In Vitro and In Vivo Testing to Determine Cd Bioaccessibility and Bioavailability in Contaminated Rice in Relation to Mouse Chow
Abstract
1. Introduction
2. Materials and Methods
2.1. Chow and Rice Preparation
2.2. In Vitro Bioaccessibility Test
2.3. In Vivo Experiment of Cd Exposure
2.4. Element Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Rice and Mouse Chow Characteristics
3.2. In Vitro Bioaccessibility of Cd
3.3. In Vivo Bioavailability of Cd
3.4. Correlation of Cd Accumulation in Biomarkers and Mineral Elements
3.5. Comparison of Cd Bioaccessibility vs Bioavailability and Implication for Exposure Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Engström, A.; Karl, M.; Yasushi, S.; Alicja, W.; Marie, V.; Agneta, Å. Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women. J. Bone Miner. Res. 2011, 26, 486–495. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Lei, M.; Sun, G.; Huang, Q.; Lu, Y.; Deacon, C.; Meharg, A.A.; Zhu, Y.G. Occurrence and partitioning of cadmium, arsenic and lead in mine impacted paddy rice: Hunan, China. Environ. Sci. Technol. 2009, 43, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Dietary exposure to cadmium at close to the current provisional tolerable weekly intake does not affect renal function among female Japanese farmers. Environ. Res. 2004, 95, 20–31. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Nutritional status affects the absorption and whole-body and organ retention of cadmium in rats fed rice-based diets. Environ. Sci. Technol. 2002, 36, 2684–2692. [Google Scholar] [CrossRef]
- Zhuang, P.; Li, Y.; Zou, B.; Su, F.; Zhang, C.; Mo, H.; Li, Z. Oral bioaccessibility and human exposure assessment of cadmium and lead in market vegetables in the Pearl River Delta, South China. Environ. Sci. Pollut. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Oomen, A.G.; Alfons, H.; Mans, M.; Evelijn, Z.; Christa, C.; Greet, S.; Willy, V.; Tom, V.D.W.; Joanna, W.; Cathy, J.M.R. Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environ. Sci. Technol. 2002, 36, 3326–3334. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; Kamp, E.V.D.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Ruby, M.V.; Davis, A.; Schoof, R.; Eberle, S.; Sellstone, C.M. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ. Sci. Technol. 1996, 30, 422–430. [Google Scholar] [CrossRef]
- Intawongse, M.; Dean, J.R. Use of the physiologically-based extraction test to assess the oral bioaccessibility of metals in vegetable plants grown in contaminated soil. Environ Pollut. 2008, 152, 60–72. [Google Scholar]
- Yang, L.S.; Zhang, X.W.; Li, Y.H.; Li, H.R.; Wang, Y.; Wang, W.Y. Bioaccessibility and risk assessment of cadmium from uncooked rice using an In Vitro digestion model. Biol. Trace Elem. Res. 2012, 145, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; He, Q.; Wu, Y.; Chen, X.; Ning, Y.L.; Chen, Y. Investigating the bioaccessibility and bioavailability of cadmium in a cooked rice food matrix by using an 11-day rapid Caco-2/HT-29 co-culture cell model combined with an in vitro digestion model. Biol. Trace Elem. Res. 2019, in press. [Google Scholar]
- Houlbrèque, F.; Hervé-Fernández, P.; Teyssié, J.; Oberhaënsli, F.; Boisson, F.; Jeffree, R. Cooking makes cadmium contained in Chilean mussels less bioaccessible to humans. Food Chem. 2011, 126, 917–921. [Google Scholar] [CrossRef]
