Eight Weeks of Aerobic Interval Training Improves Psychomotor Function in Patients with Parkinson’s Disease—Randomized Controlled Trial
Abstract
1. Introduction
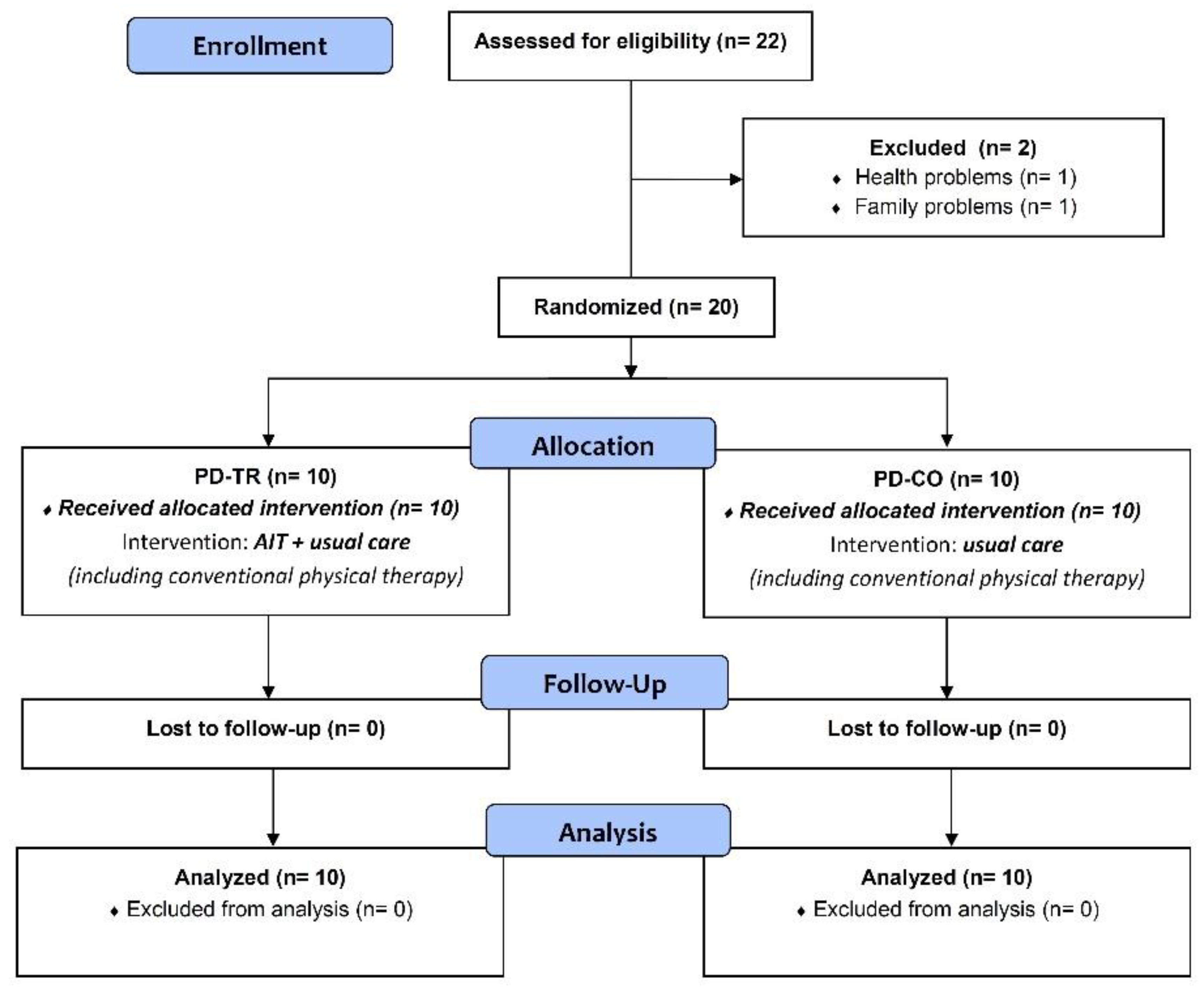
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedures
2.3. Aerobic Interval Training Protocol
2.4. Primary Outcomes
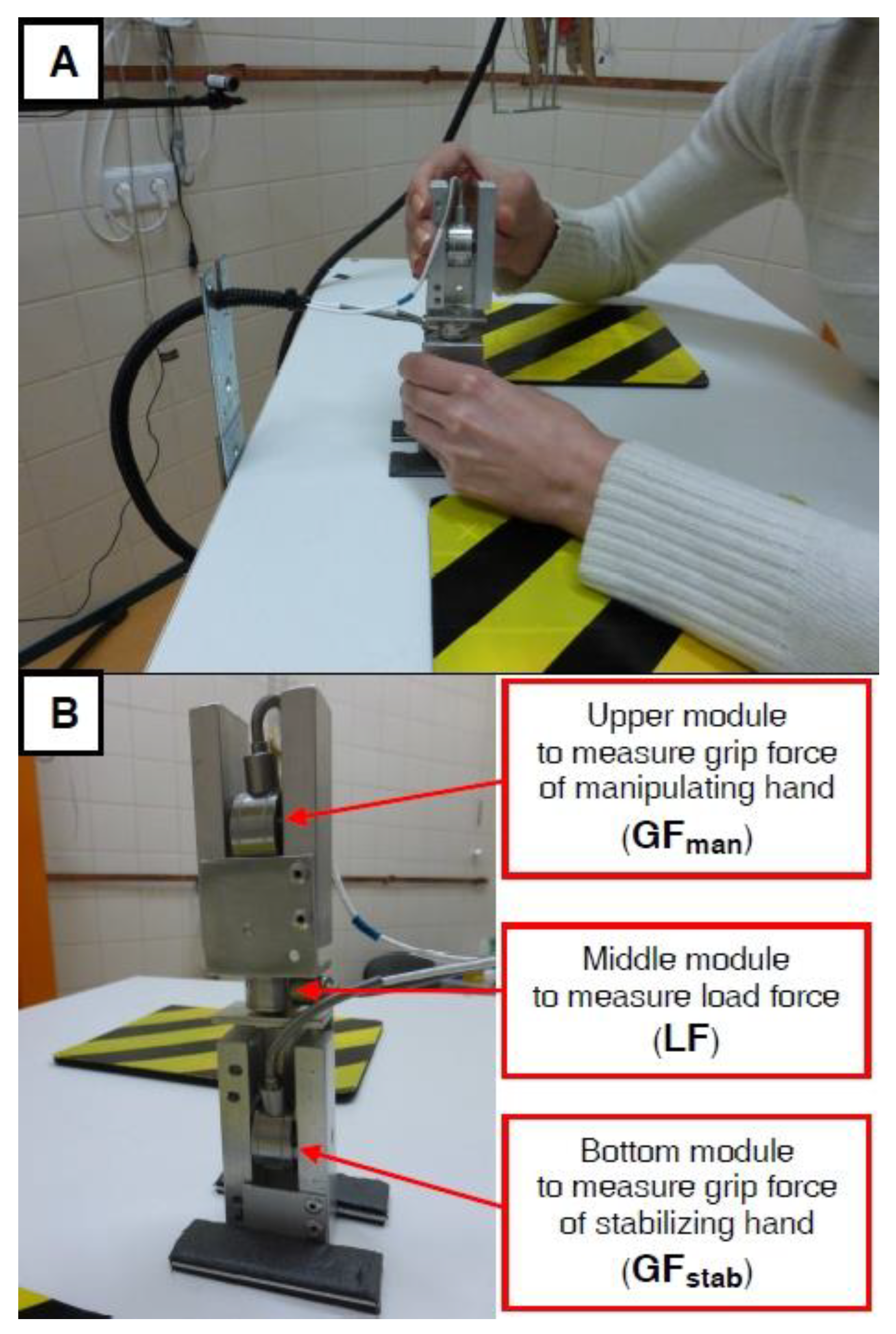
2.4.1. Bimanual Motor Control Recording and Analysis
2.4.2. Psychological Assessment of Cognitive Function
2.5. Secondary Outcomes
Neurological Assessment of Parkinson’s Disease Signs
2.6. Statistical Analysis
3. Results
3.1. Inter-Group Comparison (PD-TR vs. PD-CO) of Clinical and Anthropometric Parameters
3.2. Data on Exercise Intensity and Attendance at AIT Testing Sessions
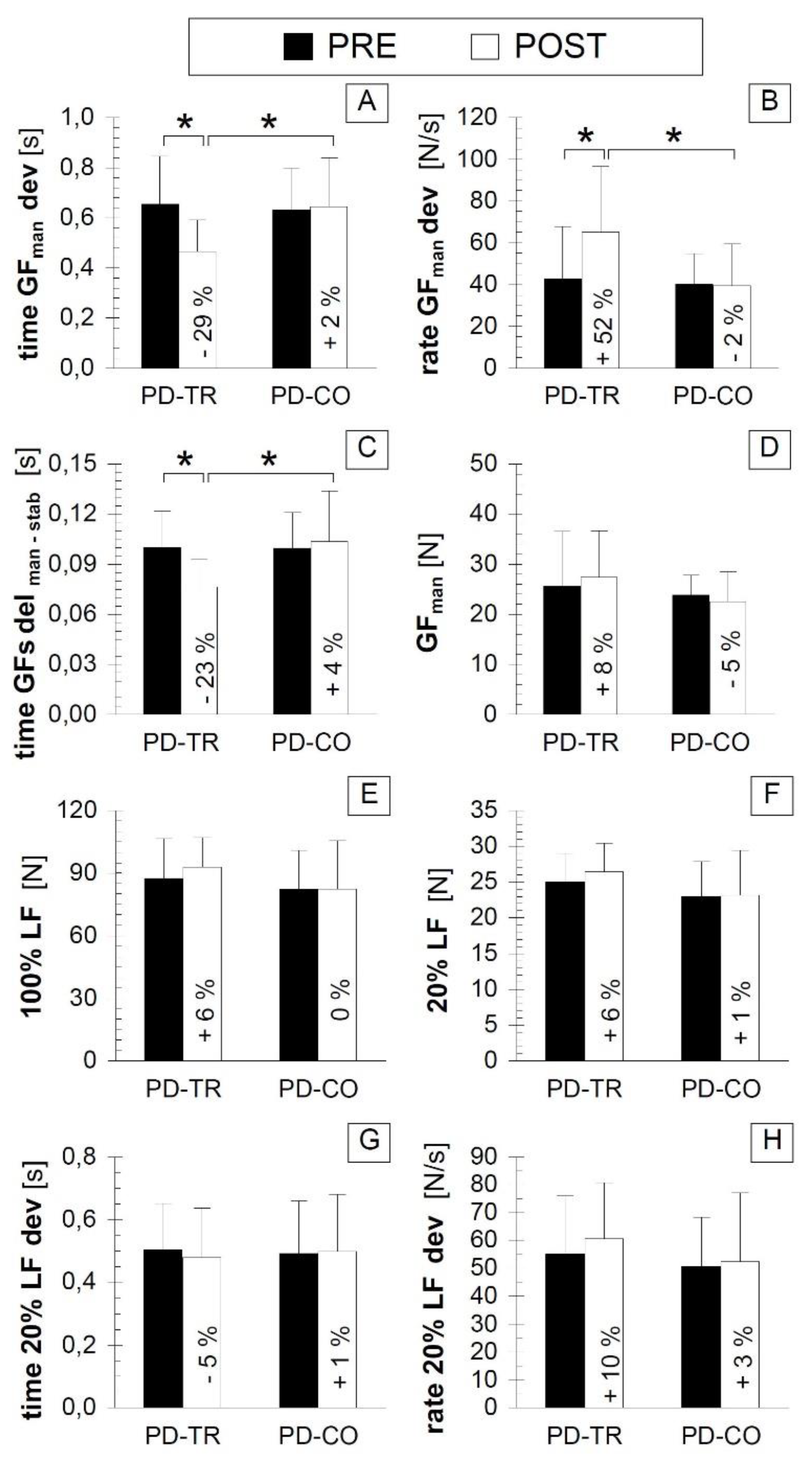
3.3. Bimanual Motor Control Outcomes
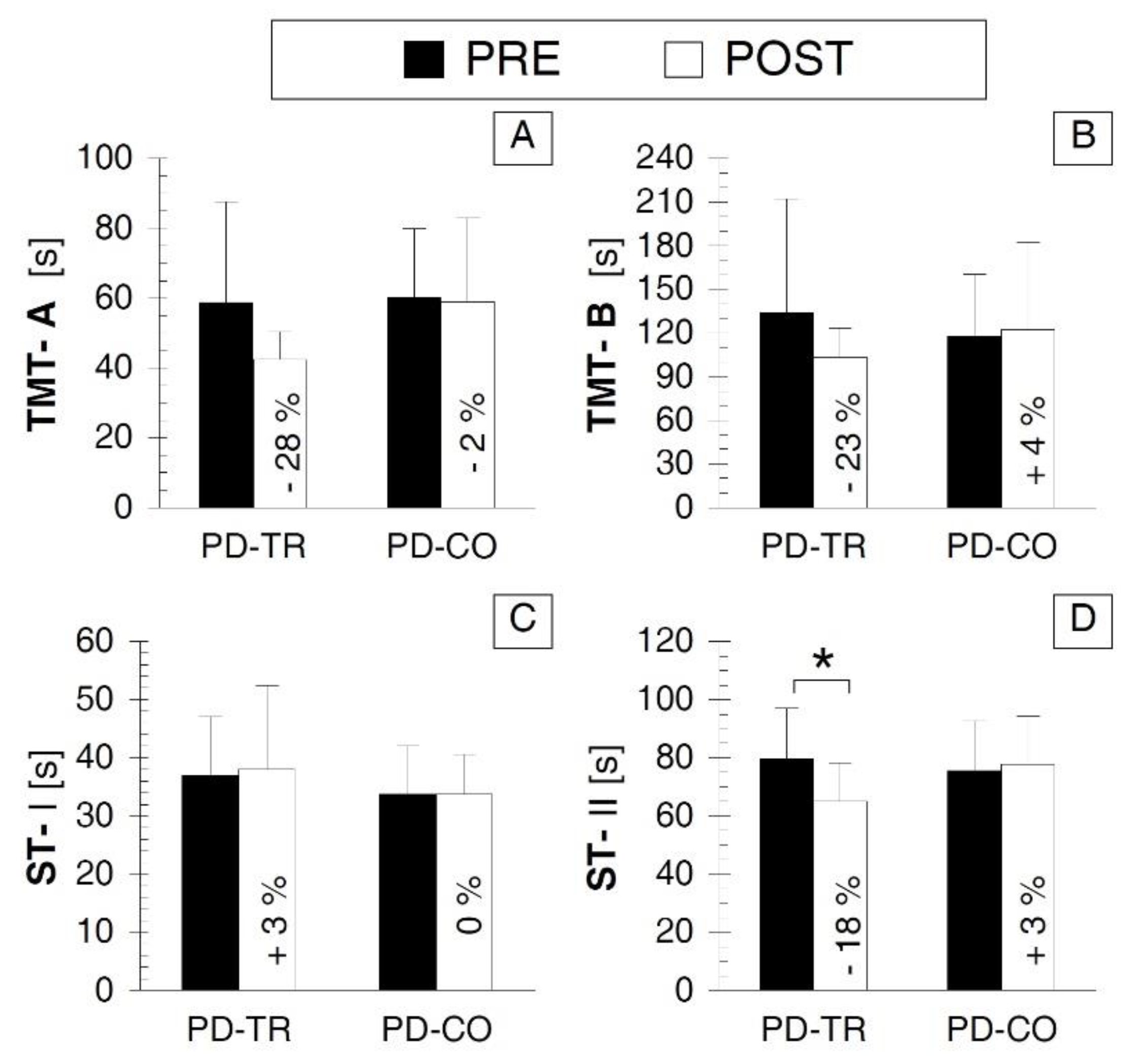
3.4. Psychological Cognitive Outcomes
3.5. Neurological Outcomes
4. Discussion
4.1. Why AIT May Be An Advantageous Treatment Approach for PD
4.2. AIT-Induced Improvement in Bimanual Motor Control
4.3. AIT-Induced Improvement in Executive Function
4.4. AIT-Induced Improvement in Motor and Non-Motor Signs of PD
4.5. Limitations and Implications of Our Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galvan, A.; Wichmann, T. Pathophysiology of parkinsonism. Clin. Neurophysiol. 2008, 119, 1459–1474. [Google Scholar] [CrossRef] [PubMed]
- Ponsen, M.M.; Daffertshofer, A.; van den Heuvel, E.; Wolters, E.Ch.; Beek, P.J.; Berendse, H.W. Bimanual coordination dysfunction in early, untreated Parkinson’s disease. Parkinsonism Relat. Disord. 2006, 12, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Sabatini, U.; Chollet, F.; Fabre, N.; Senard, J.M.; Montastruc, J.L.; Celsis, P.; Marc-Vergnes, J.P.; Rascol, A. Normal activation of the supplementary motor area in patients with Parkinson’s disease undergoing long-term treatment with levodopa. J. Neurol. Neurosurg. Psychiatry 1994, 57, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Ceballos-Baumann, A.O.; Blin, J.; Uema, T.; Boecker, H.; Passingham, R.E.; Brooks, D.J. Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain 1997, 120, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, L.; Hallett, M.; Li, K.; Chan, P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain 2010, 133, 2394–2409. [Google Scholar] [CrossRef] [PubMed]
