM Size | Main PAH Detected | Main NPAHs Detected | Source Identified | Association and Health Outcome |
|---|---|---|---|---|---|---|---|
| Kalisa et al. [34] | Kigali, Rwanda | Roadside/ambient air | PM2.5 and PM10 | BPe, Phe, Flu, BaP, and BbF | 9-NA, 2-NP+2-NFR, 6-NBaP | Wood burning and automobile emissions | The lifetime excess cancer risk exceeding the WHO guideline values and classified as definite risks. |
| Taylor et al. [121] | Western Sierra Leone | Residence/ambient air and indoor air | PM2.5 | Phe, DBA, and BPe | Burning wood | PAHs bound PM2.5 from biomass fuel from kitchens continue to be hazardous for people of developing countries. | |
| Geldenhuys et al. [119] | South Africa | Underground/ambient air | TSP | Pyr, Flu, and BaP | Diesel vehicle | Diesel exhaust emissions—recently confirmed as carcinogenic which is why the health of underground workers is of concern. | |
| Val et al. [120] | Bamako, Mali | Desert area/ambient air | PM10 | IDP, BPe, BbF, and BaP | Traffic, biomass burning, and dust | The population of Mali—highly exposed to toxic particulate pollution that could lead to strong adverse health effects. | |
| Dieme et al. [55] | Dakar (Senegal) | Urban/ambient air | PM2.5 | BbF, BPe, IDP, and BaP | Combustion of fossil fuels | PAH and Heavy metals in PM2.5 induced with dose-dependent toxicity, relying on inflammatory processes. | |
| Hassan & Khoder [128] | Dokki, Egypt | Urban/ambient air | TSP | BbF, BPe, DBA, and Chr | Unburned fossil fuels and vehicle emissions | PAHs in the particulate phase in the ambient air posing a potential health risk for the population of Egypt. | |
| Arinaitwe et al. [122] | Entebbe, Uganda | Watershed/ambient air | PM2.5 | Phe, Flu, and Pyr | Combustion of petroleum and biomass burning | Population of Uganda is likely to be exposed to toxic PAHs bound PM2.5 from biomass burning. | |
| Nassar et al. [116] | Great Cairo, Egypt | Traffic side/ambient air | TSP | Phe, Flu, BbF, and Chr | 1-NP | Gasoline engine | PAHs and NPAHs with carcinogenic and/or mutagenic health effects detected in Greater Cairo. |
| Ladji et al. [117] | Algiers, Algeria | Suburban/ambient air | PM10 | Acy, Phe, and BbF | 9-NA, 2-NFR | Motor vehicles | The population of Algeria exposed to the occurrence of nicotine in particulates associated with PAHs. |
| Muendo et al. [118] | Nairobi, Kenya | Traffic/ambient air | PM10 | Pyr, BbF, and BPe | Gasoline and diesel | Contribution of carcinogenic PAHs bound PM10 in Nairobi—approximately 30%. |
| Study | Study Location | PM Size | Biological Components Analyzed | Enumeration Techniques. | Dominant Species Identified | Association and Health Outcome |
|---|---|---|---|---|---|---|
| Abdel-Rahim et al. [138] | Assiut, Egypt | TSP | Fungi | Culture-dependent | Chaetomium globosum, Aspergillus parasiticus, Penicillium oxalicum, and Setosphaeria rostrata | The current study suggests that improvement of antimicrobial additives of paints may be a promising approach to reduce paint biodeterioration and, subsequently, air contamination of indoor environments. |
| Osman et al. [136] | Bolak, Egypt | >8 µm and <8 µm | Bacteria/Fungi | Culture-dependent | Bacillus licheniformis, Aspergillus, and Penicillium | Dust particles accumulated in air conditioning filters and floor surfaces and these would constitute important sources of airborne bacteria and fungi inside these hospitals. |
| Setlhare et al. [139] | South Africa | TSP | Bacteria/Fungi | Culture-dependent | Bacillus, Kocuria, Staphylococcus, Arthrobacter, Candida, Aureobasidium, Penicillium, and Phoma | Airborne bacteria and fungi that cause disease, especially in those populations with suppressed host immunity defenses in South Africa. Fungal genera identified (e.g., Candida), causes food spoilage and fungal infections in human |
| Rahoma [137] | Tobruk, Libya | 0.2 µm | Bacteria/Fungi | Culture-dependent | Bacillus thuringiensis and Cladosporium sp. Trichophyton sp. | Inhalation of associated pathogenic viable microorganisms and chemical contaminants such as carcinogens and small particles may trigger other physiological reactions (e.g., asthma and cardiovascular events) in humans. |
| Kellogg et al. [140] | Bamako, Mali | TSP | Bacteria/Fungi | Culture-dependent | Acinetobacter calcoaceticus, Bacillus mycoides, Bacillus pumilus, Bacillus subtilis, and Cladosporium cladosporioides | Opportunistic human pathogens were isolated from air sample and could cause severe respiratory diseases |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalisa, E.; Archer, S.; Nagato, E.; Bizuru, E.; Lee, K.; Tang, N.; Pointing, S.; Hayakawa, K.; Lacap-Bugler, D. Chemical and Biological Components of Urban Aerosols in Africa: Current Status and Knowledge Gaps. Int. J. Environ. Res. Public Health 2019, 16, 941. https://doi.org/10.3390/ijerph16060941
Kalisa E, Archer S, Nagato E, Bizuru E, Lee K, Tang N, Pointing S, Hayakawa K, Lacap-Bugler D. Chemical and Biological Components of Urban Aerosols in Africa: Current Status and Knowledge Gaps. International Journal of Environmental Research and Public Health. 2019; 16(6):941. https://doi.org/10.3390/ijerph16060941
Chicago/Turabian StyleKalisa, Egide, Stephen Archer, Edward Nagato, Elias Bizuru, Kevin Lee, Ning Tang, Stephen Pointing, Kazuichi Hayakawa, and Donnabella Lacap-Bugler. 2019. "Chemical and Biological Components of Urban Aerosols in Africa: Current Status and Knowledge Gaps" International Journal of Environmental Research and Public Health 16, no. 6: 941. https://doi.org/10.3390/ijerph16060941
APA StyleKalisa, E., Archer, S., Nagato, E., Bizuru, E., Lee, K., Tang, N., Pointing, S., Hayakawa, K., & Lacap-Bugler, D. (2019). Chemical and Biological Components of Urban Aerosols in Africa: Current Status and Knowledge Gaps. International Journal of Environmental Research and Public Health, 16(6), 941. https://doi.org/10.3390/ijerph16060941