- Li, S.W.; Sun, H.J.; Li, H.B.; Luo, J.; Ma, L.Q. Assessment of cadmium bioaccessibility to predict its bioavailability in contaminated soils. Environ. Int. 2016, 94, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Schilderman, P.A.; Moonen, E.J.; Kempkers, P.; Kleinjans, J.C. Bioavailability of soil-adsorbed cadmium in orally exposed male rats. Environ. Health Perspect. 1997, 105, 234–238. [Google Scholar] [CrossRef]
- Violet, D.; Noller, B.N.; Ng, J.C. Interaction effects of lead on bioavailability and pharmacokinetics of arsenic in the rat. Environ. Geochem. Health 2013, 35, 757–766. [Google Scholar]
- Ollson, C.J.; Smith, E.; Herde, P.; Juhasz, A.L. Influence of samplematrix on the bioavailability of arsenic, cadmium and lead during co-contaminant exposure. Sci. Total Environ. 2017, 595, 660–665. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Romarís-Hortas, V.; Moscoso-Pérez, C.; López-Mahía, P.; Muniategui-Lorenzo, S.; Bermejo-Barrera, P.; Prada-Rodríguez, D. In-vivo and in-vitro testing to assess the bioaccessibility and the bioavailability of arsenic, selenium and mercury species in food samples. TrAC Trends Anal. Chem. 2011, 30, 324–345. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci. Total Environ. 2008, 398, 13–19. [Google Scholar] [CrossRef]
- Juhasz, A.L.; John, W.; Ravi, N.; Dorota, G.; Allan, R.; Damian, T.; Euan, S. Determination of cadmium relative bioavailability in contaminated soils and its prediction using in vitro methodologies. Environ. Sci. Technol. 2010, 44, 5240. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Food and Agriculture Drganization of the United Nations; FAO: Rome, Italy, 2014. [Google Scholar]
- Ministry of Health of the People’s Republic of China. Food Safety National Standard for Maximum Levels of Contaminant in Food; GB 2762-2017; Ministry of Health of the People’s Republic of China: Beijing, China, 2017.
- Zhuang, P.; Sun, S.; Su, F.; Li, F.; Zhou, X.; Mao, P.; Li, Y.; Li, Z.; Zhang, C. Dietary strategies to reduce the oral bioaccessibility of cadmium and arsenic in rice. Environ. Sci. Pollut. Res. 2018, 25, 33353–33360. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Cherian, M.G. The influence of nutritional deficiencies on gastrointestinal uptake of cadmium and cadmium-metallothionein in rats. Toxicology 1995, 97, 71. [Google Scholar] [CrossRef]
- Fu, J.; Cui, Y. In vitro digestion/Caco-2 cell model to estimate cadmium and lead bioaccessibility/bioavailability in two vegetables: The influence of cooking and additives. Food Chem. Toxicol. 2013, 59, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Mounicou, S.; Szpunar, J.; Andrey, D.; Blake, C.; Lobinski, R. Development of a sequential enzymolysis approach for the evaluation of the bioaccessibility of Cd and Pb from cocoa. Analyst 2002, 127, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Maulvault, A.L.; Machado, R.; Afonso, C.; Lourenço, H.M.; Nunes, M.L.; Coelho, I.; Langerholc, T.; Marques, A. Bioaccessibility of Hg, Cd and As in cooked black scabbard fish and edible crab. Food Chem. Toxicol. 2011, 49, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Chaney, R.L. Mineral status of female rats affects the absorption and organ distribution of dietary cadmium derived from edible sunflower kernels (Helianthus annuus L.). Environ. Res. Sect. A 2001, 85, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Houpert, P.; Federspiel, B.; Milhaud, G. Toxicokinetics of cadmium in lactating and nonlactating ewes after oral and intravenous administration. Environ. Res. 1997, 72, 140. [Google Scholar] [CrossRef]
- WHO Environmental Health Criteria: Cadmium; World Health Organization: Geneva, Switzerland, 1992; Volume 134.