- Haslinger, B.; Erhard, P.; Kämpfe, N.; Boecker, H.; Rummeny, E.; Schwaiger, M.; Conrad, B.; Ceballos-Baumann, A.O. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 2001, 124, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.L.; Lyons, K.E.; Pahwa, R.; Sethi, K.; Stern, M.; Hauser, R.A.; Olanow, W.; Gray, A.M.; Adams, B.; Earl, N.L.; et al. Onset of dyskinesia with adjunct ropinirole prolonged-release or additional levodopa in early Parkinson’s disease. Mov. Disord. 2010, 25, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.E.; Petzinger, G.M.; Nixon, K.; Hogg, E.; Bremmer, S.; Meshul, C.K.; Jakowec, M.W. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl–1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J. Neurosci. Res. 2004, 77, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Petzinger, G.M.; Walsh, J.P.; Akopian, G.; Hogg, E.; Abernathy, A.; Arevalo, P.; Turnquist, P.; Vucković, M.; Fisher, B.E.; Togasaki, D.M.; Jakowec, M.W. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl–1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. 2007, 27, 5291–5300. [Google Scholar] [CrossRef] [PubMed]
- Vučković, M.G.; Li, Q.; Fisher, B.; Nacca, A.; Leahy, R.M.; Walsh, J.P.; Mukherjee, J.; Williams, C.; Jakowec, M.W.; Petzinger, G.M. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: In vivo imaging with [18F] fallypride. Mov. Disord. 2010, 25, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Chalimoniuk, M.; Chrapusta, S.J.; Lukačova, N.; Langfort, J. Endurance training upregulates the nitric oxide/soluble guanylyl cyclase/cyclic guanosine 3′,5′-monophosphate pathway in the striatum, midbrain and cerebellum of male rats. Brain. Res. 2015, 1618, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Langfort, J.; Chalimoniuk, M.; Kania, D.; Lukačova, N.; Chrapusta, S.J. Endurance training counteracts MPTP treatment-related changes in midbrain contents of dopamine and dopamine metabolites, and in parvalbumin expression. In Proceedings of the XX World Congress on Parkinson’s disease and Related Disorders, Geneva, Switzerland, 8–11 December 2013; p. 135. [Google Scholar]