- Groten, J.P.; Sinkeldam, E.J.; Muys, T.; Luten, J.B.; Van Bladeren, P.J. Interaction of dietary Ca, P, Mg, Mn, Cu, Fe, Zn and Se with the accumulation and oral toxicity of cadmium in rats. Food Chem. Toxicol. 1991, 29, 249–258. [Google Scholar] [CrossRef]
- Walter, A.; Rimbach, G.; Most, E.; Pallauf, J. Effect of calcium supplements to a maize-soya diet on the bioavailability of minerals and trace elements and the accumulation of heavy metals in growing rats. J. Vet. Med. 2000, 47, 367–377. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, K.Y.; Choi, B.S.; Youn, P.; Ryu, D.Y.; Klaassen, C.D.; Park, J.D. Regulation of metal transporters by dietary iron, and the relationship between body iron levels and cadmium uptake. Arch. Toxicol. 2007, 81, 327–334. [Google Scholar] [CrossRef]
- Bredderman, P.J.; Wasserman, R.H. Chemical composition, affinity for calcium, and some related properties of the vitamin D dependent calcium-binding protein. Biochemistry 1974, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. The influence of calcium content in diet on cumulation and toxicity of cadmium in the organism: Review. Arch. Toxicol. 1997, 72, 63–73. [Google Scholar] [CrossRef]
- Zhao, D.; Juhasz, A.L.; Luo, J.; Huang, L.; Luo, X.; Li, H.B.; Ma, L.Q. Mineral dietary supplements to decrease cadmium relative bioavailability in rice based on a mouse bioassay. Environ. Sci. Technol. 2017, 51, 12123–12130. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.M. The effects of whole wheat, wheat bran and zinc in the diet on the absorption and accumulation of cadmium in rats. Br. J. Nutr. 1993, 69, 199–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hooser, S.B. Cadmium. In Veterinary Toxicology, Basic and Clinical Principles, 3rd ed.; Ramesh, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 741. [Google Scholar]
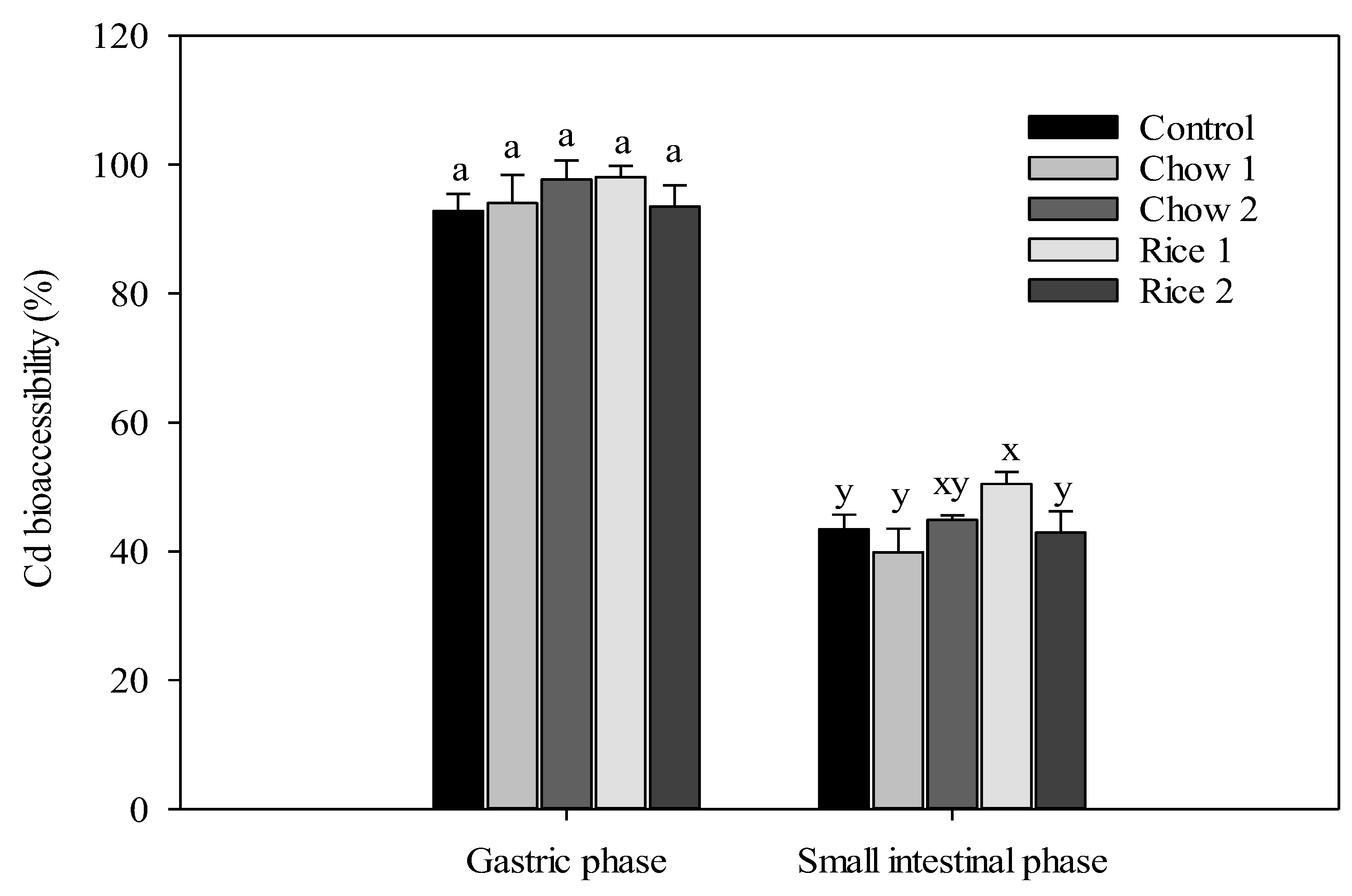
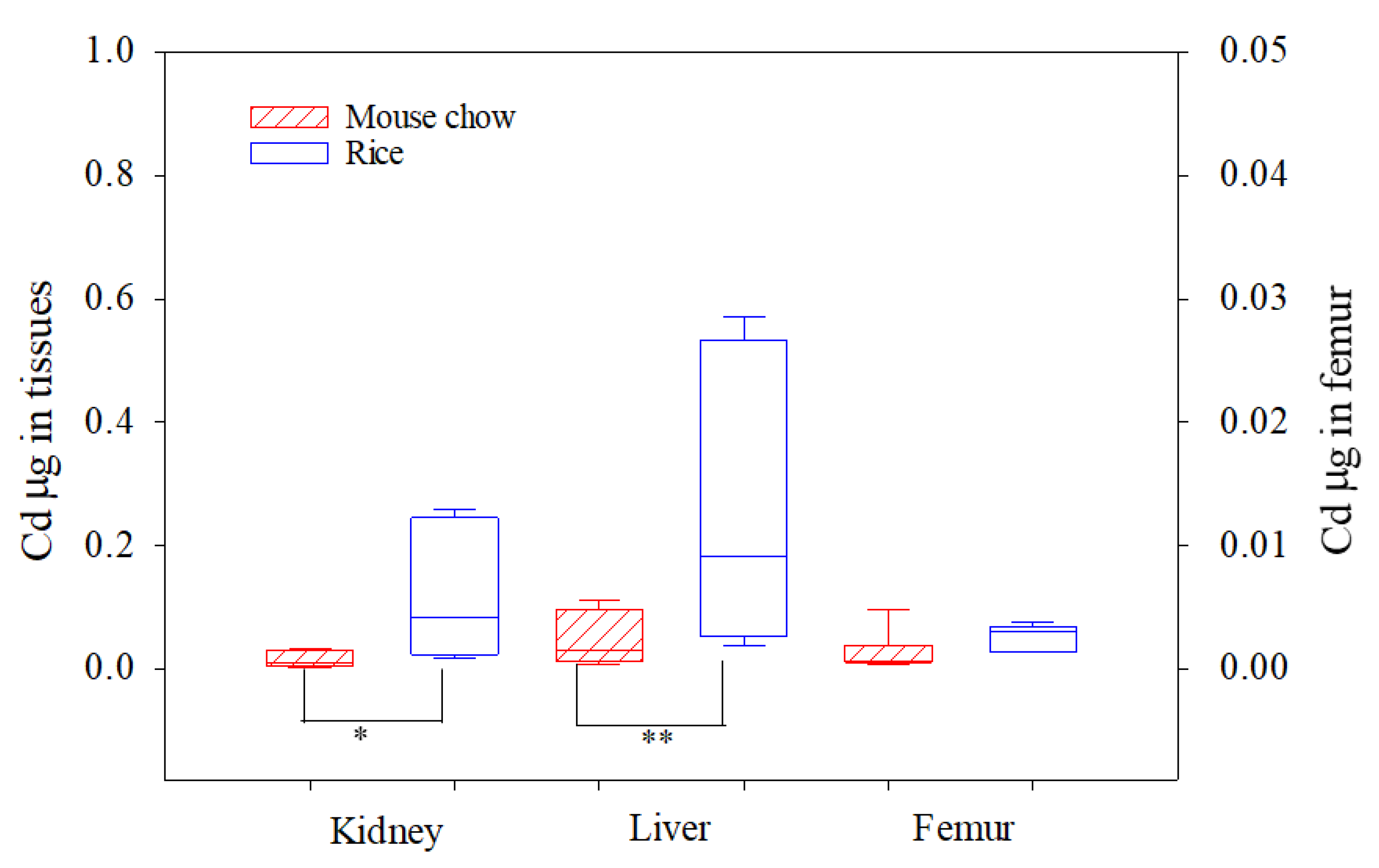
| Constituent | Basal Mouse Chow | Rice 1 | Rice 2 |
|---|---|---|---|
| Major elements (mg kg−1) | |||
| Ca | 11300 ± 200 | 240 ± 10 | 130 ± 10 |
| Mg | 2700 ± 20 | 1000 ± 100 | 1270 ± 50 |
| Fe | 110 ± 20 | 50 ± 10 | 30 ± 10 |
| Minor elements (mg kg−1) | |||
| Cd | 0.12 ± 0.01 | 1.26 ± 0.03 | 3.65 ± 0.05 |
| Pb | 0.13 ± 0.01 | 0.89 ± 0.04 | 0.05 ± 0.01 |
| Cu | 16.0 ± 0.19 | 6.03 ± 0.30 | 4.12 ± 0.15 |
| Zn | 113 ± 0.70 | 30.1 ± 0.40 | 20.2 ± 0.06 |
| Treatments | Kidney | Liver | Femur |
|---|---|---|---|
| Control | 4.90 ± 3.1d | 3.5 ± 0.3c | 1.6 ± 0.2a |
| Chow 1 | 22.5 ± 0.8d | 15.7 ± 0.6c | 1.0 ± 0.1a |
| Chow 2 | 62.8 ± 2.5c | 57.3 ± 4.1b | 4.7 ± 1.9a |
| Rice 1 | 106.8 ± 6.1b | 84.4 ± 13.7b | 2.9 ± 0.3a |
| Rice 2 | 290.7 ± 11.9a | 218.5 ± 12.3a | 3.1 ± 0.2a |
| Treatments | Kidney (%) | Liver (%) | Femur (%) |
|---|---|---|---|
| Control | 0.050 ± 0.020 b | 0.110 ± 0.001 b | 0.0090 ± 0.0010 a |
| chow 1 | 0.020 ± 0.002 c | 0.050 ± 0.005 c | 0.0010 ± 0.0001 c |
| chow 2 | 0.020 ± 0.001 c | 0.060 ± 0.004 c | 0.0020 ± 0.0010 bc |
| rice 1 | 0.090 ± 0.010 a | 0.200 ± 0.040 a | 0.0030 ± 0.0010 b |
| rice 2 | 0.080 ± 0.004 a | 0.180 ± 0.007 a | 0.0010 ± 0.0002 c |