- Toy, W.A.; Petzinger, G.M.; Leyshon, B.J.; Akopian, G.K.; Walsh, J.P.; Hoffman, M.V.; Vučković, M.G.; Jakowec, M.W. Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl–1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol. Dis. 2014, 63, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.E.; Wu, A.D.; Salem, G.J.; Song, J.; Lin, C.H.; Yip, J.; Cen, S.; Gordon, J.; Jakowec, M.; Petzinger, G. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch. Phys. Med. Rehabil. 2008, 89, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.E.; Li, Q.; Nacca, A.; Salem, G.J.; Song, J.; Yip, J.; Hui, J.S.; Jakowec, M.W.; Petzinger, G.M. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport 2013, 24, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Beall, E.B.; Frankemolle, A.M.; Penko, A.; Phillips, M.D.; Lowe, M.J.; Alberts, J.L. Exercise Therapy for Parkinson’s Disease: Pedaling Rate Is Related to Changes in Motor Connectivity. Brain. Connect. 2016, 6, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Tabak, R.; Aquije, G.; Fisher, B.E. Aerobic exercise to improve executive function in Parkinson disease: a case series. J. Neurol. Phys. Ther. 2013, 37, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tuon, T.; Valvassori, S.S.; Dal Pont, G.C.; Paganini, C.S.; Pozzi, B.G.; Luciano, T.F.; Souza, P.S.; Quevedo, J.; Souza, C.T.; Pinho, R.A. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain. Res. Bull. 2014, 108, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Uc, E.Y.; Doerschug, K.C.; Magnotta, V.; Dawson, J.D.; Thomsen, T.R.; Kline, J.N.; Rizzo, M.; Newman, S.R.; Mehta, S.; Grabowski, T.J.; et al. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 2014, 83, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Majerczak, J.; Zeligowska, E.; Mencel, J.; Jaskolski, A.; Jaskolska, A.; Marusiak, J. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J. Physiol. Pharmacol. 2014, 65, 441–448. [Google Scholar] [PubMed]
- Ridgel, A.L.; Vitek, J.L.; Alberts, J.L. Forced; not voluntary; exercise improves motor function in Parkinson’s disease patients. Neurorehabil. Neural. Repair 2009, 23, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, C.; Lungu, O.; Nadeau, A.; Robillard, M.E.; Boré, A.; Bobeuf, F.; Lafontaine, A.L.; Gheysen, F.; Bherer, L.; Doyon, J. Enhancing both motor and cognitive functioning in Parkinson’s disease: Aerobic exercise as a rehabilitative intervention. Brain. Cogn. 2015, 99, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Marusiak, J.; Żeligowska, E.; Mencel, J.; Kisiel-Sajewicz, K.; Majerczak, J.; Zoladz, J.A.; Jaskólski, A.; Jaskólska, A. Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor. J. Rehabil. Med. 2015, 47, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Close, G.L.; MacLaren, D.P.; Gregson, W.; Drust, B.; Morton, J.P. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: Implications for exercise adherence. J. Sports Sci. 2011, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A.; Angadi, S.S. High-intensity interval training for health and fitness: Can less be more? J. Appl. Physiol. 2011, 111, 1540–1541. [Google Scholar] [CrossRef] [PubMed]
- Moholdt, T.T.; Amundsen, B.H.; Rustad, L.A.; Wahba, A.; Løvø, K.T.; Gullikstad, L.R.; Bye, A.; Skogvoll, E.; Wisløff, U.; Slørdahl, S.A. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: A randomized study of cardiovascular effects and quality of life. Am. Heart J. 2009, 158, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnott, K.; Frank, J. Trail making test; part B as a measure of executive control: Validation using a set-switching paradigm. J. Clin. Exp. Neuropsychol. 2000, 22, 518–528. [Google Scholar] [CrossRef]
- Sánchez-Cubillo, I.; Periáñez, J.A.; Adrover-Roig, D.; Rodríguez-Sánchez, J.M.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching; working memory; inhibition/interference control; and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Egner, T.; Hirsch, J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 2005, 24, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Van der Elst, W.; Van Boxtel, M.P.; Van Breukelen, G.J.; Jolles, J. The Stroop color-word test: Influence of age; sex; and education; and normative data for a large sample across the adult age range. Assessment 2006, 13, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Zalonis, I.; Christidi, F.; Bonakis, A.; Kararizou, E.; Triantafyllou, N.I.; Paraskevas, G.; Kapaki, E.; Vasilopoulos, D. The stroop effect in Greek healthy population: Normative data for the Stroop Neuropsychological Screening Test. Arch. Clin. Neuropsychol. 