| Elements | Kidney | Liver | Femur |
|---|---|---|---|
| Ca | −0.799 ** | −0.790 ** | −0.062 |
| Fe | −0.921 ** | −0.921 ** | −0.266 |
| Mg | −0.679 * | −0.665 * | −0.008 |
| Cu | −0.814 ** | −0.798 ** | −0.014 |
| Zn | −0.797 ** | −0.781 ** | −0.025 |
| Pb | −0.218 | −0.218 | −0.030 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Zhou, X.; Li, Z.; Zhuang, P. In Vitro and In Vivo Testing to Determine Cd Bioaccessibility and Bioavailability in Contaminated Rice in Relation to Mouse Chow. Int. J. Environ. Res. Public Health 2019, 16, 871. https://doi.org/10.3390/ijerph16050871
Sun S, Zhou X, Li Z, Zhuang P. In Vitro and In Vivo Testing to Determine Cd Bioaccessibility and Bioavailability in Contaminated Rice in Relation to Mouse Chow. International Journal of Environmental Research and Public Health. 2019; 16(5):871. https://doi.org/10.3390/ijerph16050871
Chicago/Turabian StyleSun, Shuo, Xiaofang Zhou, Zhian Li, and Ping Zhuang. 2019. "In Vitro and In Vivo Testing to Determine Cd Bioaccessibility and Bioavailability in Contaminated Rice in Relation to Mouse Chow" International Journal of Environmental Research and Public Health 16, no. 5: 871. https://doi.org/10.3390/ijerph16050871
APA StyleSun, S., Zhou, X., Li, Z., & Zhuang, P. (2019). In Vitro and In Vivo Testing to Determine Cd Bioaccessibility and Bioavailability in Contaminated Rice in Relation to Mouse Chow. International Journal of Environmental Research and Public Health, 16(5), 871. https://doi.org/10.3390/ijerph16050871