2009, 24, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Seubert-Ravelo, A.N.; Yáñez-Téllez, M.G.; Salgado-Ceballos, H.; Escartín-Pérez, R.E.; Neri-Nani, G.A.; Velázquez-Osuna, S. Mild Cognitive Impairment in Patients with Early-Onset Parkinson’s Disease. Dement. Geriatr. Cogn. Disord. 2016, 42, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Elton, R.L.; UPDRS program members. Unified Parkinsons Disease Rating Scale. In Recent Developments in Parkinson’s Disease; Fahn, S., Marsden, C.D., Goldstein, M., Calne, D.B., Eds.; Macmillan Healthcare Information: Florham Park, NJ, USA, 1987; Volume 2, pp. 153–163. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Alberts, J.L.; Linder, S.M.; Penko, A.L.; Lowe, M.J.; Phillips, M. It is not about the bike; it is about the pedaling: Forced exercise and Parkinson’s disease. Exerc. Sport Sci. Rev. 2011, 39, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Prigent-Tessier, A.; Quirié, A.; Maguin-Gaté, K.; Szostak, J.; Mossiat, C.; Nappey, M.; Devaux, S.; Marie, C.; Demougeot, C. Physical training and hypertension have opposite effects on endothelial brain-derived neurotrophic factor expression. Cardiovasc. Res. 2013, 100, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Afzalpour, M.E.; Chadorneshin, H.T.; Foadoddini, M.; Eivari, H.A. Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol. Behav. 2015, 147, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Saucedo Marquez, C.M.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. (1985) 2015, 119, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.; Juri, C.; Baes, M.; Wyneken, U.; Rubio, F.J. Intranigral transplantation of epigenetically induced BDNF-secreting human mesenchymal stem cells: Implications for cell-based therapies in Parkinson’s disease. Biol. Blood Marrow Transplant. 2010, 16, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Slevin, J.T.; Gerhardt, G.A.; Smith, C.D.; Gash, D.M.; Kryscio, R.; Young, B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 2005, 102, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, M.; Khalid, U.; Klein, A.B.; Aznar, S.; Thomsen, G.; Jensen, P.; Knudsen, G.M. Striatal dopamine transporter binding correlates with serum BDNF levels in patients with striatal dopaminergic neurodegeneration. Neurobiol. Aging 2012, 33, 428.e1–428.e5. [Google Scholar] [CrossRef] [PubMed]
- Ridgel, A.L.; Kim, C.H.; Fickes, E.J.; Muller, M.D.; Alberts, J.L. Changes in executive function after acute bouts of passive cycling in Parkinson’s disease. J. Aging Phys. Act. 2011, 19, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Gmiąt, A.; Jaworska, J.; Micielska, K.; Kortas, J.; Prusik, K.; Prusik, K.; Lipowski, M.; Radulska, A.; Szupryczyńska, N.; Antosiewicz, J.; et al. Improvement of cognitive functions in response to a regular Nordic walking training in elderly women—A change dependent on the training experience. Exp. Gerontol. 2018, 104, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.O.; Otto, M.W.; Ellis, T.D.; Cronin-Golomb, A. The Therapeutic Potential of Exercise to Improve Mood; Cognition; and Sleep in Parkinson’s Disease. Mov. Disord. 2016, 31, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.H.; Liou, T.H.; Chou, K.R.; Chi, W.C.; Yen, C.F.; Liao, H.F.; Tseng, I.J. Large-Scale Assessment of Function and Disability in Patients with Parkinson’s Disease Using the Functioning Disability Evaluation Scale-Adult Version. Int. J. Environ. Res. Public Health 2018, 15, 2788. [Google Scholar] [CrossRef] [PubMed]
- Findley, L.; Aujla, M.; Bain, P.G.; Baker, M.; Beech, C.; Bowman, C.; Holmes, J.; Kingdom, W.K.; MacMahon, D.G.; Peto, V.; et al. Direct economic impact of Parkinson’s disease: A research survey in the United Kingdom. Mov. Disord. 2003, 18, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
| PD Patient No. | Age (Years) | Sex (F/M) | Body Mass (kg) | Height (cm) | Disease Onset/Duration (Years) | Affected Upper Limb (R/L) | Dominant Upper Limb (R/L) | Hoehn and Yahr (Points) |
|---|---|---|---|---|---|---|---|---|
| PD-TR#01 | 79 | M | 71 | 157 | 69/10 | R | R | 2.5 |
| PD-TR#02 | 65 | F | 64 | 162 | 58/7 | L | R | 2.5 |
| PD-TR#03 | 68 | M | 78 | 167 | 55/13 | R | R | 2.5 |
| PD-TR#04 | 65 | F | 61 | 155 | 63/2 | R | R | 1.5 |
| PD-TR#05 | 60 | M | 79 | 169 | 46/14 | R | R | 3.0 |
| PD-TR#06 | 84 | M | 73 | 165 | 80/4 | L | R | 1.5 |
| PD-TR#07 | 68 | M | 67 | 177 | 56/12 | L | R | 2.5 |
| PD-TR#08 | 78 | F | 67 | 159 | 70/8 | L | R | 3.0 |
| PD-TR#09 | 62 | M | 63 | 161 | 46/16 | R | R | 3.0 |
| PD-TR#10 | 88 | F | 67 | 149 | 84/4 | L | R | 2.0 |
| Mean ± SD | 72 ± 10 | - | 69 ± 9 | 162 ± 8 | 63 ± 13/9 ± 5 | - | - | 2.35 ± 0.57 |
| PD-CO#01 | 81 | F | 63 | 159 | 71/10 | L | R | 3.0 |
| PD-CO#02 | 67 | F | 64 | 156 | 63/4 | R | R | 2.0 |
| PD-CO#03 | 81 | M | 67 | 157 | 59/12 | R | R | 2.0 |
| PD-CO#04 | 90 | F | 60 | 148 | 84/6 | L | R | 2.0 |
| PD-CO#05 | 79 | F | 60 | 156 | 69/8 | L | R | 2.5 |
| PD-CO#06 | 72 | M | 78 | 182 | 61/11 | R | R | 2.0 |
| PD-CO#07 | 60 | F | 67 | 153 | 52/8 | R | R | 2.0 |
| PD-CO#08 | 74 | F | 59 | 164 | 69/5 | R | R | 1.5 |
| PD-CO#09 | 66 | F | 70 | 160 | 64/2 | L | R | 1.5 |
| PD-CO#10 | 70 | M | 76 | 166 | 55/15 | R | R | 3.0 |
| Mean ± SD | 74 ± 9 | - | 66 ± 7 | 160 ± 9 | 66 ± 9/8 ± 4 | - | - | 2.25 ± 0.53 |
| Parameters | POST vs. PRE Comparison | PD-TR vs. PD-CO Comparison | |||
|---|---|---|---|---|---|
| PD-TR | PD-CO | PRE | POST | ||
| Bimanual Motor Control Outcomes | time GFman dev | p = 0.013 * d = –1.161 | p = 0.508 d = 0.065 | p = 0.821 d = −0.113 | p = 0.025 * d = 1.097 |
| rate GFman dev | p = 0.013 * d = 0.781 | p = 0.816 d = −0.051 | p = 0.793 d = −0.119 | p = 0.044 * d = −0.970 | |
| time GFs del man-stab | p = 0.020 * d = –1.183 | p = 0.592 d = 0.152 | p = 0.993 d = −0.004 | p = 0.023 * d = 1.108 | |
| GFman | p = 0.148 d = 0.189 | p = 0.288 d = −0.252 | p = 0.646 d = −0.209 | p = 0.173 d = −0.635 | |
| 100% LF | p = 0.232 d = 0.304 | p = 0.993 d = 0.001 | p = 0.534 d = −0.284 | p = 0.246 d = −0.536 | |
| 20% LF | p = 0.275 d = 0.357 | p = 0.213 d = 0.026 | p = 0.330 d = −0.448 | p = 0.186 d = −0.619 | |
| time 20% LF dev | p = 0.449 d = −0.153 | p = 0.725 d = 0.034 | p = 0.880 d = −0.068 | p = 0.809 d = 0.110 | |
| rate 20% LF dev | p = 0.123 d = 0.269 | p = 0.645 d = 0.076 | p = 0.705 d = −0.223 | p = 0.424 d = −0.365 | |
| Psychological Outcomes | TMT-A | p = 0.083 d = −0.767 | p = 0.838 d = −0.055 | p = 0.650 d = 0.057 | p = 0.055 d = 0.918 |
| TMT-B | p = 0.216 d = −0.553 | p = 0.722 d = 0.082 | p = 0.558 d = −0.267 | p = 0.359 d = 0.421 | |
| ST-I | p = 0.751 d = 0.097 | p = 0.566 d = 0.000 | p = 0.363 d = −0.336 | p = 0.401 d = −0.385 | |
| ST-II | p = 0.007 * d = –0.953 | p = 0.140 d = 0.136 | p = 0.593 d = −0.244 | p = 0.073 d = 0.853 | |
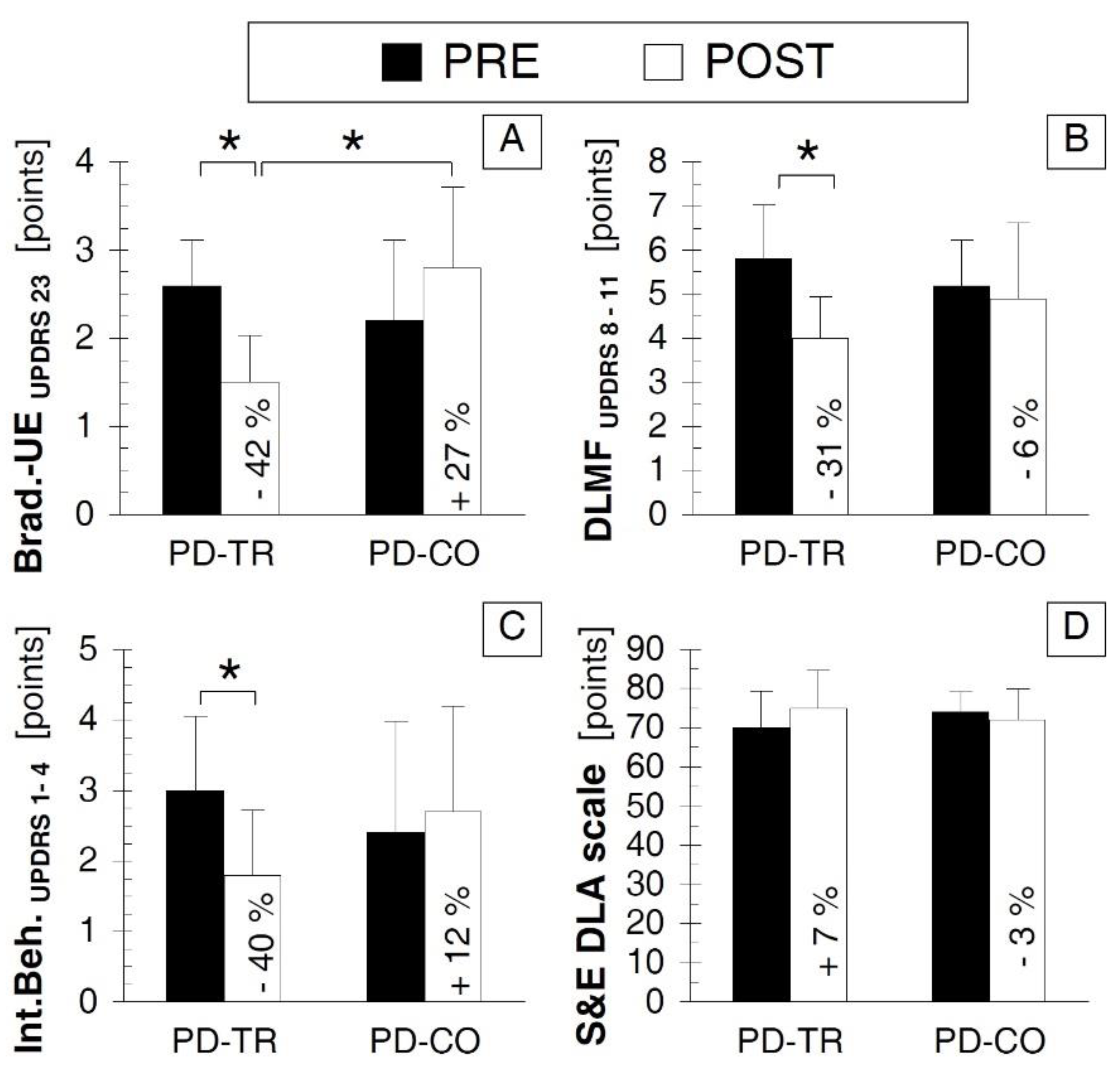
| Neurological Outcomes | Brad.-UEUPDRS 23 | p = 0.015 * d = –2.108 | p = 0.107 d = 0.653 | p = 0.354 d = −0.537 | p = 0.003 * d = 1.735 |
| DLMFUPDRS 8–11 | p = 0.004 * d = –1.643 | p = 0.616 d = 0.211 | p = 0.242 d = −0.528 | p = 0.309 d = 0.646 | |
| Int.Beh. UPDRS 1–4 | p = 0.005 * d = –1.214 | p = 0.279 d = 0.195 | p = 0.331 d = −0.447 | p = 0.122 d = 0.726 | |
| S&E DLA | p = 0.059 d = 0.522 | p = 0.317 d = −0.300 | p = 0.155 d = 0.526 | p = 0.419 d = −0.339 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marusiak, J.; Fisher, B.E.; Jaskólska, A.; Słotwiński, K.; Budrewicz, S.; Koszewicz, M.; Kisiel-Sajewicz, K.; Kamiński, B.; Jaskólski, A. Eight Weeks of Aerobic Interval Training Improves Psychomotor Function in Patients with Parkinson’s Disease—Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 880. https://doi.org/10.3390/ijerph16050880
Marusiak J, Fisher BE, Jaskólska A, Słotwiński K, Budrewicz S, Koszewicz M, Kisiel-Sajewicz K, Kamiński B, Jaskólski A. Eight Weeks of Aerobic Interval Training Improves Psychomotor Function in Patients with Parkinson’s Disease—Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2019; 16(5):880. https://doi.org/10.3390/ijerph16050880
Chicago/Turabian StyleMarusiak, Jarosław, Beth E. Fisher, Anna Jaskólska, Krzysztof Słotwiński, Sławomir Budrewicz, Magdalena Koszewicz, Katarzyna Kisiel-Sajewicz, Bartosz Kamiński, and Artur Jaskólski. 2019. "Eight Weeks of Aerobic Interval Training Improves Psychomotor Function in Patients with Parkinson’s Disease—Randomized Controlled Trial" International Journal of Environmental Research and Public Health 16, no. 5: 880. https://doi.org/10.3390/ijerph16050880
APA StyleMarusiak, J., Fisher, B. E., Jaskólska, A., Słotwiński, K., Budrewicz, S., Koszewicz, M., Kisiel-Sajewicz, K., Kamiński, B., & Jaskólski, A. (2019). Eight Weeks of Aerobic Interval Training Improves Psychomotor Function in Patients with Parkinson’s Disease—Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 16(5), 880. https://doi.org/10.3390/ijerph16050880





